Antitumor, Antiviral, and Anti-Inflammatory Efficacy of Essential Oils from Atractylodes macrocephala Koidz. Produced with Different Processing Methods
Abstract
1. Introduction
2. Results
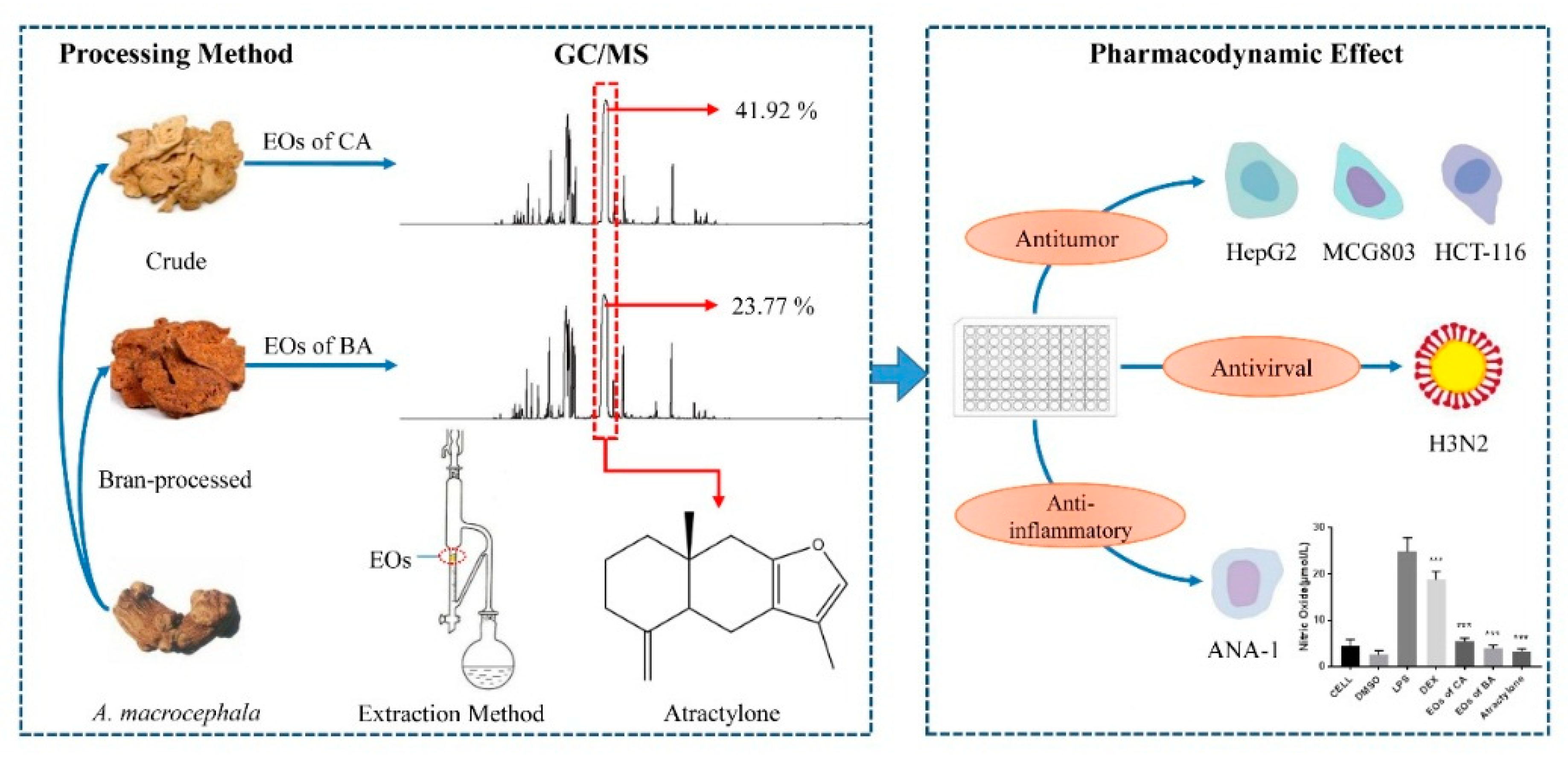
2.1. Chemical Composition of Essential Oils
2.2. Antitumor Activity of Essential Oil from A. macrocephala
2.3. Antiviral Activity of Essential Oil from A. macrocephala
2.4. Anti-Inflammatory Activity of EOs from A. macrocephala
3. Discussion
4. Materials and Methods
4.1. Plant Materialsand Chemicals
4.2. Extraction Methods
4.3. Chemical Components Analysis by GC/MS
4.4. Effects on Liver Cancer, Gastric Cancer, and Intestinal Cancer Cells
4.5. Anti-H3N2 Virucidal Activity
4.5.1. Sample Cytotoxicity Test
4.5.2. In vitro Antiviral Virus Test
4.6. Anti-Inflammatory Activity
4.6.1. MTT Method to Investigate the Effect of Test Drugs on Cell Viability
4.6.2. Inhibition of ANA-1 Cell Inflammatory Model Induced by LPS Stimulation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoang, L.S.; Tran, M.H.; Lee, J.S.; Ngo, Q.M.; Woo, M.H.; Min, B.S. Inflammatory inhibitory activity of sesquiterpenoids from Atractylodes macrocephala rhizomes. Chem. Pharm. Bull. (Tokyo) 2016, 64, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Xu, Z.; Luo, S.; Zhang, W.; Cao, G.; Liu, X.; Lou, Y.; Ma, X.; Qin, K.; Cai, B. Study on chemical fingerprinting of crude and processed Atractylodes macrocephala from different locations in Zhejiang province by reversed-phase high-performance liquid chromatography coupled with hierarchical cluster analysis. Pharm. Mag. 2012, 8, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Jia, T.; Qian, C. Rearch on the chemical references of Atractylodes macrocephala Koidz. Asia-Pac. Tradit. Med. 2010, 6, 36–39. [Google Scholar]
- Liu, P.; Teng, J.; Zhang, Y.W.; Takaishi, Y.; Duan, H.Q. Chemical constituents from rhizome of Phlomis umbrosa. Yao Xue Xue Bao Acta Pharm. Sin. 2007, 42, 401–404. [Google Scholar]
- Pharmacopoeia Commission of the Ministry of Health of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2015. [Google Scholar]
- Wang, X.T.; Li, L.H.; Ran, X.K.; Dou, D.Q.; Li, B.; Yang, B.Y.; Li, W.; Koike, K.; Kuang, H.X. What caused the changes in the usage of Atractylodis macrocephalae Rhizoma from ancient to current times? J. Nat. Med. Tokyo 2016, 70, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.D.; Cao, G.; Xia, Y.H.; Wen, C.P.; Fan, Y.S. Fast analysis of principal volatile compounds in crude and processed Atractylodes macrocephala by an automated static headspace gas chromatography-mass spectrometry. Pharm. Mag. 2014, 10, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.Q.; Chen, R.Q.; Wang, L. Anti-inflammatory activity of atractylenolide III through inhibition of nuclear factor-kappa B and mitogen-activated protein kinase pathways in mouse macrophages. Immunopharm. Immunot. 2016, 38, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Dou, D.Q. Bidirectional effective components of Atractylodis macrocephalae Rhizoma on gastrointestinal peristalsis. Int. J. Pharm. 2016, 12, 108–115. [Google Scholar] [CrossRef]
- Song, H.P.; Hou, X.Q.; Li, R.Y.; Yu, R.; Li, X.; Zhou, S.N.; Huang, H.Y.; Cai, X.; Zhou, C. Atractylenolide I stimulates intestinal epithelial repair through polyamine-mediated Ca2+ signaling pathway. Phytomedicine 2017, 28, 27–35. [Google Scholar] [CrossRef]
- Huang, H.L.; Lin, T.W.; Huang, Y.L.; Huang, R.L. Induction of apoptosis and differentiation by atractylenolide-1 isolated from Atractylodes macrocephala in human leukemia cells. Bioorg. Med. Chem. Lett. 2016, 26, 1905–1909. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhu, Y.J.; Zhang, T.; Zhao, Z.G.; Zhao, Y.; Cheng, P.; Li, H.; Gao, H.; Su, X.M. Anti-tumor effects of Atractylenolide I isolated from Atractylodes macrocephala in human lung carcinoma cell lines. Molecules 2013, 18, 13357–13368. [Google Scholar] [CrossRef] [PubMed]
- Long, F.Y.; Wang, T.; Jia, P.; Wang, H.F.; Qing, Y.; Xiong, T.T.; He, M.J.; Wang, X.L. Anti-tumor effects of Atractylenolide-I on human ovarian cancer cells. Med. Sci. Monit. 2017, 23, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.R.; Yuan, X.R.; Li, L.; Zhang, T.; Wang, B. Comparison of different methods for extraction of Cinnamomi ramulus: Yield, chemical composition and in vitro antiviral activities. Nat. Prod. Res. 2017, 31, 2909–2913. [Google Scholar] [CrossRef] [PubMed]
- Comelli, N.C.; Romero, O.E.; Diez, P.A.; Marinho, C.F.; Schliserman, P.; Carrizo, A.; Ortiz, E.V.; Duchowicz, P.R. QSAR study of biologically active essential oils against beetles infesting the walnut in Catamarca, Argentina. J. Agr. Food Chem. 2018, 66, 12855–12865. [Google Scholar] [CrossRef] [PubMed]
- Perez-Outeiral, J.; Elcoroaristizabal, S.; Amigo, J.M.; Vidal, M. Development and validation of a method for the determination of regulated fragrance allergens by High-Performance Liquid Chromatography and parallel factor analysis 2. J. Chromatography A 2017, 1526, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Szoke, E.; Maday, E.; Kiss, S.A.; Sonnewend, L.; Lemberkovics, E. Effect of magnesium on essential oil formation of genetically transformed and non-transformed chamomile cultures. J. Am. Coll. Nutr. 2004, 23, 763s–767s. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Li, K.F.; Liu, H.H.; Yang, L. Extraction of biomedical compounds from the wood of Pterocarpus macarocarpus Kurz heartwood. Pak. J. Pharm. Sci. 2018, 31, 913–918. [Google Scholar]
- Li, X.F.; Mou, Z.; Wang, X.P.; Wu, H.M.; Xu, F.; Zhu, C.X.; Zhang, M. Geographic analysis of the cultivation region of Ai pian derived from Blumea balsamifera through the determination of volatiles in the medicinal product and blood of treated mice by gas chromatography-mass spectrometry (GC-MS). Instrum. Sci. Technol. 2019, 47, 597–610. [Google Scholar] [CrossRef]
- Sedaghat Doost, A.; Stevens, C.V.; Claeys, M.; Van Der Meeren, P. Fundamental study on the salt tolerance of oregano essential oil-in-water nanoemulsions containing tween 80. Langmuir: ACS J. Surf. Colloids 2019. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Cheng, Z.; Ma, L.; Zhao, L.; Li, J. A comparison of electronic nose and gas chromatography-mass spectrometry on discrimination and prediction of ochratoxin A content in Aspergillus carbonarius cultured grape-based medium. Food Chem. 2019, 297, 124850. [Google Scholar] [CrossRef]
- El Mokni, R.; Majdoub, S.; Chaieb, I.; Jlassi, I.; Joshi, R.K.; Hammami, S. Chromatographic analysis, antimicrobial and insecticidal activities of the essential oil of Phlomis floccosa D. Don. Biomed. Chromatogr. BMC 2019. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jia, J.; Zhang, Q.; Gu, H.; Yang, L. A modified approach for isolation of essential oil from fruit of Amorpha fruticosa Linn using microwave-assisted hydrodistillation concatenated liquid-liquid extraction. J. Chromatography A 2017, 1524, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Zhang, Q.; Wang, B.T. Chemical characterization of herbal formula yupingfeng powder and its single herbs (i) volatile components. Anal. Lett. 2009, 42, 2610–2624. [Google Scholar] [CrossRef]
- Andriana, Y.; Xuan, T.D.; Quy, T.N.; Tran, H.D.; Le, Q.T. Biological activities and chemical constituents of essential oils from Piper cubeba Bojer and Piper nigrum L. Molecules 2019, 24, 1876. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Cai, H.; Cong, X.; Liu, X.; Ma, X.; Lou, Y.; Qin, K.; Cai, B. Global detection and analysis of volatile components from sun-dried and sulfur-fumigated herbal medicine by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Analyst 2012, 137, 3828–3835. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; Koca, M.; Yilmaz, S.V.; Yildirim, K.; Pinar, N.M.; Demirci, B.; Brestic, M.; Sytar, O. Molecular docking studies of coumarins isolated from extracts and essential oils of zosima absinthifolia link as potential inhibitors for Alzheimer’s disease. Molecules 2019, 24, 722. [Google Scholar] [CrossRef] [PubMed]
- Le, T.B.; Beaufay, C.; Nghiem, D.T.; Pham, T.A.; Mingeot-Leclercq, M.P.; Quetin-Leclercq, J. Evaluation of the anti-trypanosomal activity of vietnamese essential oils, with emphasis on curcuma longa L. and its components. Molecules 2019, 24, 1158. [Google Scholar] [CrossRef]
- Stoev, G. Application and chiral recognition of heptakis (2,6-di-O-methyl-3-O-trifluoroacetyl)-beta-cyclodextrin as a stationary phase for the gas-chromatographic separation of enantiomers. J Chromatogr. 1992, 589, 257–263. [Google Scholar] [CrossRef]
- Guo, F.Q.; Huang, L.F.; Zhou, S.Y.; Zhang, T.M.; Liang, Y.Z. Comparison of the volatile compounds of Atractylodes medicinal plants by headspace solid-phase microextraction-gas chromatography-mass spectrometry. Anal. Chim. Acta 2006, 570, 73–78. [Google Scholar] [CrossRef]
- Kapetanos, C.; Karioti, A.; Bojovic, S.; Marin, P.; Veljic, M.; Skaltsa, H. Chemical and principal-component analyses of the essential oils of apioideae taxa (Apiaceae) from central Balkan. Chem. Biodivers. 2008, 5, 101–119. [Google Scholar] [CrossRef]
- Borisov, R.S.; Esparza, C.; Goriainov, S.V.; Zaikin, V.G. Suitable in-situ derivatization of alcohols by reaction with basic amines in direct analysis in real time mass spectrometry. Talanta 2019, 200, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Chen, H.M.; Shi, B.F. Synthesis of phthalic acid derivatives via Pd-catalyzed alkoxycarbonylation of aromatic C–H bonds with alkyl chloroformates. Chem. Commun. 2018, 54, 10859–10862. [Google Scholar] [CrossRef] [PubMed]
- Hamad, Y.K.; Abobakr, Y.; Salem, M.Z.M.; Ali, H.M.; Al-Sarar, A.S.; Al-Zabib, A.A. Activity of plant extracts/essential oils against three plant pathogenic fungi and mosquito larvae: GC/MS analysis of bioactive compounds. Bioresources 2019, 14, 4489–4511. [Google Scholar] [CrossRef]
- El-Gawad, A.A.; Elshamy, A.; El Gendy, A.E.; Gaara, A.; Assaeed, A. Volatiles profiling, allelopathic activity, and antioxidant potentiality of xanthium strumarium leaves essential oil from egypt: Evidence from chemometrics analysis. Molecules 2019, 24, 584. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, G.H.; Yuan, K. Extraction and GC-MS analysis of the volatile constituents of a tractylodes macrocephala. Asian J. Chem. 2011, 23, 551–555. [Google Scholar]
- Zhou, Q.; Liu, S.; Liu, Y.; Song, H. Comparative analysis of volatiles of 15 brands of extra-virgin olive oils using solid-phase micro-extraction and solvent-assisted flavor evaporation. Molecules 2019, 24, 1512. [Google Scholar] [CrossRef] [PubMed]
- He, X.W.; Zhang, L.T.; Chen, J.P.; Sui, J.L.; Yi, G.H.; Wu, J.Y.; Ma, Y.Z. Correlation between chemical composition and antifungal activity of clausena lansium essential oil against candida spp. Molecules 2019, 24, 1394. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; das Gracas Cardoso, M.; de Andrade, J.; Silva, L.F.; Teixeira, M.L.; Valerio Resende, J.M.; da Silva Figueiredo, A.C.; Barroso, J.G. Chemical composition and antioxidant activity of essential oils from cinnamodendron dinisii schwacke and siparuna guianensis aublet. Antioxidants 2013, 2, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ge, L.; Mo, J.G.; Su, L.; Li, Y.J.; Yang, K.D. Essential oils and ethanol extract from Camellia nitidissima and evaluation of their biological activity. J. Food Sci. Tech. Mys. 2018, 55, 5075–5081. [Google Scholar] [CrossRef]
- Mahanta, B.P.; Sut, D.; Kemprai, P.; Paw, M.; Lal, M.; Haldar, S. A (1) H-NMR spectroscopic method for the analysis of thermolabile chemical markers from the essential oil of black turmeric (Curcuma caesia) rhizome: Application in post-harvest analysis. Phytochem. Anal. PCA 2019. [Google Scholar] [CrossRef]
- Benedetto, C.; D’Auria, M.; Mecca, M.; Prasad, P.; Singh, P.; Singh, S.; Sinisgalli, C.; Milella, L. Chemical and biological evaluation of essential oil from Saussurea costus (Falc.) Lipsch. from Garhwal Himalaya collected at different harvesting periods. Nat. Prod. Res. 2019, 33, 2355–2358. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.X.; Huang, X.Y.; Lv, R.Q.; Zhang, Z.C.; Sun, J.; Aheto, J.H. Analysis of volatile compounds of Tremella aurantialba fermentation via electronic nose and HS-SPME-GC-MS. J. Food Saf. 2018, 38. [Google Scholar] [CrossRef]
- Mirza, B.; Samiei, S.S.; Taherkhani, M.; Fathizadeh, M. Composition of the essential oils of Anthemis hyalina DC., Achillea nobilis L. and Cichorium intybus L. Three asteraceae herbs growing wild in Iran. Asian J. Chem. 2012, 24, 1151–1154. [Google Scholar]
- Ling, Y.; Wang, X.M.; Wang, C.N.; Xu, C.J.; Zhang, W.; Zhang, Y.H.; Zhang, Y.N. Hybrids from farnesylthiosalicylic acid and hydroxamic acid as dual ras-related signaling and histone deacetylase (hdac) inhibitors: Design, synthesis and biological evaluation. Chemmedchem 2015, 10, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Elmassry, M.M.; Kormod, L.; Labib, R.M.; Farag, M.A. Metabolome based volatiles mapping of roasted umbelliferous fruits aroma via HS-SPME GC/MS and peroxide levels analyses. J. Chromatogr. B 2018, 1099, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, Z.; Huang, Z.; Zhao, M.; Li, P.; Zhou, W.; Zhang, K.; Zheng, X.; Lin, L.; Tang, J.; et al. Variation in essential oil and bioactive compounds of curcuma kwangsiensis collected from natural habitats. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, R.F.; Lai, P.X. Chemical composition of the essential oil of chloranthus serratus from China. Chem. Nat. Compd. 2017, 53, 159–161. [Google Scholar] [CrossRef]
- Xu, S.Z.; Qi, X.J.; Liu, Y.Q.; Liu, Y.H.; Lv, X.; Sun, J.Z.; Cai, Q. UPLC-MS/MS of Atractylenolide I, Atractylenolide II, Atractylenolide III, and Atractyloside A in rat plasma after oral administration of raw and wheat bran-processed atractylodis rhizoma. Molecules 2018, 23, 3234. [Google Scholar] [CrossRef]
- Su, H.H.; Wang, W.D.; Bao, L.Z.; Wang, S.S.; Cao, X.F. Synthesis and evaluation of essential oil-derived beta-methoxyacrylate derivatives as high potential fungicides. Molecules 2017, 22, 763. [Google Scholar] [CrossRef]
- Zeng, M.N.; Li, M.; Li, M.; Zhang, B.B.; Li, B.K.; Zhang, L.; Feng, W.S.; Zheng, X.K. 2-Phenylacetamide isolated from the seeds of lepidium apetalum and its estrogen-like effects in vitro and in vivo. Molecules 2018, 23, 2293. [Google Scholar] [CrossRef]
- Guo, Z.B.; Liu, Z.Z.; Yue, H.F.; Wang, J.Y. Beta-elemene increases chemosensitivity to 5-fluorouracil through down-regulating microRNA-191 expression in colorectal carcinoma cells. J. Cell Biochem. 2018, 119, 7032–7039. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Wang, T.Y.; Xu, S.T.; Lin, A.J.; Yao, H.Q.; Xie, W.J.; Zhu, Z.Y.; Xu, J.Y. Novel hybrids of natural beta-elemene bearing isopropanolamine moieties: Synthesis, enhanced anticancer profile, and improved aqueous solubility. Fitoterapia 2017, 120, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Ning, L.P.; Xiong, Y.; Shi, H.; Wang, T.S.; An, R.M. Essential oils extracted from Phoebe hui Cheng ex Yang: Chemical constituents, antitumor and antibacterial activities, and potential use as a species identifier. J. Wood Chem. Technol. 2017, 37, 201–210. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Franca, H.S.; Kuster, R.M.; Teixeira, L.A.; Rocha, L.M. Chemical composition and antibacterial activity of Brazilian propolis essential oil. J. Venom. Anim. Toxins 2010, 16, 121–130. [Google Scholar] [CrossRef]
- Jaradat, N.; Al-Lahham, S.; Abualhasan, M.N.; Bakri, A.; Zaide, H.; Hammad, J.; Hussein, F.; Issa, L.; Mousa, A.; Speih, R. Chemical constituents, antioxidant, cyclooxygenase inhibitor, and cytotoxic activities of teucrium pruinosum boiss. essential oil. Biomed. Res. Int. 2018, 2018, 4034689. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Nam, S.Y.; Hwang, S.Y.; Kim, H.M.; Jeong, H.J. Atractylone, an active constituent of KMP6, attenuates allergic inflammation on allergic rhinitis in vitro and in vivo models. Mol. Immunol. 2016, 78, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117–3126. [Google Scholar] [CrossRef]
- Vivinus-Nebot, M.; Frin-Mathy, G.; Bzioueche, H.; Dainese, R.; Bernard, G.; Anty, R.; Filippi, J.; Saint-Paul, M.C.; Tulic, M.K.; Verhasselt, V.; et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: Role of epithelial barrier disruption and low-grade inflammation. Gut 2014, 63, 744–752. [Google Scholar] [CrossRef]
- Schulzke, J.D.; Ploeger, S.; Amasheh, M.; Fromm, A.; Zeissig, S.; Troeger, H.; Richter, J.; Bojarski, C.; Schumann, M.; Fromm, M. Epithelial tight junctions in intestinal inflammation. Ann. New York Acad. Sci. 2009, 1165, 294–300. [Google Scholar] [CrossRef]
- Ying, S.; Qing, S.; Li, C.Y. The effect of gentian violet on virulent properties of candida albicans. Mycopathologia 2010, 169, 279–285. [Google Scholar] [CrossRef]
- Amparo, T.R.; Seibert, J.B.; Mathias, F.A.S.; Vieira, J.F.P.; Soares, R.; Freitas, K.M.; Cabral, V.A.R.; Brandao, G.C.; Santos, O.; de Souza, G.H.B.; et al. Anti-inflammatory activity of Protium spruceanum (Benth.) Engler is associated to immunomodulation and enzymes inhibition. J. Ethnopharmacol. 2019, 241, 112024. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, C.; Chen, X.; Liu, J.; Yu, Q.; Liu, Y.; Liu, J. Drug delivery system based on near-infrared light-responsive molybdenum disulfide nanosheets controls the high-efficiency release of dexamethasone to inhibit inflammation and treat osteoarthritis. ACS Appl. Mater. Interf. 2019, 11, 11587–11601. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of atractylone is available from the authors. |



| No. | Retention (min) | Compounds | Molecular Formula | EOs of CA | EOs of BA | CAS No. | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Area (%) | Similarity | Area (%) | Similarity | ||||||
| 1 | 21.085 | α-Guaiene | C15H24 | ND | 0.13 | 87.5 | 3691-12-1 | [15] | |
| 2 | 22.327 | α-Amylcinnamyl alcohol | C14H20O | 0.35 | 87.8 | 0.29 | 87.9 | 101-85-9 | [16] |
| 3 | 22.561 | Berkheyaradulene | C15H24 | 0.54 | 87.8 | 0.42 | 87.7 | 65372-78-3 | [17] |
| 4 | 22.769 | Eremophylene | C15H24 | ND | 0.15 | 83.7 | 10219-75-7 | [18] | |
| 5 | 22.987 | α-Gurjunene | C15H24 | 0.48 | 93.1 | 0.97 | 92.8 | 489-40-7 | [19] |
| 6 | 23.189 | β-Isocomene | C15H24 | ND | 0.26 | 71.1 | 74311-15-2 | [17] | |
| 7 | 23.236 | (−)-β-Caryophyllene | C15H24 | ND | 0.28 | 70 | 87-44-5 | [20] | |
| 8 | 23.674 | Isoledene | C15H24 | 1.85 | 96.3 | 1.75 | 96.3 | 95910-36-4 | [7] |
| 9 | 24.135 | γ-Elemene | C15H24 | 0.64 | 93.5 | 1.14 | 93.7 | 29873-99-2 | [21] |
| 10 | 24.753 | α-Caryophyllene | C15H24 | 1.21 | 92.5 | 1.07 | 92.5 | 6753-98-6 | [22] |
| 11 | 25.622 | 1,2,3,4,4a,7-Hexahydro-1,6-dimethyl-4-(1-methylethyl)naphthalene | C15H24 | 0.33 | 81.1 | 0.34 | 83.2 | 16728-99-7 | [23] |
| 12 | 25.850 | Cedrol | C15H26O | ND | 0.14 | 51.6 | 77-53-2 | [24] | |
| 13 | 25.864 | β-Selinene | C15H24 | 4.88 | 95.4 | 4.36 | 95.3 | 17066-67-0 | [25] |
| 14 | 26.085 | trans-Nuciferol | C15H22O | 0.69 | 76 | 0.67 | 78.6 | 39599-18-3 | [26] |
| 15 | 26.829 | Calarene | C15H24 | 0.84 | 91.5 | 0.74 | 91.7 | 17334-55-3 | [27] |
| 16 | 27.136 | Zingiberene | C15H24 | 0.33 | 76.1 | 0.25 | 65.8 | 495-60-3 | [28] |
| 17 | 27.441 | (−)-Norbornenone | C7H8O | 1.06 | 57.5 | ND | 16620-79-4 | [29] | |
| 18 | 27.459 | 1,2,3,6-Tetramethylbicyclo[2.2.2] octa-2,5-diene | C12H18 | 1.68 | 58.5 | ND | 62338-43-6 | [30] | |
| 19 | 27.486 | 2-Methoxy-4-methyl-1-(1-methylethyl)benzene | C11H16O | ND | 0.55 | 60.9 | 1076-56-8 | [30] | |
| 20 | 27.486 | 3,7-Guaiadiene | C15H24 | 9.57 | 75.7 | ND | 6754-04-7 | [31] | |
| 21 | 27.497 | Eudesma-4(14),11-diene | C15H24 | 5.34 | 72.6 | 5.38 | 70.2 | 17066-67-0 | [30] |
| 22 | 27.500 | 1-Heptanal | C7H14O | ND | 1.74 | 57.9 | 111-71-7 | [24] | |
| 23 | 27.507 | 1-Adamantylethanol | C12H20O | ND | 3.66 | 61.4 | 6240-11-5 | [32] | |
| 24 | 27.519 | 2-Phenylacetamide | C8H9NO | ND | 6.32 | 64.3 | 103-81-1 | [33] | |
| 25 | 27.674 | Eudesma-3,7(11)-diene | C15H24 | 5.57 | 80.6 | 4.36 | 79.6 | 6813-21-4 | [34] |
| 26 | 27.708 | Caryophyllene | C15H24 | 0.57 | 61.9 | ND | 87-44-5 | [30] | |
| 27 | 27.721 | β-Himachalene | C15H24 | ND | 0.19 | 61.4 | 1461-03-6 | [30] | |
| 28 | 27.723 | Isolongifolene | C15H24 | 4.33 | 81.3 | 3.04 | 75.9 | 1135-66-6 | [35] |
| 29 | 27.732 | β-Eudesmol | C15H26O | ND | 0.77 | 54.3 | 473-15-4 | [36] | |
| 30 | 27.738 | trans-2-Heptenal | C7H12O | ND | 0.21 | 61.8 | 18829-55-5 | [37] | |
| 31 | 27.756 | (9E,12E)-9,12-Octadecadienoic acid methylester | C19H34O2 | ND | 0.41 | 76.8 | 2566-97-4 | [24] | |
| 32 | 28.089 | γ-Gurjunene | C15H24 | 1.31 | 95 | 4.37 | 94.6 | 22567-17-5 | [38] |
| 33 | 28.297 | Aromadendrene | C15H24 | 3.23 | 91.1 | 3.29 | 91.9 | 489-39-4 | [7] |
| 34 | 29.978 | β-Vatirenene | C15H22 | 0.37 | 78.5 | 0.35 | 77.4 | 27840-40-0 | [7] |
| 35 | 31.173 | Atractylone | C15H20O | 41.92 | 95 | 23.77 | 95 | 6989-21-5 | [39] |
| 36 | 31.239 | (Z)-3-decen-1-ol | C10H20O | ND | 8.99 | 78.2 | 10340-22-4 | [30] | |
| 37 | 31.514 | Agarospirol | C15H26O | ND | 6.25 | 77.9 | 1460-73-7 | [40] | |
| 38 | 31.559 | β-Elemene | C15H24 | 0.25 | 79 | ND | 515-13-9 | [41] | |
| 39 | 31.616 | 4,11,11-Trimethyl-8-methylenebicyclo[7.2.0] undec-4-ene | C15H24 | ND | 0.16 | 80.3 | 13877-93-5 | [42] | |
| 40 | 32.066 | 10S,11S-Himachala-3(12),4-diene | C15H24 | 1.54 | 94.3 | 1.38 | 94.4 | 60909-28-6 | [43] |
| 41 | 32.611 | Dehydroaromadendrene | C15H22 | 0.29 | 81.5 | 0.24 | 83.1 | 698388-95-3 | [30] |
| 42 | 33.039 | 4,5-Dehydroisolongifolene | C15H22 | ND | 0.45 | 79.5 | 1246777-02-5 | [36] | |
| 43 | 33.072 | Aristolone | C15H22O | 2.69 | 87.1 | 3.58 | 82.7 | 6831-17-0 | [44] |
| 44 | 33.283 | 3,7,11-Trimethyl-dodeca-2,4,6,10-tetraenal | C15H22O | 0.34 | 78.2 | 0.28 | 81.2 | 13832-89-8 | [45] |
| 45 | 33.865 | Spathulenol | C15H24O | 0.34 | 76.7 | ND | 6750-60-3 | [7] | |
| 46 | 33.874 | 3,4,7,8-Tetrahydro-8,8,9,9-tetramethyl-2H-2,4a-methanonaphthalene | C15H22 | ND | 0.25 | 77.9 | 67517-14-0 | [46] | |
| 47 | 36.250 | Velleral | C15H20O2 | 1.02 | 85.3 | 0.70 | 84.2 | 50656-61-6 | [47] |
| 48 | 37.741 | α-Curcumene | C15H22 | 3.65 | 89.2 | 3.95 | 88.8 | 644-30-4 | [7] |
| 49 | 39.958 | Procerin | C15H18O2 | 0.83 | 72.3 | 0.67 | 74.2 | 552-96-5 | [48] |
| 50 | 40.439 | 8,9-dehydro-9-formyl-Cycloisolongifolene | C16H22O | 0.31 | 69 | 0.25 | 68.8 | 1206188-76-2 | [7] |
| 51 | 41.022 | 3a,5,6,7,8,8a,9,9a-Octahydro-5,8a-dimethyl-3-methylenenaphtho[2-b]furan-2(3H)-one | C15H20O2 | 0.72 | 89.8 | 0.36 | 89.4 | 80367-94-8 | [48] |
| 52 | 41.861 | 1,2,3,3a,4,5-Hexahydro-1,1,4,4-tetramethyl-2,3b-methano-3bH-cyclopenta[1,3]cyclopropa[1,2]benzene-6-carboxaldehyde | C16H22O | 0.23 | 68.7 | 0.13 | 67.9 | 59820-24-5 | [48] |
| Total | 98.44 | 98.02 | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, S.; Li, L.; Huang, H.; Wang, B.; Zhang, T. Antitumor, Antiviral, and Anti-Inflammatory Efficacy of Essential Oils from Atractylodes macrocephala Koidz. Produced with Different Processing Methods. Molecules 2019, 24, 2956. https://doi.org/10.3390/molecules24162956
Gu S, Li L, Huang H, Wang B, Zhang T. Antitumor, Antiviral, and Anti-Inflammatory Efficacy of Essential Oils from Atractylodes macrocephala Koidz. Produced with Different Processing Methods. Molecules. 2019; 24(16):2956. https://doi.org/10.3390/molecules24162956
Chicago/Turabian StyleGu, Sihao, Ling Li, Hai Huang, Bing Wang, and Tong Zhang. 2019. "Antitumor, Antiviral, and Anti-Inflammatory Efficacy of Essential Oils from Atractylodes macrocephala Koidz. Produced with Different Processing Methods" Molecules 24, no. 16: 2956. https://doi.org/10.3390/molecules24162956
APA StyleGu, S., Li, L., Huang, H., Wang, B., & Zhang, T. (2019). Antitumor, Antiviral, and Anti-Inflammatory Efficacy of Essential Oils from Atractylodes macrocephala Koidz. Produced with Different Processing Methods. Molecules, 24(16), 2956. https://doi.org/10.3390/molecules24162956






