Neuroprotective Effects of Ginseng Phytochemicals: Recent Perspectives
Abstract
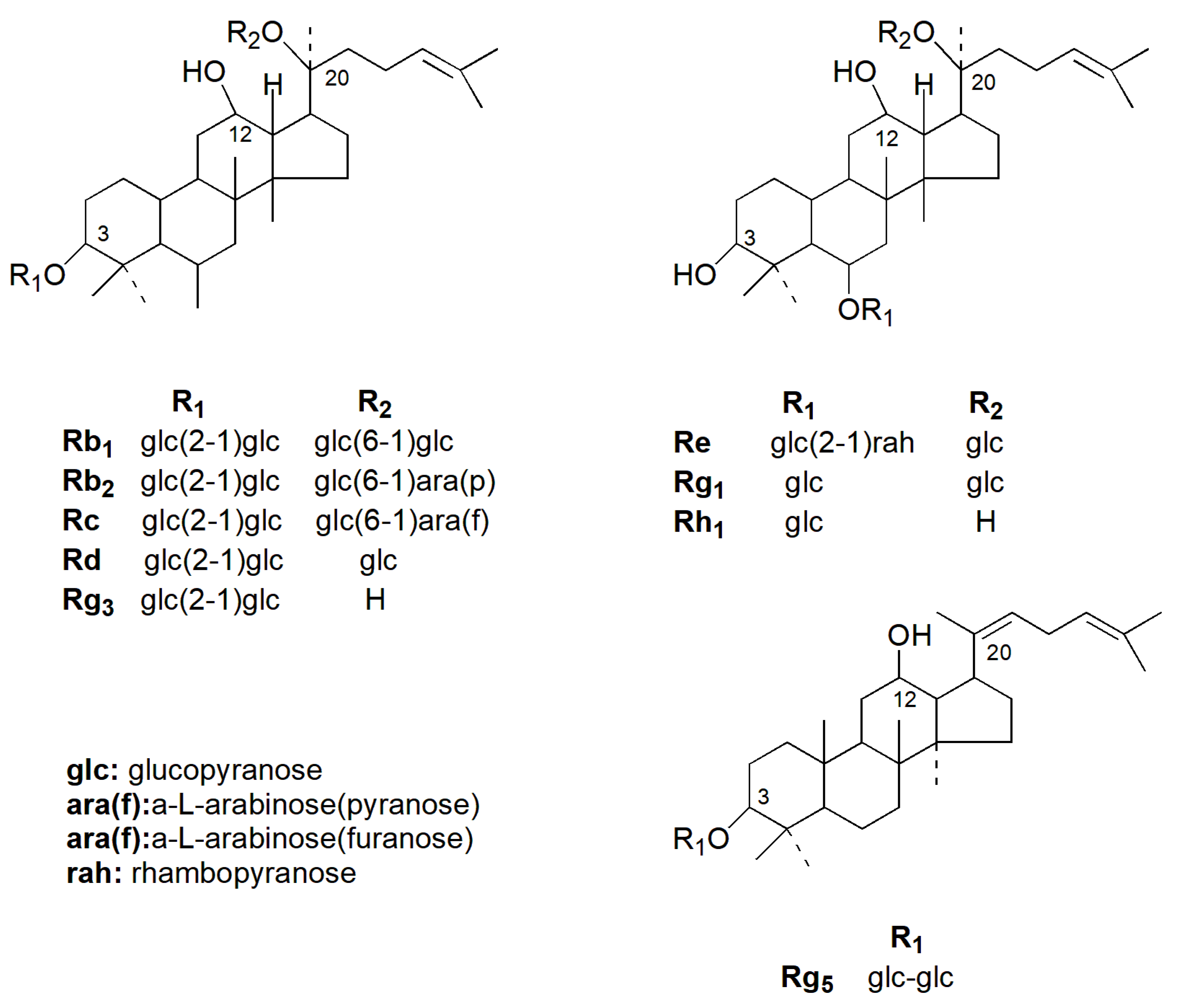
:1. Introduction
2. Chronic Neurodegenerative Diseases
2.1. Alzheimer’s Disease
2.2. Parkinson’s Disease
2.3. Huntington’s Disease
3. Acute Neurodegenerative Diseases
3.1. Cerebral Infarction
3.2. Cerebral Ischemia-Reperfusion Injury
4. Depression
5. Stress
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Liu, F.F.; Zhang, Z.; Chen, W.; Gu, H.Y.; Yan, Q.J. Regulatory mechanism of microRNA-377 on CDH13 expression in the cell model of Alzheimer’s disease. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2801–2808. [Google Scholar] [PubMed]
- Zhong, L.L.; Song, Y.Q.; Cao, H.; Ju, K.J.; Yu, L. The non-motor symptoms of Parkinson’s disease of different motor types in early stage. Eur. Rev. Med Pharmacol. Sci. 2017, 21, 5745–5750. [Google Scholar] [PubMed]
- Baquero, M.; Martin, N. Depressive symptoms in neurodegenerative diseases. World J. Clin. Cases 2015, 3, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Sung, J.H.; An, R.; Hernandez, M.E.; Sosnoff, J.J. Gait variability in people with neurological disorders: A systematic review and meta-analysis. Hum. Mov. Sci. 2016, 47, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; León, R. Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol. Ther. 2016, 157, 84–104. [Google Scholar] [CrossRef] [PubMed]
- Ciechanover, A.; Kwon, Y.T. Degradation of misfolded proteins in neurodegenerative diseases: Therapeutic targets and strategies. Exp. Mol. Med. 2015, 47, e147. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Im, K.; Kim, G.; Min, H. Antiviral activity of 20(R)-ginsenoside Rh2 against murine gammaherpesvirus. J. Ginseng Res. 2017, 41, 496–502. [Google Scholar] [CrossRef]
- Hong, B.N.; Ji, M.G.; Kang, T.H. The Efficacy of Red Ginseng in Type 1 and Type 2 Diabetes in Animals. Evid. Based Complement. Altern. Med. 2013, 2013, 593181. [Google Scholar] [CrossRef]
- Jeong, C.S. Effect of butanol fraction of Panax ginseng head on gastric lesion and ulcer. Arch. Pharmacal. Res. 2002, 25, 61. [Google Scholar] [CrossRef]
- Kiefer, D.; Pantuso, T. Panax ginseng. Am. Fam. Physician 2003, 68, 1539–1542. [Google Scholar]
- Liu, X.; Wang, L.; Wen, A.; Yang, J.; Yan, Y.; Song, Y.; Liu, X.; Ren, H.; Wu, Y.; Li, Z.; et al. Ginsenoside-Rd improves outcome of acute ischaemic stroke—A randomized, double-blind, placebo-controlled, multicenter trial. Eur. J. Neurol. 2012, 19, 855–863. [Google Scholar] [CrossRef]
- Strong, M.J.; Grace, G.M.; Freedman, M.; Lomen-Hoerth, C.; Woolley, S.; Goldstein, L.H.; Murphy, J.; Shoesmith, C.; Rosenfeld, J.; Leigh, P.N. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Mot. Neuron Disord. 2009, 10, 131–146. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Wang, Z.; Li, S.; Min, G.; Wang, L.; Chen, J.; Cheng, J.; Wu, Y. Inhibitory effect of ginsenoside-Rd on carrageenan-induced inflammation in rats. Can. J. Physiol. Pharmacol. 2012, 90, 229–236. [Google Scholar] [CrossRef]
- Yang, X.L.; Guo, T.K.; Wang, Y.H.; Huang, Y.H.; Liu, X.; Wang, X.X.; Li, W.; Zhao, X.; Wang, L.P.; Yan, S. Ginsenoside Rd attenuates the inflammatory response via modulating p38 and JNK signaling pathways in rats with TNBS-induced relapsing colitis. Int. Immunopharmacol. 2012, 12, 408–414. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Gillis, C.N. Panax ginseng pharmacology: A nitric oxide link? Biochem. Pharmacol. 1997, 54, 1–8. [Google Scholar] [CrossRef]
- Peña, I.D.; Yoon, S.Y.; Kim, H.J.; Park, S.; Hong, E.Y.; Ryu, J.H.; Park, I.H.; Cheong, J.H. Effects of ginseol k-g3, an Rg3-enriched fraction, on scopolamine-induced memory impairment and learning deficit in mice. J. Ginseng Res. 2014, 38, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Christensen, L.P. Chapter 1 Ginsenosides: Chemistry, Biosynthesis, Analysis, and Potential Health Effects. Adv. Food Nutr. Res. 2008, 55, 1–99. [Google Scholar]
- Shin, B.K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem. Toxicol. 2017, 107, 362–372. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, Y.; Han, S.H.; Jeon, J.Y.; Hwang, M.; Im, Y.J.; Kim, J.H.; Sun, Y.L.; Chae, S.W.; Kim, M.G. Development and validation of an LC-MS/MS method for determination of compound K in human plasma and clinical application. J. Ginseng Res. 2013, 37, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Koh, E.; Jang, O.H.; Hwang, K.H.; An, Y.N.; Moon, B.K. Effects of Steaming and Air-Drying on Ginsenoside Composition of Korean Ginseng (Panax ginseng C.A. Meyer). J. Food Process. Preserv. 2015, 39, 207–213. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, L.; Chen, K. Roles and mechanisms of ginsenoside in cardiovascular diseases: Progress and perspectives. Sci. China Life Sci. 2016, 59, 292–298. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, C.; Zhang, H.; Wu, R. Autophagy in glaucoma: Crosstalk with apoptosis and its implications. Brain Res. Bull. 2015, 117, 1–9. [Google Scholar] [CrossRef]
- Lee, B.; Sandhu, S.; McArthur, G. Cell cycle control as a promising target in melanoma. Curr. Opin. Oncol. 2015, 27, 141–150. [Google Scholar] [CrossRef]
- Brassai, A.; Suvanjeiev, R.G.; Ban, E.G.; Lakatos, M. Role of synaptic and nonsynaptic glutamate receptors in ischaemia induced neurotoxicity. Brain Res. Bull. 2015, 112, 1–6. [Google Scholar] [CrossRef]
- Dong, X.; Zheng, L.; Lu, S.; Yang, Y. Neuroprotective effects of pretreatment of ginsenoside Rb1 on severe cerebral ischemia-induced injuries in aged mice: Involvement of anti-oxidant signaling. Geriatr. Gerontol. Int. 2017, 17, 338–345. [Google Scholar] [CrossRef]
- Lv, H.; Wang, L.; Shen, J.; Hao, S.; Ming, A.; Wang, X.; Su, F.; Zhang, Z. Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Res. Bull. 2015, 115, 30–36. [Google Scholar] [CrossRef]
- Liu, C.X.; Xiao, P.G. Recent advances on ginseng research in China. J. Ethnopharmacol. 1992, 36, 27–38. [Google Scholar]
- Nah, S.Y.; Park, H.J.; Mccleskey, E.W. A trace component of ginseng that inhibits Ca2+ channels through a pertussis toxin-sensitive G protein. Proc. Natl. Acad. Sci. USA 1995, 92, 8739–8743. [Google Scholar] [CrossRef]
- Nocerino, E.; Amato, M.; Izzo, A.A. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia 2000, 71, S1–S5. [Google Scholar] [CrossRef]
- Van, J.K.; Robertson, H.; Hagg, T.; Drobitch, R. Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson’s disease. Exp. Neurol. 2003, 184, 521–529. [Google Scholar]
- Liu, L.; Anderson, G.A.; Fernandez, T.G.; Dore, S. Efficacy and Mechanism of Panax Ginseng in Experimental Stroke. Front. Neurosci. 2019, 13, 294. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, E.; Fernandez-Moriano, C.; Gomez-Serranillos, M.P. Potential neuroprotective activity of Ginseng in Parkinson’s disease: A review. J. Neuroimmune Pharmacol. 2015, 10, 14–29. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Veselov, V.V.; Zakharenko, A.M.; Golokhvast, K.S.; Nosyrev, A.E.; Cravotto, G.; Tsatsakis, A.; Spandidos, D.A. Panax ginseng components and the pathogenesis of Alzheimer’s disease (Review). Mol. Med. Rep. 2019, 19, 2975–2998. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, D.; Lee, H.L.; Kim, C.E.; Jung, K.; Kang, K.S. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: Past findings and future directions. J. Ginseng Res. 2018, 42, 239–247. [Google Scholar] [CrossRef]
- Rajabian, A.; Rameshrad, M.; Hosseinzadeh, H. Therapeutic potential of Panax ginseng and its constituents, ginsenosides and gintonin, in neurological and neurodegenerative disorders: A patent review. Expert Opin. Ther. Pat. 2018, 29, 55–72. [Google Scholar] [CrossRef]
- Mattson, M.P. Oxidative Stress, Perturbed Calcium Homeostasis, and Immune Dysfunction in Alzheimer’s Disease. J. NeuroVirol. 2002, 8, 539–550. [Google Scholar] [CrossRef]
- Harrop, J.S.; Sharan, A.D.; Vaccaro, A.R.; Przybylski, G.J. The cause of neurologic deterioration after acute cervical spinal cord injury. Spine 2001, 26, 340–346. [Google Scholar] [CrossRef]
- Appel, S.H.; Smith, R.G.; Le, W.D. Immune-mediated cell death in neurodegenerative disease. Adv. Neurol. 1996, 69, 153–159. [Google Scholar]
- Hardy, J. Pathways to Primary Neurodegenerative Disease. Ann. N. Y. Acad. Sci. 2000, 924, 29–34. [Google Scholar] [CrossRef]
- Braak, H.; Tredici, K.D.; Rüb, U.; Vos, R.A.I.D.; Steur, E.N.H.J.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Hebert, L.E.; Jennifer, W.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef]
- Bolognin, S.; Blanchard, J.; Wang, X.; Basurtoislas, G.; Tung, Y.C.; Kohlbrenner, E.; Grundkeiqbal, I.; Iqbal, K. An experimental rat model of sporadic Alzheimer’s disease and rescue of cognitive impairment with a neurotrophic peptide. Acta Neuropathol. 2012, 123, 133–151. [Google Scholar] [CrossRef]
- Praticò, D. Oxidative stress hypothesis in Alzheimer’s disease: A reappraisal. Trends Pharmacol. Sci. 2008, 29, 609–615. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.; Li, W.; Zhou, L.; Li, Q.; Wang, X.; He, P. A UPLC/MS-based metabolomics investigation of the protective effect of ginsenosides Rg1 and Rg2 in mice with Alzheimer’s disease. J. Ginseng Res. 2016, 40, 9–17. [Google Scholar] [CrossRef]
- Li, N.; Liu, B.; Dluzen, D.E.; Jin, Y. Protective effects of ginsenoside Rg 2 against glutamate-induced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2007, 111, 458–463. [Google Scholar] [CrossRef]
- Liu, L.; Huang, J.; Hu, X.; Li, K.; Sun, C. Simultaneous determination of ginsenoside (G-Re, G-Rg1, G-Rg2, G-F1, G-Rh1) and protopanaxatriol in human plasma and urine by LC-MS/MS and its application in a pharmacokinetics study of G-Re in volunteers. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2011–2017. [Google Scholar] [CrossRef]
- Jun, Y.E.; Yao, J.P.; Wang, X.U.; Zheng, M.; Peng, L.I.; Chengwei, H.E.; Wan, J.B.; Yao, X.; Huanxing, S.U. Neuroprotective effects of ginsenosides on neural progenitor cells against oxidative injury. Mol. Med. Rep. 2016, 13, 3083–3091. [Google Scholar]
- Peng, L.H.; Ko, C.H.; Siu, S.W.; Koon, C.M.; Yue, G.L.; Cheng, W.H.; Lau, T.W.; Han, Q.B.; Ng, K.M.; Fung, K.P.; et al. In vitro & in vivo assessment of a herbal formula used topically for bone fracture treatment. J. Ethnopharmacol. 2010, 131, 282–289. [Google Scholar]
- Fang, F.; Chen, X.; Huang, T.; Lue, L.F.; Luddy, J.S.; Yan, S.S. Multi-faced neuroprotective effects of Ginsenoside Rg1 in an Alzheimer mouse model. Biochim. Biophys. Acta 2012, 1822, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.M. Effect of Dosage Form and Administration Route to Distribution of Ginsenoside Rg1 in Serum and Brain of Rats. Chin. J. Exp. Tradit. Med Formulae 2011, 17, 43–46. [Google Scholar]
- Bai-Yin, H.; Chang-Qing, L.; You-Liang, X.; Dong-Xu, J.; Jian-Nan, C.; Fang-Yi, L. Study on percutaneous absorption of ginsenoside Rg1 in total saponins from Panax notoginseng through intact skin. J. Guangdong Pharm. Coll. 2010, 26, 564–567. [Google Scholar]
- Jia, L.; Wei, C.; Zi-Min, Y.; Xue-Tao, L. In vitro transdermal absorption studies of ginsenoside Rg1 in traumatology spray. Chin. J. Exp. Tradit. Chin. Med. 2010, 18, 18–20. [Google Scholar]
- Yuan, S.; Bo, L.; Xiu-Mei, T. Experimental study on in vitro permeation of swollen and painful cataplasm. Yunnan J. Tradit. Chin. Med. 2012, 33, 54. [Google Scholar]
- Hong-Cai, W.; Yu-Meng, J.; Xue, Z.; Ning, W.; Di, G.; Ming, G.; Jing-Yu, Z.; Zi-Chao, Y. Protective effects of ginsenoside Rb1 on Aβ amyloid-induced hippocampal neuronal injury in rats. J. Jilin Univ. Med. Ed. 2012, 38, 447–450. [Google Scholar]
- Zhao, H.H.; Di, J.; Liu, W.S.; Liu, H.L.; Lai, H.; Lu, Y.L. Involvement of GSK3 and PP2A in ginsenoside Rb1′s attenuation of aluminum-induced tau hyperphosphorylation. Behav. Brain Res. 2013, 241, 228–234. [Google Scholar] [CrossRef]
- Schapira, A.H. Pathogenesis of Parkinson’s disease. Baillières Clin. Neurol. 2011, 258, 307–310. [Google Scholar]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Badger, J.L.; Corderollana, O.; Hartfield, E.M.; Wademartins, R. Parkinson’s disease in a dish—Using stem cells as a molecular tool. Neuropharmacology 2014, 76, 88–96. [Google Scholar] [CrossRef]
- Kim, D.; Jeon, H.; Ryu, S.; Koo, S.; Ha, K.T.; Kim, S. Proteomic Analysis of the Effect of Korean Red Ginseng in the Striatum of a Parkinson’s Disease Mouse Model. PLoS ONE 2016, 11, e0164906. [Google Scholar] [CrossRef]
- Zhou, T.; Zu, G.; Zhang, X.; Wang, X.; Li, S.; Gong, X.; Liang, Z.; Zhao, J. Neuroprotective effects of ginsenoside Rg1 through the Wnt/beta-catenin signaling pathway in both in vivo and in vitro models of Parkinson’s disease. Neuropharmacology 2016, 101, 480–489. [Google Scholar] [CrossRef]
- Ardah, M.T.; Paleologou, K.E.; Lv, G.; Menon, S.A.; Abul Khair, S.B.; Lu, J.H.; Safieh-Garabedian, B.; Al-Hayani, A.A.; Eliezer, D.; Li, M.; et al. Ginsenoside Rb1 inhibits fibrillation and toxicity of alpha-synuclein and disaggregates preformed fibrils. Neurobiol. Dis. 2015, 74, 89–101. [Google Scholar] [CrossRef]
- Radad, K.; Gille, G.; Moldzio, R.; Saito, H.; Ishige, K.; Rausch, W.D. Ginsenosides Rb1 and Rg1 effects on survival and neurite growth of MPP+-affected mesencephalic dopaminergic cells. J. Neural Transm. 2004, 111, 37–45. [Google Scholar] [CrossRef]
- Heng, Y.; Zhang, Q.S.; Mu, Z.; Hu, J.F.; Yuan, Y.H.; Chen, N.H. Ginsenoside Rg1 attenuates motor impairment and neuroinflammation in the MPTP-probenecid-induced parkinsonism mouse model by targeting alpha-synuclein abnormalities in the substantia nigra. Toxicol. Lett. 2016, 243, 7–21. [Google Scholar] [CrossRef]
- Zhou, T.T.; Zu, G.; Wang, X.; Zhang, X.G.; Li, S.; Liang, Z.H.; Zhao, J. Immunomodulatory and neuroprotective effects of ginsenoside Rg1 in the MPTP(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) -induced mouse model of Parkinson’s disease. Int. Immunopharmacol. 2015, 29, 334–343. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Cao, F.; Miao, X.; Chen, T.; Chang, Q.; Zheng, Y. Formulation of 20(S)-protopanaxadiol nanocrystals to improve oral bioavailability and brain delivery. Int. J. Pharm. 2016, 497, 239–247. [Google Scholar] [CrossRef]
- Sun, X.C.; Ren, X.F.; Chen, L.; Gao, X.Q.; Xie, J.X.; Chen, W.F. Glucocorticoid receptor is involved in the neuroprotective effect of ginsenoside Rg1 against inflammation-induced dopaminergic neuronal degeneration in substantia nigra. J. Steroid Biochem. Mol. Biol. 2016, 155, 94–103. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Mourad, I.M.; Mohammed, H.S.; Noor, N.A.; Ezz, H.S.A. A study on the possible therapeutic role of Panax ginseng extract against a rat model of Parkinson’s disease induced by intrastriatal rotenone injection. Int. J. Clin. Exp. Med. 2016, 9, 3831–3841. [Google Scholar]
- Chen, X.; Huang, T.; Zhang, J.; Song, J.; Chen, L.; Zhu, Y. Involvement of calpain and p25 of CDK5 pathway in ginsenoside Rb1′s attenuation of beta-amyloid peptide25-35-induced tau hyperphosphorylation in cortical neurons. Brain Res. 2008, 1200, 99–106. [Google Scholar] [CrossRef]
- Chong, M.S.; Goh, L.K.; Lim, W.S.; Chan, M.; Tay, L.; Chen, G.; Feng, L.; Ng, T.P.; Tan, C.H.; Lee, T.S. Gene expression profiling of peripheral blood leukocytes shows consistent longitudinal downregulation of TOMM40 and upregulation of KIR2DL5A, PLOD1, and SLC2A8 among fast progressors in early Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2013, 34, 399–405. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Shim, J.S.; Song, M.Y.; Yim, S.V.; Lee, S.E.; Park, K.S. Proteomic analysis reveals that the protective effects of ginsenoside Rb1 are associated with the actin cytoskeleton in beta-amyloid-treated neuronal cells. J. Ginseng Res. 2016, 40, 278–284. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Zhang, Z.; Pei, X.; Wang, J.; Li, Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009, 1256, 111–122. [Google Scholar] [CrossRef]
- Baake, V.; Reijntjes, R.; Dumas, E.M.; Thompson, J.C.; Roos, R.A.C.; REGISTRY Investigators of the European Huntington’s Disease Network. Cognitive decline in Huntington’s disease expansion gene carriers. Cortex 2017, 95, 51–62. [Google Scholar] [CrossRef]
- Mehrabi, N.F.; Waldvogel, H.J.; Tippett, L.J.; Hogg, V.M.; Synek, B.J.; Faull, R.L. Symptom heterogeneity in Huntington’s disease correlates with neuronal degeneration in the cerebral cortex. Neurobiol. Dis. 2016, 96, 67–74. [Google Scholar] [CrossRef]
- Rosas, H.D.; Salat, D.H.; Lee, S.Y.; Zaleta, A.K.; Hevelone, N.; Hersch, S.M. Complexity and Heterogeneity: What Drives the Ever-changing Brain in Huntington’s Disease? Ann. N. Y. Acad. Sci. 2008, 1147, 196–205. [Google Scholar] [CrossRef]
- Waldvogel, H.J.; Thu, D.; Hogg, V.; Tippett, L.; Faull, R.L.M. Selective neurodegeneration, neuropathology and symptom profiles in Huntington’s disease. Adv. Exp. Med. Biol. 2012, 769, 141–152. [Google Scholar]
- Wu, J.; Jeong, H.K.; Bulin, S.E.; Kwon, S.W.; Park, J.H.; Bezprozvanny, I. Ginsenosides protect striatal neurons in a cellular model of Huntington’s disease. J. Neurosci. Res. 2009, 87, 1904–1912. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.; Yoon, I.S.; Lee, J.H.; Jang, B.J.; Jeong, S.M.; Lee, J.H.; Lee, B.H.; Han, J.S.; Oh, S.; et al. Protective effects of ginseng saponins on 3-nitropropionic acid-induced striatal degeneration in rats. Neuropharmacology 2005, 48, 743–756. [Google Scholar] [CrossRef]
- Johnston, S.C.; Mendis, S.; Mathers, C.D. Global variation in stroke burden and mortality: Estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009, 8, 345–354. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Thundyil, J.; Tang, S.C.; Sobey, C.G.; Taylor, S.M.; Arumugam, T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011, 6, 11. [Google Scholar] [CrossRef]
- Gomes, J.; Wachsman, A.M. Types of Strokes. In Handbook of Clinical Nutrition and Stroke; Humana Press: Totowa, NJ, USA, 2013; pp. 15–31. [Google Scholar]
- Callahan, A.; Amarenco, P.; Goldstein, L.B.; Sillesen, H.; Messig, M.; Samsa, G.P.; Altafullah, I.; Ledbetter, L.Y.; Macleod, M.J.; Scott, R. Risk of Stroke and Cardiovascular Events After Ischemic Stroke or Transient Ischemic Attack in Patients With Type 2 Diabetes or Metabolic Syndrome: Secondary Analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Trial. Arch. Neurol. 2011, 68, 1245–1251. [Google Scholar] [CrossRef]
- Zheng, G.Q.; Cheng, W.; Wang, Y.; Wang, X.M.; Zhao, S.Z.; Zhou, Y.; Liu, S.J.; Wang, X.T. Ginseng total saponins enhance neurogenesis after focal cerebral ischemia. J. Ethnopharmacol. 2011, 133, 724–728. [Google Scholar] [CrossRef]
- Liu, X.; Xia, J.; Wang, L.; Song, Y.; Yang, J.; Yan, Y.; Ren, H.; Zhao, G. Efficacy and safety of ginsenoside-Rd for acute ischaemic stroke: A randomized, double-blind, placebo-controlled, phase II multicenter trial. Eur. J. Neurol. 2009, 16, 569–575. [Google Scholar] [CrossRef]
- Sutherland, B.A.; Minnerup, J.; Balami, J.S.; Arba, F.; Buchan, A.M.; Kleinschnitz, C. Neuroprotection for ischaemic stroke: Translation from the bench to the bedside. Int. J. Stroke 2012, 7, 407–418. [Google Scholar] [CrossRef]
- Wahlgren, N.G.; Ahmed, N. Neuroprotection in cerebral ischaemia: Facts and fancies—the need for new approaches. Cerebrovasc. Dis. 2004, 17, 153–166. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Akiyoshi, Y.; Susumu, T.; Tokado, H.; Fukuzaki, K.; Nagata, R.; Samukawa, K.; Iwao, H.; Kito, G. Ginsenoside Rb1 Reduces Neurodegeneration in the Peri-infarct Area of a Thromboembolic Stroke Model in Non-human Primates. J. Pharmacol. Sci. 2008, 107, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tang, J.; Khatibi, N.H.; Zhu, M.; Chen, D.; Tu, L.; Chen, L.; Wang, S. Treatment with ginsenoside rb1, a component of panax ginseng, provides neuroprotection in rats subjected to subarachnoid hemorrhage-induced brain injury. Acta Neurochir. Suppl. 2011, 110, 75–79. [Google Scholar]
- Jiang, Z.; Wang, Y.; Zhang, X.; Peng, T.; Lu, Y.; Leng, J.; Xie, Q. Preventive and therapeutic effects of ginsenoside Rb1 for neural injury during cerebral infarction in rats. Am. J. Chin. Med. 2013, 41, 341–352. [Google Scholar] [CrossRef]
- Wan, F.; Si, Y.C.; Niu, X. Effects of astrocyte following treatment with ginsenoside on proliferation and differentiation of neural stem cells after stroke. China J. Tradit. Chin. Med. Pharm. 2016, 5, 1617–1624. [Google Scholar]
- Chen, J.; Bai, Q.; Zhao, Z.; Sui, H.; Xie, X. Ginsenoside Represses Symptomatic Intracerebral Hemorrhage after Recombinant Tissue Plasminogen Activator Therapy by Promoting Transforming Growth Factor-beta1. J. Stroke Cerebrovasc. Dis. 2016, 25, 549–555. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Sciacca, R.D.; Raimondo, D.D.; Pedone, C.; Placa, S.L.; Pinto, A.; Licata, G. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: A retrospective chart review from the GIFA study. Int. J. Cardiol. 2011, 151, 318–322. [Google Scholar] [CrossRef]
- Guo, Z.; Cao, G.; Yang, H.; Zhou, H.; Li, L.; Cao, Z.; Yu, B.; Kou, J. A combination of four active compounds alleviates cerebral ischemia-reperfusion injury in correlation with inhibition of autophagy and modulation of AMPK/mTOR and JNK pathways. J. Neurosci. Res. 2014, 92, 1295–1306. [Google Scholar] [CrossRef]
- Gao, X.Q.; Yang, C.X.; Chen, G.J.; Wang, G.Y.; Chen, B.; Tan, S.K.; Liu, J.; Yuan, Q.L. Ginsenoside Rb1 regulates the expressions of brain-derived neurotrophic factor and caspase-3 and induces neurogenesis in rats with experimental cerebral ischemia. J. Ethnopharmacol. 2010, 132, 393–399. [Google Scholar] [CrossRef]
- Rui, W.; Li, Y.N.; Wang, G.J.; Hao, H.P.; Wu, X.L.; Fang, Z. Neuroprotective effects and brain transport of ginsenoside Rg1. Chin. J. Nat. Med. 2009, 7, 315–320. [Google Scholar]
- Sierra, C.; Coca, A.; Schiffrin, E.L. Vascular mechanisms in the pathogenesis of stroke. Curr. Hypertens. Rep. 2011, 13, 200–207. [Google Scholar] [CrossRef]
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke 2009, 4, 461–470. [Google Scholar] [CrossRef]
- Kim, J.S.; Yun, I.; Choi, Y.B.; Lee, K.S.; Kim, Y.I. Ramipril protects from free radical induced white matter damage in chronic hypoperfusion in the rat. J. Clin. Neurosci. 2008, 15, 174–178. [Google Scholar] [CrossRef]
- Sun, Z.G.; Chen, L.P.; Wang, F.W.; Xu, C.Y.; Geng, M. Protective effects of ginsenoside Rg1 against hydrogen peroxide-induced injur y in human neuroblastoma cells. Neural Regen. Res. 2016, 11, 1159–1164. [Google Scholar]
- Xie, Z.; Shi, M.; Zhang, C.; Zhao, H.; Hui, H.; Zhao, G. Ginsenoside Rd Protects Against Cerebral Ischemia-Reperfusion Injury Via Decreasing the Expression of the NMDA Receptor 2B Subunit and its Phosphorylated Product. Neurochem. Res. 2016, 41, 2149–2159. [Google Scholar] [CrossRef]
- Xie, C.L.; Li, J.H.; Wang, W.W.; Zheng, G.Q.; Wang, L.X. Neuroprotective effect of ginsenoside-Rg1 on cerebral ischemia/reperfusion injury in rats by downregulating protease-activated receptor-1 expression. Life Sci. 2015, 121, 145–151. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Fan, X.J.; Li, X.; Peng, L.L.; Wang, G.H.; Ke, K.F.; Jiang, Z.L. Ginsenoside Rg1 protects neurons from hypoxic-ischemic injury possibly by inhibiting Ca2+ influx through NMDA receptors and L-type voltage-dependent Ca2+ channels. Eur. J. Pharmacol. 2008, 586, 90–99. [Google Scholar] [CrossRef]
- Ye, R.; Kong, X.; Yang, Q.; Zhang, Y.; Han, J.; Li, P.; Xiong, L.; Zhao, G. Ginsenoside rd in experimental stroke: Superior neuroprotective efficacy with a wide therapeutic window. Neurotherapeutics 2011, 8, 515–525. [Google Scholar] [CrossRef]
- Ye, R.; Kong, X.; Yang, Q.; Zhang, Y.; Han, J.; Zhao, G. Ginsenoside Rd attenuates redox imbalance and improves stroke outcome after focal cerebral ischemia in aged mice. Neuropharmacology 2011, 61, 815–824. [Google Scholar] [CrossRef]
- Ye, R.; Zhang, X.; Kong, X.; Han, J.; Yang, Q.; Zhang, Y.; Chen, Y.; Li, P.; Liu, J.; Shi, M.; et al. Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience 2011, 178, 169–180. [Google Scholar] [CrossRef]
- Ye, R.; Yang, Q.; Kong, X.; Han, J.; Zhang, X.; Zhang, Y.; Li, P.; Liu, J.; Shi, M.; Xiong, L.; et al. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem. Int. 2011, 58, 391–398. [Google Scholar] [CrossRef]
- Ye, R.; Li, N.; Han, J.; Kong, X.; Cao, R.; Rao, Z.; Zhao, G. Neuroprotective effects of ginsenoside Rd against oxygen-glucose deprivation in cultured hippocampal neurons. Neurosci. Res. 2009, 64, 306–310. [Google Scholar] [CrossRef]
- Strunk, D.R.; Adler, A.D.; Hollars, S.N. Cognitive Therapy Skills Predict Cognitive Reactivity to Sad Mood Following Cognitive Therapy of Depression. Cognitive Ther. Res. 2013, 37, 1214–1219. [Google Scholar] [CrossRef]
- Harzheim, D.; Klose, H.; Pinado, F.P.; Ehlken, N.; Nagel, C.; Fischer, C.; Ghofrani, A.; Rosenkranz, S.; Seyfarth, H.J.; Halank, M. Anxiety and depression disorders in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Respir. Res. 2013, 14, 104. [Google Scholar] [CrossRef]
- Jin, C.; Wang, Z.Z.; Zhou, H.; Lou, Y.X.; Chen, J.; Zuo, W.; Tian, M.T.; Wang, Z.Q.; Du, G.H.; Kawahata, I.; et al. Ginsenoside Rg1-induced antidepressant effects involve the protection of astrocyte gap junctions within the prefrontal cortex. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2017, 75, 183–191. [Google Scholar] [CrossRef]
- Jeong, H.G.; Ko, Y.H.; Oh, S.Y.; Han, C.; Kim, T.; Joe, S.H. Effect of Korean Red Ginseng as an adjuvant treatment for women with residual symptoms of major depression. Asia Pac. Psychiatry 2015, 7, 330–336. [Google Scholar] [CrossRef]
- Lee, K.J.; Ji, G.E. The effect of fermented red ginseng on depression is mediated by lipids. Nutr. Neurosci. 2014, 17, 7–15. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.O.; Lee, M.; Park, Y.; Kim, D.; Cho, K.H.; Kim, S.Y.; Lee, E.H. Effects of ginsenoside Rb1 on the stress-induced changes of BDNF and HSP70 expression in rat hippocampus. Environ. Toxicol. Pharmacol. 2014, 38, 257–262. [Google Scholar] [CrossRef]
- Sang, H.L.; Jung, B.H.; Sang, Y.C.; Sun, Y.K.; Lee, E.H.; Chung, B.C. Influence of ginsenoside Rb1 on brain neurosteroid during acute immobilization stress. Arch. Pharmacal. Res. 2006, 29, 566–569. [Google Scholar]
- Lee, S.H.; Jung, B.H.; Kim, S.Y.; Lee, E.H.; Chung, B.C. The antistress effect of ginseng total saponin and ginsenoside Rg3 and Rb1 evaluated by brain polyamine level under immobilization stress. Pharmacol. Res. 2006, 54, 46–49. [Google Scholar] [CrossRef]
- Sang, H.L.; Hur, J.; Lee, E.H.; Sun, Y.K. Ginsenoside Rb1 Modulates Level of Monoamine Neurotransmitters in Mice Frontal Cortex and Cerebellum in Response to Immobilization Stress. Biomol. Ther. 2012, 20, 482–486. [Google Scholar] [Green Version]
- Jia, D.Y.; Zhang, M.X.; Yao, Y.R.; Jin, W.; Liu, J.W.; Wang, J.L.; Chen, B.C. Effects of ginsenoside Rb1 on the mRNA expression of tyrosine kinase B in the hippocampus of acute immobilization stress rats. Chin. J. Integr. Tradit. West. Med. 2013, 33, 376–379. [Google Scholar]
- Dong, J.; Wang, J.; Fang, J.; Feng, R.; Yuan, Z.; Lu, K.; Jin, Y.; Zeng, L. Effects of ginsenosides Rb1 on learning and memory and expression of somatostatin in sleep deprivation rats. J. Zhejiang Univ. 2013, 42, 197–204. [Google Scholar]
- Liang, J.; Yu, Y.; Wang, B.; Lu, B.; Zhang, J.; Zhang, H.; Ge, P. Ginsenoside Rb1 attenuates oxygen-glucose deprivation-induced apoptosis in SH-SY5Y cells via protection of mitochondria and inhibition of AIF and cytochrome c release. Molecules 2013, 18, 12777–12792. [Google Scholar] [CrossRef]
- Wu, H.T.; Chen, X.X.; Xiong, L.J. Experimental study of proliferation of schwann cells cultured with ginsenoside rb_1. Chin. J. Repar. Reconstr. Surg. 2003, 17, 26–29. [Google Scholar]

| Ginsenoside | Activities | Models | Dosing and Administration | Mode of Action | Ref. |
|---|---|---|---|---|---|
| 20(S)-proto-panaxadiol nanocrystals | Neurodegenerative disease | A polyethylene catheter was cannulated into the right jugular vein of the rats under anesthesia | 20 mg/kg Oral | Increased plasma Cmax and increased bioavailability | [67] |
| Korean red ginseng extract | PD | The rats were administered 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)–HCl by i.p. | 100 mg/kg Oral | Restored MPTP-induced protein downregulation | [61] |
| Rg1 | PD | The rats were administered MPTP–HCl by i.p. | 5mg/kg, 10mg/kg, and 20mg/kg i.p. | Decreased MPP+-induced cytotoxicity Protected PC12 cells from MPP+-induced apoptosis. | [62] |
| Rg1 | PD | The chronic MPTP/probenecid model | 10 mg/kg, 20 mg/kg, or 40 mg/kg Oral | Improved high MPTP-induced behavior defects, loss of dopamine neurons, mortality, and abnormal ultrastructure changes in the SNpc | [65] |
| Rg1 | PD | Two weeks after ovariectomy, unilateral infusion of lipopolysaccharide into the right side of substantia nigra pars compacta (SNpc) under anesthesia | Rg1 10 mg/kg, 10 mg/mL i.p. | Showed protective effects on mesencephalic dopaminergic neurons | [68] |
| Panax ginseng extract | PD | Injected a solution of rotenone in the right striatum of rat bregma | 100 mg/kg Oral | Improved the midbrain and striatal changes and showed a partial ameliorative effect against a rat model of PD | [69] |
| Rb1 | AD | SH-SY5Y cells used stable isotope labeling with amino acids in cell culture | 100 mM/day Rb1 pretreatment | Prevented β-amyloid-induced neurotoxicity in SH-SY5Y cells and apoptotic cells; increased the expression of actin cytoskeleton proteins | [70,71,72] |
| Ginseng total saponins | AD | SAM, senescence-accelerated mouse; SAMP, senescence-accelerated mouse prone substrain; SAMR, senescence-accelerated mouse-resistant substrain | 50,100, and 200 mg/kg/day Oral | Prevented memory loss in aged SAMP8 mice by upregulating the increase in antioxidant capacity in the hippocampus and upregulating plasticity-related proteins | [73] |
| Ginsenoside | Models | Dosing/Administration | Mode of Action | Ref. |
|---|---|---|---|---|
| Rd | Induced by transient MCAO | 50 mg·kg−1 i.p. | Improved behavior score, viability, and infarct volume of the cultured neurons after ischemia and protected Sprague Dawley rats and cultured neurons from I/R. | [101] |
| Ginseng total saponins | Induced by transient MCAO | 25mg·kg−1·d−1 i.p. | Improved the regeneration of the central nervous system in adults, thereby improving neurological deficits after focal cerebral ischemia. | [84] |
| Rg1 | Induced by transient MCAO | 45 mg·kg−1 i.v. | Showed effective neuroprotection by reducing the brain infarct volume and neurological scores. | [96] |
| Rg1 | Induced by transient MCAO | 40 mg·kg−1·d−1 i.p. | Showed it was neuroprotective by improving neurological damage, BBB permeability, and the brain infarct volume. | [102] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Li, N.; Pu, Y.; Zhang, T.; Wang, B. Neuroprotective Effects of Ginseng Phytochemicals: Recent Perspectives. Molecules 2019, 24, 2939. https://doi.org/10.3390/molecules24162939
Huang X, Li N, Pu Y, Zhang T, Wang B. Neuroprotective Effects of Ginseng Phytochemicals: Recent Perspectives. Molecules. 2019; 24(16):2939. https://doi.org/10.3390/molecules24162939
Chicago/Turabian StyleHuang, Xing, Ning Li, Yiqiong Pu, Tong Zhang, and Bing Wang. 2019. "Neuroprotective Effects of Ginseng Phytochemicals: Recent Perspectives" Molecules 24, no. 16: 2939. https://doi.org/10.3390/molecules24162939






