Broad-Spectrum Antiviral Natural Products from the Marine-Derived Penicillium sp. IMB17-046
Abstract
:1. Introduction
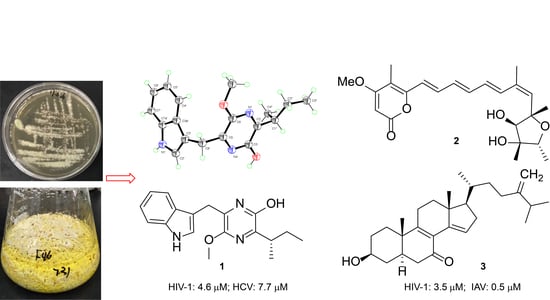
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation and Isolation
3.4. Crystallographic Analysis of 1
3.5. ECD Calculation of (14S,15R,16R,17R)-2
3.6. Anti-HIV Assay
3.7. Anti-Influenza A Virus Assay
3.8. Anti-HCV Assay
3.9. Cytotoxicity Assay
3.10. Antibacterial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Global Hepatitis Report; World Health Organization: Geneva, Switzerland, 2017.
- De Clercq, E.; Li, G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2012–2013: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2017, 15, 273. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Hao, X.; Tan, J.; Li, F.; Qiao, X.; Chen, S.; Xiao, C.; Chen, M.; Peng, Z.; et al. Raistrickindole A, an anti-HCV oxazinoindole alkaloid from Penicillium raistrickii IMB17-034. J. Nat. Prod. 2019, 82, 1391–1395. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, M.; Wu, C.; Tan, Y.; Li, J.; Hao, X.; Duan, Y.; Guan, Y.; Shang, X.; Wang, Y.; et al. Identification and proposed relative and absolute configurations of niphimycins C–E from the marine-derived Streptomyces sp. IMB7-145 by genomic analysis. J. Nat. Prod. 2018, 81, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wang, M.; Tan, Y.; Hu, X.; He, H.; Xiao, C.; You, X.; Wang, Y.; Gan, M. Neo-actinomycins A and B, natural actinomycins bearing the 5H-oxazolo[4,5-b]phenoxazine chromophore, from the marine-derived Streptomyces sp. IMB094. Sci. Rep. 2017, 7, 3591. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Hu, Y.; Wang, Q.; Zhou, H.; Wang, Y.; Gan, M. Tetrocarcins N and O, glycosidic spirotetronates from a marine-derived Micromonospora sp. identified by PCR-based screening. RSC Advances 2016, 6, 91773–91778. [Google Scholar] [CrossRef]
- Wu, C.; Tan, Y.; Gan, M.; Wang, Y.; Guan, Y.; Hu, X.; Zhou, H.; Shang, X.; You, X.; Yang, Z.; et al. Identification of elaiophylin derivatives from the marine-derived actinomycete Streptomyces sp. 7-145 using PCR-based screening. J. Nat. Prod. 2013, 76, 2153–2157. [Google Scholar] [CrossRef]
- Nishiyama, S.; Shizuri, Y.; Toshima, H.; Ozaki, M.; Yamamura, S.; Kawai, K.; Kawai, N.; Furukawa, H. Isolation, structural elucidation, and total synthesis of epiisocitreoviridinol. Chem. Lett. 1987, 16, 515–518. [Google Scholar] [CrossRef]
- Asai, T.; Luo, D.; Yamashita, K.; Oshima, Y. Structures and biomimetic synthesis of novel α-pyrone polyketides of an endophytic Penicillium sp. In catharanthus roseus. Org. Lett. 2013, 15, 1020–1023. [Google Scholar] [CrossRef]
- Muhammad, I.; Choudhary, S.G.M. Talat Mukhmoor, Farzana Shaheen, Shamsher Ali, and Atta-ur-Rahman. Isolation of bioactive compounds from Aspergillus terreus. Z. Naturforsch. 2004, 59b, 324–328. [Google Scholar]
- Cui, C.M.; Li, X.M.; Meng, L.; Li, C.S.; Huang, C.G.; Wang, B.G. 7-nor-ergosterolide, a pentalactone-containing norsteroid and related steroids from the marine-derived endophytic Aspergillus ochraceus EN-31. J. Nat. Prod. 2010, 73, 1780–1784. [Google Scholar] [CrossRef]
- Piccialli, V.; Sica, D. Four new trihydroxylated sterols from the sponge Spongionella gracilis. J. Nat. Prod. 1987, 50, 915–920. [Google Scholar] [CrossRef]
- Motohashi, K.; Inaba, K.; Fuse, S.; Doi, T.; Izumikawa, M.; Khan, S.T.; Takagi, M.; Takahashi, T.; Shin-ya, K. JBIR-56 and JBIR-57, 2(1H)-pyrazinones from a marine sponge-derived Streptomyces sp. SPD081030SC-03. J. Nat. Prod. 2011, 74, 1630–1635. [Google Scholar] [CrossRef]
- Goetz, M.A.; Zhang, C.; Zink, D.L.; Arocho, M.; Vicente, F.; Bills, G.F.; Polishook, J.; Dorso, K.; Onishi, R.; Gill, C.; et al. Coelomycin, a highly substituted 2,6-dioxo-pyrazine fungal metabolite antibacterial agent discovered by Staphylococcus aureus fitness test profiling. J. Antibiot. 2010, 63, 512–518. [Google Scholar] [CrossRef]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Crystallogr. Sect. A 1983, 39, 876–881. [Google Scholar] [CrossRef]
- Hooft, R.W.W.; Straver, L.H.; Spek, A.L. Determination of absolute structure using Bayesian statistics on Bijvoet differences. J. Appl. Crystallogr. 2008, 41, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Badrinarayanan, S.; Sperry, J. Pyrazine alkaloids via dimerization of amino acid-derived alpha-amino aldehydes: Biomimetic synthesis of 2,5-diisopropylpyrazine, 2,5-bis(3-indolylmethyl)pyrazine and actinopolymorphol C. Org. Biomol. Chem. 2012, 10, 2126–2132. [Google Scholar] [CrossRef]
- Nawrath, T.; Dickschat, J.S.; Kunze, B.; Schulz, S. The biosynthesis of branched dialkylpyrazines in myxobacteria. Chem. Biodivers. 2010, 7, 2129–2144. [Google Scholar] [CrossRef]
- Franck, B.; Gehrken, H.P. Citreoviridins from Aspergillus terreus. Angew. Chem. Int. Ed. Engl. 1980, 19, 461–462. [Google Scholar] [CrossRef]
- Steyn, P.S.; Vleggaar, R.; Wessels, P.L.; Woudenberg, M. Biosynthesis of citreoviridin. A carbon-13 N.M.R. Study. J. Chem. Soc. Perkin Trans. 1 1982, 2175–2178. [Google Scholar] [CrossRef]
- Sakabe, N.; Goto, T.; Hirata, Y. Structure of citreoviridin, a mycotoxin produced by Penicillium citreo-viride molded on rice. Tetrahedron 1977, 33, 3077–3081. [Google Scholar] [CrossRef]
- Arai, K.; Masuda, K.; Kiriyama, N.; Nitta, K.; Yamamoto, Y.; Shimizu, S. Metabolic products of Aspergillus terreus. IV. Metabolites of the strain ifo 8835. (2). The isolation and chemical structure of indolyl benzoquinone pigments. Chem. Pharm. Bull. 1981, 29, 961–969. [Google Scholar] [CrossRef]
- Nagel, D.W.; Steyn, P.S.; Scott, D.B. Production of citreoviridin by Penicillium pulvillorum. Phytochemistry 1972, 11, 627–630. [Google Scholar] [CrossRef]
- Ge, Y.Z.; Zhou, B.; Xiao, R.X.; Yuan, X.J.; Zhou, H.; Xu, Y.C.; Wainberg, M.A.; Han, Y.S.; Yue, J.M. A new class of HIV-1 inhibitors and the target identification via proteomic profiling. Sci. China Chem. 2018, 61, 1430–1439. [Google Scholar] [CrossRef]
- Perwitasari, O.; Yan, X.; O’Donnell, J.; Johnson, S.; Tripp, R.A. Repurposing kinase inhibitors as antiviral agents to control influenza A virus replication. Assay Drug Dev. Technol. 2015, 13, 638–649. [Google Scholar] [CrossRef]
- Mohr, E.L.; McMullan, L.K.; Lo, M.K.; Spengler, J.R.; Bergeron, É.; Albariño, C.G.; Shrivastava-Ranjan, P.; Chiang, C.-F.; Nichol, S.T.; Spiropoulou, C.F.; et al. Inhibitors of cellular kinases with broad-spectrum antiviral activity for hemorrhagic fever viruses. Antiviral Res. 2015, 120, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Fock, K.M.; Graham, D.Y.; Malfertheiner, P. Helicobacter pylori research: Historical insights and future directions. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 495. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of shelx. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision a.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. Specdis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, J.; Yang, Z.; Ma, L.; Mi, Z.; Wu, Y.; Guo, J.; Zhou, J.; Li, X.; Guo, Y.; et al. Microbial natural product alternariol 5-O-methyl ether inhibits HIV-1 integration by blocking nuclear import of the pre-integration complex. Viruses 2017, 9, 105. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, Z.; Liu, Z.; Li, X.; Zhang, Y.; Zhang, Z.; Cen, S. A cell-based high-throughput approach to identify inhibitors of influenza A virus. Acta. Pharm. Sin. B 2014, 4, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Hang, X.; Jiang, X.; Zeng, L.; Jia, J.; Xie, Y.; Li, F.; Bi, H. In vitro and in vivo activities of zinc linolenate, a selective antibacterial agent against Helicobacter pylori. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Miniyar, P.B.; Murumkar, P.R.; Patil, P.S.; Barmade, M.A.; Bothara, K.G. Unequivocal role of pyrazine ring in medicinally important compounds: A review. Mini. Rev. Med. Chem. 2013, 13, 1607–1625. [Google Scholar] [CrossRef]
- Dolezal, M.; Zitko, J. Pyrazine derivatives: A patent review (june 2012–present). Expert Opin. Ther. Pat. 2015, 25, 33–47. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–8 are available from the authors. |





| No. | 1 | No. | 2 | 3 | |||
|---|---|---|---|---|---|---|---|
| δC, Type | δH, Mult. (J in Hz) | δC, Type | δH, Mult. (J in Hz) | δC, Type | δH, Mult. (J in Hz) | ||
| 2 | 153.3, C | 1 | 164.0, C | 34.8, CH2 | 1.92, m; 1.46, m | ||
| 3 | 149.8, C | 2 | 88.6, CH2 | 5.51, s | 31.3, CH2 | 1.94, m; 1.54, m | |
| 5 | 148.7, C | 3 | 170.8, C | 69.9, CH | 3.67, dddd (11.4, 11.4, 4.8, 4.2) | ||
| 6 | 128.7, C | 4 | 108.0, C | 37.3, CH2 | 1.75, m; 1.42, m | ||
| 1′ | 8.04, s | 5 | 154.5, C | 38.8, CH | 2.00, m | ||
| 2′ | 123.5, CH | 7.22, d (2.4) | 6 | 119.0, CH | 6.35, d (15.0) | 42.1, CH2 | 2.37, dd (10.8, 16.8) |
| 3′ | 111.4, CH | 2.35, dd (10.8, 4.8) | |||||
| 3a′ | 127.3, C | 7 | 136.0, CH | 7.18, dd (10.8, 15.0) | 197.5, C | ||
| 4′ | 119.3, CH | 7.68, brd (7.8) | 8 | 131.8, CH | 6.40, dd (10.8, 15.0) | 127.1, C | |
| 5′ | 119.8, CH | 7.09, ddd (7.8, 7.2, 1.2) | 9 | 139.0, CH | 6.57, dd (10.8, 15.6) | 165.2, C | |
| 6′ | 122.4, CH | 7.18, ddd (7.8, 7.2, 1.2) | 10 | 129.9, CH | 6.31, dd (10.8, 15.0) | 38.0, C | |
| 7′ | 111.3, CH | 7.34, brd (7.8) | 11 | 133.2, CH | 7.09, d (15.6) | 24.2, CH2 | 2.54, dd (20.4, 5.4) |
| 7a′ | 136.3, C | 2.41, ddd (20.4, 12.0, 6.0) | |||||
| 8′ | 24.9, CH2 | 4.13, d (15.6) | 12 | 132.0, C | 36.0, CH2 | 2.10, dd (12.0, 6.0); 1.47, m | |
| 4.10, d (15.6) | 13 | 138.7, CH | 5.75, s | 45.7, C | |||
| 1′′ | 36.2, CH | 3.15, sextet (6.6) | 14 | 84.5, C | 141.3, C | ||
| 2′′ | 28.0, CH2 | 1.81, m | 15 | 86.2, CH | 4.02, s | 126.6, CH | 6.46, brs |
| 1.55, m | 16 | 81.0, C | 36.9, CH2 | 2.48, ddd (16.2, 7.2, 3.0) | |||
| 3′′ | 12.2, CH3 | 0.87, t (7.2) | 2.19, dd (16.2, 6.0) | ||||
| 4′′ | 18.4, CH3 | 1.20, d (6.6) | 17 | 77.6, CH | 3.84, q (6.6) | 55.8, CH | 1.52, m |
| OMe | 54.4, CH3 | 3.98, s | 18 | 12.3, CH3 | 1.20, d (6.6) | 15.5, CH3 | 0.80, s |
| 19 | 17.4, CH3 | 1.23, s | 17.6, CH3 | 1.14, s | |||
| 20 | 20.7, CH3 | 1.40, s | 34.0, CH | 1.65, m | |||
| 21 | 22.6, CH3 | 1.85, s | 19.0, CH3 | 0.99, d (6.6) | |||
| 22 | 8.9, CH3 | 1.96, s | 34.5, CH2 | 1.61, m; 1.22, m | |||
| 23 | 30.9, CH2 | 2.13, m; 1.93, m | |||||
| 24 | 156.7, C | ||||||
| 25 | 33.8, CH | 2.24, m | |||||
| 26 | 22.0, CH3 | 1.04, d (7.2) | |||||
| 27 | 21.9, CH3 | 1.03, d (7.2) | |||||
| 28 | 106.1, CH2 | 4.73, d (1.8); 4.68, d (1.8) | |||||
| OMe | 56.3, CH3 | 3.83, s | |||||
| Compound | HIV-1 | IAV | HCV | H. pylori (MIC, μg/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | CC50 (μM) | IC50 (μM) | CC50 (μM) | IC50 (μM) | CC50 (μM) | G27 | 159 | |
| 1 | 4.6 ± 0.3 | 44.3 ± 1.6 | 20.4 ± 0.3 | 76.7 ± 4.6 | 7.7 ± 0.2 | 116.1 ± 4.9 | 4 | 16 |
| 2 | >10 | >100 | 3.6 ± 0.2 | >100 | NT | NT | 4 | 1 |
| 3 | 3.5 ± 0.8 | 51.2 ± 3.5 | 0.5 ± 0.02 | >100 | NT | NT | NT | NT |
| 7 | >10 | >100 | >10 | >100 | NT | NT | NT | NT |
| 8 | 6.2 ± 0.2 | 26.0 ± 0.2 | >10 | >100 | NT | NT | NT | NT |
| Efavirenz | 0.0005 ± 0.0002 | >100 | NT | NT | NT | NT | NT | NT |
| Ribavirin | NT | NT | 15.4 ± 0.9 | >100 | NT | NT | NT | NT |
| VX-950 | NT | NT | NT | NT | 0.05 ± 0.03 | 25.8 ± 3.4 | NT | NT |
| Metronidazole | NT | NT | NT | NT | NT | NT | 1 | 16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, Y.; Hao, X.; Li, S.; Jia, J.; Guan, Y.; Peng, Z.; Bi, H.; Xiao, C.; Cen, S.; et al. Broad-Spectrum Antiviral Natural Products from the Marine-Derived Penicillium sp. IMB17-046. Molecules 2019, 24, 2821. https://doi.org/10.3390/molecules24152821
Li J, Wang Y, Hao X, Li S, Jia J, Guan Y, Peng Z, Bi H, Xiao C, Cen S, et al. Broad-Spectrum Antiviral Natural Products from the Marine-Derived Penicillium sp. IMB17-046. Molecules. 2019; 24(15):2821. https://doi.org/10.3390/molecules24152821
Chicago/Turabian StyleLi, Jiao, Yujia Wang, Xiaomeng Hao, Shasha Li, Jia Jia, Yan Guan, Zonggen Peng, Hongkai Bi, Chunling Xiao, Shan Cen, and et al. 2019. "Broad-Spectrum Antiviral Natural Products from the Marine-Derived Penicillium sp. IMB17-046" Molecules 24, no. 15: 2821. https://doi.org/10.3390/molecules24152821
APA StyleLi, J., Wang, Y., Hao, X., Li, S., Jia, J., Guan, Y., Peng, Z., Bi, H., Xiao, C., Cen, S., & Gan, M. (2019). Broad-Spectrum Antiviral Natural Products from the Marine-Derived Penicillium sp. IMB17-046. Molecules, 24(15), 2821. https://doi.org/10.3390/molecules24152821