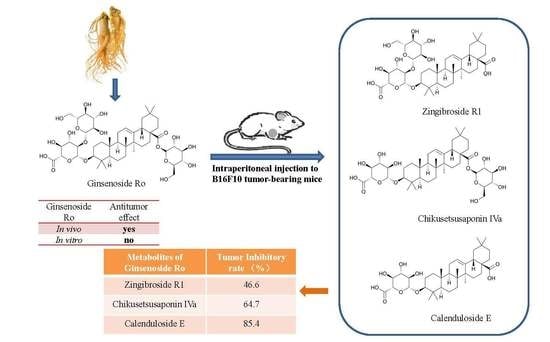
Inhibitory Effects of Ginsenoside Ro on the Growth of B16F10 Melanoma via Its Metabolites
Abstract
:1. Introduction
2. Results
2.1. Anti-Tumour Effects of Ro In Vivo
2.2. Effects of Ro on the Viability of B16F10 Melanoma Cells In Vitro
2.3. Determination and Identification of Metabolites in the Plasma of Tumour-Bearing Mice Treated with Ro
2.4. Effects of R1, IVa, and E on the Viability of B16F10 Melanoma Cells In Vitro
2.5. Anti-Tumour Effects of R1, IVa, and E In Vivo
2.6. Effects of R1, IVa, and E on Tube Formation of Human Umbilical Vein Endothelial Cells
2.7. Effects of Ro in the Haemolysis Test
3. Discussion
4. Materials and Methods
4.1. Reagents, Cell Lines, and Animals
4.2. Tumour Xenograft Experiment
4.3. Effects of Ro on B16F10 Cell Viability
4.4. Determination of Ro Metabolites by HPLC-MS
4.5. Cell Viability Assay for the Ro Metabolites
4.6. Anti-Tumour Assay for the Metabolites of Ro In Vivo
4.7. Tube Formation Assay
4.8. Haemolysis Test
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, P.; Montes de Oca, M.K.; Alkeswani, A.R.; McClees, S.F.; Das, T.; Elmets, C.A.; Afaq, F. Tea polyphenols for the prevention of UVB-induced skin cancer. Photodermatol. Photoimmunol. 2018, 34, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.A.; Wilhite, T.J.; Balboni, T.A.; Alexander, B.M.; Spektor, A.; Ott, P.A.; Ng, A.K.; Hodi, F.S.; Schoenfeld, J.D. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology 2015, 4, e1046028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duh, P.-D.; Chen, Z.-T.; Lee, S.-W.; Lin, T.-P.; Wang, Y.-T.; Yen, W.-J.; Kuo, L.-F.; Chu, H.-L. Antiproliferative activity and apoptosis induction of Eucalyptus Citriodora resin and its major bioactive compound in melanoma B16F10 cells. J. Agr. Food Chem. 2012, 60, 7866–7872. [Google Scholar] [CrossRef] [PubMed]
- Gray-Schopfer, V.C.; Karasarides, M.; Hayward, R.; Marais, R. Tumor necrosis factor-alpha blocks apoptosis in melanoma cells when BRAF signaling is inhibited. Cancer Res. 2007, 67, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Vakharia, P.; Yousif, R.; Patel, K.; Rangel, S.; West, D.; Nardone, B. Overall risk of second primary malignancies in patients with malignant melanoma: A national US population-based study. J. Am. Acad. Dermatol. 2018, 79, AB212. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- National Cancer Institute: Surveillance, Epidemiology and End Results Program (SEER). Cancer Stat Facts: Melanoma of the Skin. 2019. Available online: https://seer.cancer.gov/statfacts/html/melan.html (accessed on 22 July 2019).
- Tedesco, I.; Russo, G.L. Panax ginseng: More Than an Adaptogen Remedy. In Nonvitamin and Nonmineral Nutritional Supplements; Academic Press: Cambridge, MA, USA, 2019; pp. 251–256. [Google Scholar]
- Espenel, S.; Vallard, A.; Rancoule, C.; Garcia, M.A.; Guy, J.B.; Chargari, C.; Deutsch, E.; Magne, N. Melanoma: Last call for radiotherapy. Crit. Rev. Oncol. Hematol. 2017, 110, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, A.; Patel, V.L.; Dagoglu, N. Radiation therapy in the management of malignant melanoma. Oncology 2015, 29, 743–751. [Google Scholar]
- Cui, Q.; Yang, D.H.; Chen, Z.S. Special Issue: Natural Products: Anticancer and Beyond. Molecules 2018, 23, 1246. [Google Scholar] [CrossRef]
- Wang, C.Y.; Bai, X.Y.; Wang, C.H. Traditional Chinese medicine: A treasured natural resource of anticancer drug research and development. Am. J. Chin. Med. 2014, 42, 543–559. [Google Scholar] [CrossRef]
- Mitra, S.; Dash, R. Natural Products for the Management and Prevention of Breast Cancer. Evid. Based Complement. Altern. Med. 2018, 2018, 8324696. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Calway, T.; Yuan, C.-S. Herbal medicines as adjuvants for cancer therapeutics. Am. J. Chin. Med. 2012, 40, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Zablocka, M.; Shi, X.; Caminade, A.M.; Majoral, J.P. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 2018, 47, 514–532. [Google Scholar] [CrossRef] [PubMed]
- AlQathama, A.; Prieto, J. Natural products with therapeutic potential in melanoma metastasis. Nat. Prod. Rep. 2015, 32, 1170–1182. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Kim, B.G.; Kang, J.S.; Park, S.-K.; Lee, K.; Hyun, D.-H.; Kim, H.M.; In, M.-J.; Kim, D.C. Lipid-Soluble Ginseng Extract Inhibits Invasion and Metastasis of B16F10 Melanoma Cells. J. Med. Food 2015, 18, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamohara, S.; Kageyama, M.; Sunayama, S.; Denpo, K. Safety and efficacy of a dietary supplement containing functional food ingredients for erectile dysfunction. Personal. Med. Univ. 2014, 3, 38–41. [Google Scholar] [CrossRef]
- Ramesh, T.; Kim, S.-W.; Sung, J.-H.; Hwang, S.-Y.; Sohn, S.-H.; Yoo, S.-K.; Kim, S.-K. Effect of fermented Panax ginseng extract (GINST) on oxidative stress and antioxidant activities in major organs of aged rats. Exp. Gerontol. 2012, 47, 77–84. [Google Scholar] [CrossRef]
- Chen, T.; Li, B.; Qiu, Y.; Qiu, Z.; Qu, P. Functional mechanism of Ginsenosides on tumor growth and metastasis. Saudi J. Biol. Sci. 2018, 25, 917–922. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Zeng, J.Z.; Wong, A.S.T. Chemical Structures and Pharmacological Profiles of Ginseng Saponins. Molecules 2019, 24, 2443. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.; Yu, Y.; Chen, B.; Tang, C.; Li, X. Antitumor effects of ginsenoside Rg3 on human hepatocellular carcinoma cells. Mol. Med. Rep. 2012, 5, 1295–1298. [Google Scholar]
- Chen, F.; Sun, Y.; Zheng, S.-L.; Qin, Y.; Julian McClements, D.; Hu, J.-N.; Deng, Z.-Y. Antitumor and immunomodulatory effects of ginsenoside Rh2 and its octyl ester derivative in H22 tumor-bearing mice. J. Funct. Foods 2017, 32, 382–390. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Khan, M.N.; Zhang, L.; Chen, Q.; Zhao, Y.; Yan, Q.; Fu, L.; Liu, J. Ginsenoside Rg3 sensitizes hypoxic lung cancer cells to cisplatin via blocking of NF-κB mediated epithelial–mesenchymal transition and stemness. Cancer Lett. 2018, 415, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Yan, Y.; Cai, H. Ginsenoside Rh2 inhibited proliferation by inducing ROS mediated ER stress dependent apoptosis in lung cancer cells. Biol. Pharm. Bull. 2017, 40, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, R.; Yang, X.; Zhang, Z.; Kang, N.; Bao, L.; Shen, Y.; Yan, H.; Zheng, F. Ginsenoside Rg3 suppresses the proliferation of prostate cancer cell line PC3 through ROS-induced cell cycle arrest. Oncol. Lett. 2019, 17, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, H.; Han, Z.; Li, W.; Mai, Z.; Yuan, R. Ginsenoside Rh2 Inhibits Angiogenesis in Prostate Cancer by Targeting CNNM1. J. Nanosci. Nanotechnol. 2019, 19, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Meng, Y.; Sun, Q.; Zhang, Z.; Guo, X.; Sheng, X.; Tai, G.; Cheng, H.; Zhou, Y. Ginsenoside compound K sensitizes human colon cancer cells to TRAIL-induced apoptosis via autophagy-dependent and-independent DR5 upregulation. Cell Death Dis. 2016, 7, e2334. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, T.; Zhao, L.; Chen, W.; Hou, H.; Ye, Z.; Li, X. Ginsenoside 20 (S)-Rg3 inhibits the Warburg effect through STAT3 pathways in ovarian cancer cells. Int. J. Oncol. 2015, 46, 775–781. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, X.; Lu, J.; Chen, W.; Li, X.; Zhao, L. Ginsenoside 20 (S)-Rg3 inhibits the warburg effect via modulating DNMT3A/MiR-532-3p/HK2 pathway in ovarian cancer cells. Cell. Physiol. Biochem. 2018, 45, 2548–2559. [Google Scholar] [CrossRef]
- Mao, Q.; Zhang, P.-H.; Wang, Q.; Li, S.-L. Ginsenoside F2 induces apoptosis in humor gastric carcinoma cells through reactive oxygen species-mitochondria pathway and modulation of ASK-1/JNK signaling cascade in vitro and in vivo. Phytomedicine 2014, 21, 515–522. [Google Scholar] [CrossRef]
- Qian, J.; Li, J.; Jia, J.-G.; Jin, X.; Yu, D.-J.; Guo, C.-X.; Xie, B.; Qian, L.-Y. Ginsenoside-Rh2 inhibits proliferation and induces apoptosis of human gastric cancer SGC-7901 side population cells. Asian Pac. J Cancer Prev. 2016, 17, 1817–1821. [Google Scholar] [CrossRef]
- Gu, B.; Wang, J.; Song, Y.; Wang, Q.; Wu, Q. The inhibitory effects of ginsenoside Rd on the human glioma U251 cells and its underlying mechanisms. J. Cell. Biochem. 2019, 120, 4444–4450. [Google Scholar] [CrossRef] [PubMed]
- Li, K.F.; Kang, C.M.; Yin, X.F.; Li, H.X.; Chen, Z.Y.; Li, Y.; Zhang, Q.; Qiu, Y.R. Ginsenoside Rh2 inhibits human A172 glioma cell proliferation and induces cell cycle arrest status via modulating Akt signaling pathway. Mol. Med. Rep. 2018, 17, 3062–3068. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.-S.; Cho, S.-H.; Shin, J.-S.; Kim, D.-H.; Choi, J.-H.; Choi, S.Y.; Rhee, Y.K.; Hong, H.-D.; Lee, K.-T. Ginsenoside Rh2 induces cell cycle arrest and differentiation in human leukemia cells by upregulating TGF-β expression. Carcinogenesis 2013, 34, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Yin, J.; Xu, C.; Mu, Y.; Lv, S. 20 (S)-ginsenoside Rh2 induce the apoptosis and autophagy in U937 and K562 Cells. Nutrients 2018, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Kim, A.K. Anti-breast cancer activity of Fine Black ginseng (Panax ginseng Meyer) and ginsenoside Rg5. J. Ginseng Res. 2015, 39, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, S.; Jeong, D.; Kim, S.J. Ginsenoside Rh2 epigenetically regulates cell-mediated immune pathway to inhibit proliferation of MCF-7 breast cancer cells. J. Ginseng Res. 2018, 42, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.W.; Ahn, K.S.; Lee, J.-C.; Kim, S.-H.; Chung, B.C.; Choi, M.H. Validated quantification for selective cellular uptake of ginsenosides on MCF-7 human breast cancer cells by liquid chromatography–mass spectrometry. Anal. Bioanal. Chem. 2010, 396, 3017–3025. [Google Scholar] [CrossRef] [PubMed]
- Quan, K.; Liu, Q.; Wan, J.Y.; Zhao, Y.J.; Guo, R.Z.; Alolga, R.N.; Li, P.; Qi, L.W. Rapid preparation of rare ginsenosides by acid transformation and their structure-activity relationships against cancer cells. Sci Rep. 2015, 5, 8598. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, S.; Ma, L.; Wang, X.; Su, G.; Zhao, Y. Structure activity relationships and antinociceptive activity of two novel dammarane-type sapogenins with notable anticancer effect. Phytochem. Lett. 2018, 27, 49–54. [Google Scholar] [CrossRef]
- Zhu, S.; Zou, K.; Cai, S.; Meselhy, M.R.; Komatsu, K. Simultaneous determination of triterpene saponins in ginseng drugs by high-performance liquid chromatography. Chem. Pharm. Bull. 2004, 52, 995–998. [Google Scholar] [CrossRef]
- Zheng, K.; Li, Y.; Wang, S.; Wang, X.; Liao, C.; Hu, X.; Fan, L.; Kang, Q.; Zeng, Y.; Wu, X. Inhibition of autophagosome-lysosome fusion by ginsenoside Ro via the ESR2-NCF1-ROS pathway sensitizes esophageal cancer cells to 5-fluorouracil-induced cell death via the CHEK1-mediated DNA damage checkpoint. Autophagy 2016, 12, 1593–1613. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.J.; Oh, Y.; Lee, S.; Ryu, I.W.; Kim, K.; Lim, C.-J. Antioxidative properties of ginsenoside Ro against UV-B-induced oxidative stress in human dermal fibroblasts. Biosci. Biotechnol. Biochem. 2015, 79, 2018–2021. [Google Scholar] [CrossRef]
- Kim, D.S.; Oh, S.R.; Jung, K.Y.; Park, J.D.; Kim, S.I.; Lee, H.-K. Anticomplementary activity of ginseng saponins and their degradation products. Phytochemistry 1998, 47, 397–399. [Google Scholar] [CrossRef]
- ZHANG, X.-H.; Xian-Xiang, X.; Tao, X. Ginsenoside Ro suppresses interleukin-1β-induced apoptosis and inflammation in rat chondrocytes by inhibiting NF-κB. Chin. J. Nat. Med. 2015, 13, 283–289. [Google Scholar] [CrossRef]
- Matsuda, H.; Samukawa, K.; Kubo, M. Anti-inflammatory activity of ginsenoside Ro. Planta Med. 1990, 56, 19–23. [Google Scholar] [CrossRef]
- Matsuda, H.; Samukawa, K.; Kubo, M. Anti-hepatitic activity of ginsenoside Ro. Planta Med. 1991, 57, 523–526. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Matsuda, H. Antidiabetogenic activity of oleanolic acid glycosides from medicinal foodstuffs. Biofactors 2000, 13, 231–237. [Google Scholar] [CrossRef]
- Murata, K.; Takeshita, F.; Samukawa, K.; Tani, T.; Matsuda, H. Effects of ginseng rhizome and ginsenoside Ro on testosterone 5α-reductase and hair re-growth in testosterone-treated mice. Phytother. Res. 2012, 26, 48–53. [Google Scholar] [CrossRef]
- Li, J.; Guo, W.J.; Yang, Q.Y. Effects of ursolic acid and oleanolic acid on humancolon carcinoma cell line HCT15. World J. Gastroenterol. 2002, 8, 493–495. [Google Scholar] [CrossRef]
- Yan, S.; Huang, C.; Wu, S.; Yin, M. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef]
- Shyu, M.-H.; Kao, T.-C.; Yen, G.-C. Oleanolic acid and ursolic acid induce apoptosis in HuH7 human hepatocellular carcinoma cells through a mitochondrial-dependent pathway and downregulation of XIAP. J. Agric. Food Chem. 2010, 58, 6110–6118. [Google Scholar] [CrossRef]
- Wu, J.; Yang, C.; Guo, C.; Li, X.; Yang, N.; Zhao, L.; Hang, H.; Liu, S.; Chu, P.; Sun, Z. SZC015, a synthetic oleanolic acid derivative, induces both apoptosis and autophagy in MCF-7 breast cancer cells. Chem. Biol. Interact. 2016, 244, 94–104. [Google Scholar] [CrossRef]
- Kim, G.-J.; Jo, H.-J.; Chung, K.-H.; Lee, K.-J.; An, J.H. Oleanolic Acid Induces p53 Dependent Apoptosis via the ERK/JNK/AKT Pathway in Cancer Cell Lines. Oncotarget 2018, 9, 26370–26386. [Google Scholar] [CrossRef]
- Li, L.; Wei, L.; Shen, A.; Chu, J.; Lin, J.; Peng, J. Oleanolic acid modulates multiple intracellular targets to inhibit colorectal cancer growth. Int. J. Oncol. 2015, 47, 2247–2254. [Google Scholar] [CrossRef] [Green Version]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Takemura, M.; Endo, S.; Matsunaga, T.; Soda, M.; Zhao, H.-T.; El-Kabbani, O.; Tajima, K.; Iinuma, M.; Hara, A. Selective inhibition of the tumor marker aldo-keto reductase family member 1B10 by oleanolic acid. J. Nat. Prod. 2011, 74, 1201–1206. [Google Scholar] [CrossRef]
- Lúcio, K.A.; Rocha Gda, G.; Monção-Ribeiro, L.C.; Fernandes, J.; Takiya, C.M.; Gattass, C.R. Oleanolic acid initiates apoptosis in non-small cell lung cancer cell lines and reduces metastasis of a B16F10 melanoma model in vivo. PLoS ONE 2011, 6, e28596. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, Z.; Li, J.; Li, Q.; Hu, S.; Li, J.; Sun, M.; Cai, Z. Oleanolic acid derivative Dex-OA has potent anti-tumor and anti-metastatic activity on osteosarcoma cells in vitro and in vivo. Investig. New Drugs 2011, 29, 258–265. [Google Scholar] [CrossRef]
- Baek, N.-I.; Kim, D.S.; Lee, Y.H.; Park, J.D.; Lee, C.B.; Kim, S.I. Cytotoxicities of ginseng saponins and their degradation products against some cancer cell lines. Arch. Pharm. Res. 1995, 18, 164–168. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, P.; Zheng, S.; Wang, Y.; Zhu, Y.; Zhou, H.; Wang, Y.; Xiao, S. Profiling and identification of the metabolites of ginsenoside Ro in rat faeces and urine after oral administration. Eur. Food Res. Technol. 2016, 242, 199–210. [Google Scholar] [CrossRef]
- Du, Q.; Fu, C.; Tie, J.; Liu, T.; Li, L.; Ren, X.; Huang, Z.; Liu, H.; Tang, F.; Li, L. Gelatin microcapsules for enhanced microwave tumor hyperthermia. Nanoscale 2015, 7, 3147–3154. [Google Scholar] [CrossRef]
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Isolation and analysis of ginseng: Advances and challenges. Nat. Prod. Rep. 2011, 28, 467–495. [Google Scholar] [CrossRef]
- Yu, C.; Wang, C.Z.; Zhou, C.J.; Wang, B.; Han, L.; Zhang, C.F.; Wu, X.H.; Yuan, C.S. Adulteration and cultivation region identification of American ginseng using HPLC coupled with multivariate analysis. J. Pharm. Biomed. Anal. 2014, 99, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Dai, D.; Zhang, C.F.; Williams, S.; Yuan, C.S.; Wang, C.Z. Ginseng on Cancer: Potential Role in Modulating Inflammation-Mediated Angiogenesis. Am. J. Chin. Med. 2017, 45, 13–22. [Google Scholar] [CrossRef]
- Jin, Y.; Kim, Y.J.; Jeon, J.N.; Wang, C.; Min, J.W.; Noh, H.Y.; Yang, D.C. Effect of white, red and black ginseng on physicochemical properties and ginsenosides. Plant Foods Hum. Nutr. 2015, 70, 141–145. [Google Scholar] [CrossRef]
- Yun, T.-K. Panax ginseng—A non-organ-specific cancer preventive? Lancet Oncol. 2001, 2, 49–55. [Google Scholar] [CrossRef]
- Saba, E.; Jeon, B.R.; Jeong, D.-H.; Lee, K.; Goo, Y.-K.; Kim, S.-H.; Sung, C.-K.; Roh, S.-S.; Kim, S.D.; Kim, H.-K. Black ginseng extract ameliorates hypercholesterolemia in rats. J. Ginseng Res. 2016, 40, 160–168. [Google Scholar] [CrossRef]
- Qi, L.-W.; Wang, C.-Z.; Yuan, C.-S. American ginseng: Potential structure–function relationship in cancer chemoprevention. Biochem. Pharmacol. 2010, 80, 947–954. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Zhang, J.W.; Ai, C.Z.; Xiang, N.; Liu, H.X.; Yang, L. Anti-androgen-independent prostate cancer effects of ginsenoside metabolites in vitro: Mechanism and possible structure-activity relationship investigation. Arch. Pharm. Res. 2009, 32, 49–57. [Google Scholar] [CrossRef]
- Cho, S.-H.; Chung, K.-S.; Choi, J.-H.; Kim, D.-H.; Lee, K.-T. Compound K, a metabolite of ginseng saponin, induces apoptosis via caspase-8-dependent pathway in HL-60 human leukemia cells. BMC Cancer 2009, 9, 449. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Li, N.; Zheng, F.; Dai, Y.; Ge, Y.; Yue, H.; Yu, S. Mechanism of antidiabetic and synergistic effects of ginseng polysaccharide and ginsenoside Rb1 on diabetic rat model. J. Pharm. Biomed. Anal. 2018, 158, 451–460. [Google Scholar] [CrossRef]
- Yao, H.; Wan, J.Y.; Zeng, J.; Huang, W.H.; Sava-Segal, C.; Li, L.; Niu, X.; Wang, Q.; Wang, C.Z.; Yuan, C.S. Effects of compound K, an enteric microbiome metabolite of ginseng, in the treatment of inflammation associated colon cancer. Oncol. Lett. 2018, 15, 8339–8348. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hua, H.Y.; Liu, X.Y.; Liu, J.H.; Yu, B.Y. In vitro biotransformation of red ginseng extract by human intestinal microflora: Metabolites identification and metabolic profile elucidation using LC-Q-TOF/MS. J. Pharm. Biomed. Anal. 2014, 98, 296–306. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Zhang, Y.; Li, S.P.; Yue, H.; Chen, C.B.; Liu, S.Y. Multicomponent assessment and ginsenoside conversions of Panax quinquefolium L. roots before and after steaming by HPLC-MS(n). J. Ginseng Res. 2019, 43, 27–37. [Google Scholar] [CrossRef]
- Park, J.C.; Lee, Y.J.; Choi, H.Y.; Shin, Y.K.; Kim, J.D.; Ku, S.K. In Vivo and In Vitro Antitumor Effects of Platycodin D, a Saponin Purified from Platycodi Radix on the H520 Lung Cancer Cell. Evid. Based Complement. Altern. Med. 2014, 2014, 478653. [Google Scholar] [CrossRef]
- Gerlier, D.; Thomasset, N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods 1986, 94, 57–63. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Zhang, L.; Chen, L.; Du, Y.; Ye, T.; Shi, X. Naringenin exerts anti-angiogenic effects in human endothelial cells: Involvement of ERRalpha/VEGF/KDR signaling pathway. Fitoterapia 2016, 111, 78–86. [Google Scholar] [CrossRef]
- Zheng, S.; Li, W.; Wang, J.; Chen, Y.; Hou, W.; Gao, W.; Liu, Q.; Wang, Y. Platycodin D inhibits B16F10 melanoma metastasis via antiangiogenic activity. RSC Adv. 2016, 6, 10606–10614. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |









| Groups | Dosage (mg/kg) | Weight (g) | Organ Indices (×100, mg/g) | ||
|---|---|---|---|---|---|
| Before | After | Thymus | Spleen | ||
| Normal | - | 23.11 ± 0.67 | 24.27 ± 0.99 | 1.97 ± 0.14 | 3.46 ± 0.33 |
| Model | - | 22.27 ± 0.71 | 24.89 ± 1.26 | 1.23 ± 0.27 ## | 4.74 ± 1.21 # |
| CTX | 20 | 22.29 ± 0.85 | 22.27 ± 1.02 | 0.56 ± 0.09 ** | 2.98 ± 0.86 * |
| Ro | 25 | 22.91 ± 1.12 | 24.73 ± 1.43 | 1.67 ± 0.25 * | 3.54 ± 1.03 |
| Ro (μg/mL) | Haemolysis Rate (%) |
|---|---|
| 1 | 0.15 ± 0.13 |
| 3 | 0.31 ± 0.13 |
| 10 | 0.23 ± 0.23 |
| 30 | 0.15 ± 0.13 |
| 100 | 0.31 ± 0.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.-w.; Xiao, S.-y.; Wang, J.; Hou, W.; Wang, Y.-p. Inhibitory Effects of Ginsenoside Ro on the Growth of B16F10 Melanoma via Its Metabolites. Molecules 2019, 24, 2985. https://doi.org/10.3390/molecules24162985
Zheng S-w, Xiao S-y, Wang J, Hou W, Wang Y-p. Inhibitory Effects of Ginsenoside Ro on the Growth of B16F10 Melanoma via Its Metabolites. Molecules. 2019; 24(16):2985. https://doi.org/10.3390/molecules24162985
Chicago/Turabian StyleZheng, Si-wen, Sheng-yuan Xiao, Jia Wang, Wei Hou, and Ying-ping Wang. 2019. "Inhibitory Effects of Ginsenoside Ro on the Growth of B16F10 Melanoma via Its Metabolites" Molecules 24, no. 16: 2985. https://doi.org/10.3390/molecules24162985
APA StyleZheng, S. -w., Xiao, S. -y., Wang, J., Hou, W., & Wang, Y. -p. (2019). Inhibitory Effects of Ginsenoside Ro on the Growth of B16F10 Melanoma via Its Metabolites. Molecules, 24(16), 2985. https://doi.org/10.3390/molecules24162985