Qualitative and Quantitative Evaluation of Heat-Induced Changes in Polyphenols and Antioxidant Capacity in Prunus domestica L. By-products
Abstract
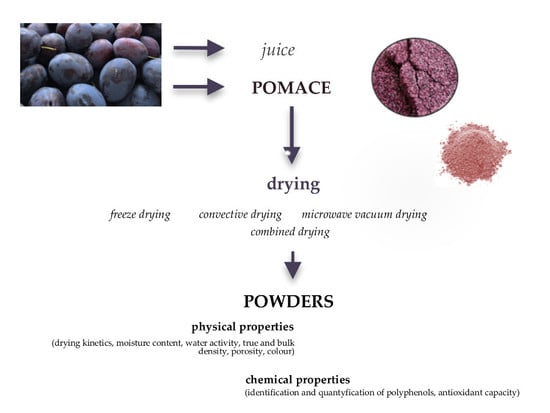
:1. Introduction
2. Results
2.1. Properties of Plum Pomace Powders
2.1.1. Drying Kinetics
2.1.2. Physical Properties
2.1.3. Chemical Properties
2.1.4. Antioxidant Capacity
2.1.5. Multiple Linear Regression Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Drying methods
3.2.2. Physical properties
Moisture Content
- MR: moisture ratio
- Mi: current moisture content,
- M0: initial moisture content.
Water Activity
True and Bulk Density
- m: mass of the sample,
- V: total sample volume.
- mc: sample mass in cylinder,
- Vc: sample volume in cylinder.
Porosity
- ρb: bulk density,
- ρt: true density.
Color
3.2.3. Chemical properties
Identification and Quantification of Polyphenols
Antioxidant Capacity
3.2.4. Statistical analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT Food Sci. Technol. 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Sikora, E.; Szczech, M.; Kowalska, B. The waste from vegetable and fruit processing-Possibilities of using. Przemysł Fermentacyjny i Owocowo Warzywny 2013, 57, 47–49. [Google Scholar]
- Tarko, T.; Duda-Chodak, A.; Bebak, A. Biological activity of selected fruit and vegetable pomaces. Żywność: Nauka Technologia Jakość 2012, 4, 55–65. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food. Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Kapasakalidis, P.G.; Rastall, R.A.; Gordon, M.H. Extraction of polyphenols from processed black currant (Ribes nigrum L.) residues. J. Agric. Food Chem. 2006, 54, 4016–4021. [Google Scholar] [CrossRef] [PubMed]
- Statistics Poland. Available online: www.stat.gov.pl (accessed on 20 April 2019).
- Michalska, A.; Łysiak, G. Applicability of plums grown in Poland to drying process in terms of changes in bioactive compounds and of Maillard reaction products being formed. Żywność Nauka Technologia Jakość 2014, 21, 29–38. [Google Scholar] [CrossRef]
- Majerska, J.; Michalska, A.; Figiel, A. A review of new directions in managing fruit and vegetable processing by-products. Trends Food Sci. Technol. 2019, 88, 207–219. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Lech, K.; Nowicka, P.; Oszmiański, J. Effect of convective and vacuum–microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food Bioprocess. Technol. 2014, 7, 829–841. [Google Scholar] [CrossRef]
- de Jesus Junqueira, J.R.; Corrêa, J.L.G.; de Oliveira, H.M.; Ivo Soares Avelar, R.; Salles Pio, L.A. Convective drying of cape gooseberry fruits: Effect of pretreatments on kinetics and quality parameters. LWT Food Sci. Technol. 2017, 82, 404–410. [Google Scholar] [CrossRef]
- Méndez-Lagunas, L.; Rodríguez-Ramírez, J.; Cruz-Gracida, M.; Sandoval-Torres, S.; Barriada-Bernal, G. Convective drying kinetics of strawberry (Fragaria ananassa): Effects on antioxidant activity, anthocyanins and total phenolic content. Food Chem. 2017, 230, 174–181. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Effect of different drying techniques on physical properties, total polyphenols and antioxidant capacity of blackcurrant pomace powders. LWT Food Sci. Technol. 2017, 78, 114–121. [Google Scholar] [CrossRef]
- Figiel, A. Drying kinetics and quality of beetroots dehydrated by combination of convective and vacuum-microwave methods. J. Food Eng. 2010, 98, 461–470. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Physicochemical properties of whole fruit plum powders obtained using different drying technologies. Food Chem. 2016, 207, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Musielak, G.; Kieca, A. Influence of Varying Microwave Power during Microwave–Vacuum Drying on the Drying Time and Quality of Beetroot and Carrot Slices. Drying Technol. 2014, 32, 1326–1333. [Google Scholar] [CrossRef]
- Ratti, C. 3-Freeze drying for food powder production. In Handbook of Food Powders; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 57–84. [Google Scholar]
- Pałacha, Z. Water activity as an important parameter of food stability. Przemysł Spożywczy 2008, 62, 22–26. [Google Scholar]
- Michalska, A.; Lech, K. The effect of carrier quantity and drying method on the physical properties of apple juice powders. Beverages 2018, 4, 2. [Google Scholar] [CrossRef]
- Cuq, B.; Mandato, S.; Jeantet, R.; Saleh, K.; Ruiz, T. 7-Agglomeration/granulation in food powder production. In Handbook of Food Powders; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 150–177. [Google Scholar]
- Jaiswal, R.; Karaköse, H.; Rühmann, S.; Goldner, K.; Neumüller, M.; Treutter, D.; Kuhnert, N. Identification of phenolic compounds in plum fruits (Prunus salicina L. and Prunus domestica L.) by high-performance liquid chromatography/tandem mass spectrometry and characterization of varieties by quantitative phenolic fingerprints. J. Agric. Food Chem. 2013, 61, 12020–12031. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Łysiak, G.P.; Figiel, A. Chemical composition and antioxidant properties of powders obtained from different plum juice formulations. Int. J. Mol. Sci. 2017, 18, 176. [Google Scholar] [CrossRef]
- Piga, A.; Del Caro, A.; Corda, G. From plums to prunes: influence of drying parameters on polyphenols and antioxidant activity. J. Agric. Food Chem. 2003, 51, 3675–3681. [Google Scholar] [CrossRef]
- Treutter, D.; Wang, D.; Farag, M.A.; Baires, G.D.A.; Rühmann, S.; Neumüller, M. Diversity of phenolic profiles in the fruit skin of Prunus domestica plums and related species. J. Agric. Food Chem. 2012, 60, 12011–12019. [Google Scholar] [CrossRef]
- Raynal, J.; Moutounet, M.; Souquet, J.M. Intervention of phenolic compounds in plum technology. 1. Changes during drying. J. Agric. Food Chem. 1989, 37, 1046–1050. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC−DAD−ESIMS analysis of phenolic compounds in nectarines, peaches, and plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.-O.; Moon, H.Y.; Kang, H.G.; Lee, C.Y. Contribution of individual polyphenolics to total antioxidant capacity of plums. J. Agric. Food Chem. 2003, 51, 7240–7245. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Typek, R. Transformation of 5-O-caffeoylquinic acid in blueberries during high-temperature processing. J. Agric. Food Chem. 2014, 62, 10889–10895. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Łysiak, G.P.; Lech, K.; Figiel, A. Functional relationships between phytochemicals and drying conditions during the processing of blackcurrant pomace into powders. Adv. Powder Technol. 2017, 28, 1340–1348. [Google Scholar] [CrossRef]
- Cui, Z.-W.; Xu, S.-Y.; Sun, D.-W. Microwave–vacuum drying kinetics of carrot slices. J. Food Eng. 2004, 65, 157–164. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Lech, K.; Michalska, A.; Wasilewska, M.; Figiel, A.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Influence of osmotic dehydration pre-treatment and combined drying method on physico-chemical and sensory properties of pomegranate arils, cultivar Mollar de Elche. Food Chem. 2017, 232, 306–315. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med., 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: New York, NY, USA, 1999; Volume 299, pp. 15–27. [Google Scholar]
- Uyanik, G.K.; Guler, N. A study on multiple linear regression analysis. Proc. Soc. Beh. Sci. 2013, 106, 234–240. [Google Scholar] [CrossRef]


| Drying Method | Drying Conditions | Constants | Statistics | ||||
|---|---|---|---|---|---|---|---|
| A | k | n | c | RMSE | R2 | ||
| CD | 50 °C | 1 | 0.00218 | 1.38 | 0.0184 | 0.0066 | 0.9997 |
| 60 °C | 1 | 0.00218 | 1.40 | 0.0143 | 0.0062 | 0.9997 | |
| 70 °C | 1 | 0.00218 | 1.49 | 0.0115 | 0.0058 | 0.9998 | |
| 80 °C | 1 | 0.00218 | 1.54 | 0.0096 | 0.0161 | 0.9982 | |
| 90 °C | 1 | 0.00218 | 1.60 | 0.0053 | 0.0089 | 0.9994 | |
| MVD | 120 W | 1 | 0.0447 | 0.97 | 0.0040 | 0.0346 | 0.9867 |
| 240 W | 1 | 0.0447 | 1.24 | 0.0130 | 0.0106 | 0.9987 | |
| 360 W | 1 | 0.0447 | 1.46 | 0.0121 | 0.016 | 0.9973 | |
| 480 W | 1 | 0.0447 | 1.49 | 0.0001 | 0.0206 | 0.9957 | |
| CD/MVD | 50 °C/120 W | 0.2230 | 0.046 | 1.09 | 0.0120 | 0.0001 | 0.9997 |
| 60 °C/120 W | 0.1878 | 0.046 | 1.12 | 0.0001 | 0.0026 | 0.9981 | |
| 70 °C/120 W | 0.2016 | 0.046 | 1.12 | 0.0106 | 0.0012 | 0.9996 | |
| 80 °C/120 W | 0.2290 | 0.046 | 1.10 | 0.0090 | 0.0024 | 0.9988 | |
| 90 °C/120 W | 0.1990 | 0.046 | 1.13 | 0.0060 | 0.0013 | 0.9995 | |
| Drying Method | Process Conditions | Drying Time (min) | Max Temperature (°C) | Moisture Content (kg H2O∙kg −1 dm) | Water Activity (-) | True Density (g∙cm−3) | Bulk Density (g∙cm−3) | Porosity (%) | Color | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||||||
| FD | −60°C/30 °C | 1440 ± 20 a* | - | 0.067 ± 0.016 a | 0.1617 ± 0.007 j | 1.515 ± 0.028 a | 0.467 ± 0.04 b | 69.48 ± 0.7 a | 47.32 ± 0.16 i | 14.68 ± 0.24 a | 13.63 ± 0.26 h |
| CD | 50 °C | 690 ± 15 b | 50 ± 2 g | 0.056 ± 0.001 b | 0.3465 ± 0.001 a | 1.455 ± 0.016 a | 0.656 ± 0.012 ab | 54.92 ± 1.51 ab | 47.50 ± 0.26 i | 12.40 ± 0.21 e | 16.58 ± 0.46 g |
| 60 °C | 600 ± 20 c | 60 ± 2 f | 0.045 ± 0.002 b | 0.2977 ± 0.001 e | 1.464 ± 0.025 a | 0.876 ± 0.030 a | 40.24 ± 3.47 b | 50.78 ± 0.34 e | 11.45 ± 0.04 f | 17.76 ± 0.11 cde | |
| 70 °C | 450 ± 15 d | 70 ± 2 e | 0.031 ± 0.004 c | 0.2414 ± 0.001 g | 1.438 ± 0.021 a | 0.765 ± 0.138 ab | 46.89 ± 8.51 ab | 54.42 ± 0.37 a | 10.87 ± 0.16 g | 17.54 ± 0.21 def | |
| 80 °C | 360 ± 15 e | 80 ± 2 d | 0.024 ± 0.002 dc | 0.2098 ± 0.002 h | 1.452 ± 0.026 a | 0.714 ± 0.014 ab | 50.80 ± 0.32 ab | 52.54 ± 0.13 b | 10.34 ± 0.04 h | 16.61 ± 0.21 g | |
| 90 °C | 330 ± 10 f | 90 ± 2 c | 0.017 ± 0.004 d | 0.1833 ± 0.002 i | 1.407 ± 0.024 a | 0.749 ± 0.069 ab | 46.72 ± 6.21 b | 53.80 ± 0.26 a | 10.16 ± 0.12 h | 18.46 ± 0.36 bc | |
| MVD | 120 W | 120 ± 8 h | 85 ± 3 cd | 0.044 ± 0.002 b | 0.2935 ± 0.001 e | 1.433 ± 0.025 a | 0.761 ± 0.106 ab | 46.83 ± 8.66 ab | 48.58 ± 0.45 gh | 12.58 ± 0.24 d | 16.88 ± 0.37 fg |
| 240 W | 56 ± 4 i | 101 ± 4b | 0.040 ± 0.008 b | 0.2845 ± 0.001 f | 1.438 ± 0.027 a | 0.805 ± 0.019 a | 43.98 ± 0.17 b | 49.02 ± 0.13 efg | 13.14 ± 0.19 bc | 17.07 ± 0.37 efg | |
| 360 W | 42 ± 3 i | 121 ± 4 a | 0.021 ± 0.004 dc | 0.1917 ± 0.002 i | 1.465 ± 0.046 a | 0.790 ± 0.122 ab | 46.24 ± 5.94 b | 50.49 ± 0.39 e | 11.61 ± 0.19 f | 16.87 ± 0.19 fg | |
| 480 W | 32 ± 2 i | 127 ± 5 a | 0.014 ± 0.001 e | 0.1690 ± 0.002 j | 1.469 ± 0.033 a | 0.775 ± 0.115 ab | 47.31 ± 6.18 ab | 51.70 ± 0.13 bc | 10.80 ± 0.24 g | 17.84 ± 0.43 cd | |
| CD/MVD | 50 °C /120 W | 222 ± 4 g | 78 ± 2 de | 0.051 ± 0.008 b | 0.3344 ± 0.001 b | 1.433 ± 0.040 a | 0.705 ± 0.010 ab | 51.09 ± 2.64 ab | 45.97 ± 0.57 j | 12.13 ± 0.04 e | 16.37 ±0.13 g |
| 60 °C/120 W | 139 ± 4 h | 85 ± 3 dc | 0.051 ± 0.002 b | 0.3184 ± 0.001 c | 1.457 ± 0.030a | 0.693 ± 0.114ab | 52.55 ± 6.45ab | 50.55 ± 0.31def | 12.36 ± 0.11de | 18.34 ± 0.37bcd | |
| 70 °C/120 W | 131 ± 4 h | 85 ± 2 dc | 0.047 ± 0.007 b | 0.2939 ± 0.001 e | 1.395 ± 0.021 a | 0.659 ± 0.125 ab | 52.69 ± 9.96 ab | 48.73 ± 0.39 fgh | 13.44 ± 0.18 b | 19.33 ± 0.47 a | |
| 80 °C/120 W | 132 ± 4 h | 81 ± 3 d | 0.046 ± 0.003 b | 0.3067 ± 0.001 d | 1.398 ± 0.017 a | 0.665 ± 0.096 ab | 52.36 ± 7.72 ab | 47.70 ± 0.64 i | 13.43 ± 0.21 b | 18.70 ± 0.256 ab | |
| 90 °C/120 W | 122 ± 4 h | 90 ± 3 c | 0.052 ± 0.003 b | 0.3107 ± 0.001 dc | 1.384 ± 0.024a | 0.790 ± 0.009ab | 42.96 ± 0.75b | 47.91 ± 0.51hi | 12.63 ± 0.07f | 17.32 ± 0.23def | |
| FD | CD | MVD | CD/MVD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD 50 °C | CD 60 °C | CD 70 °C | CD 80 °C | CD 90 °C | MVD 120 | MVD 240 | MVD 360 | MVD 480 | 50 °C/120 W | 60 °C/120 W | 70 °C/120 W | 80 °C/120 W | 90 °C/120 W | ||
| PROCYANIDINS | |||||||||||||||
| Polymeric procyanidins | 680.5 ± 11.2 cde* | 693.6 ± 0.2 cde | 673.8 ± 6.2 de | 613.1 ± 27.7 ef | 476.9 ± 10.7 g | 769.8 ± 3.1 bc | 831.1 ± 31.6 ab | 726.6 ± 9.9 cd | 532.4 ± 12.3 fg | 676.3 ± 16.6 de | 651.8 ± 8.7 de | 690.5 ± 3.5 cde | 676.5 ± 6.6 de | 712.2 ± 9.4 cd | 889.8 ± 70.3 a |
| (+)-catechin | 148.1 ± 6.1 a | 108.5 ± 0.4 e | 109.3 ± 1.8 e | 114.1 ± 0.5 cde | 113.1 ± 7.1 cde | 109 ± 6.7 e | 127.5 ± 2.7 bc | 131.9 ± 2.5 b | 136.6 ± 4.7 ab | 125.1 ± 3.3 bcd | 116.5 ± 4.9 cde | 110.5 ± 2.0 de | 111.2 ± 2.2 de | 106.4 ± 2.1 e | 115.7 ± 1 cde |
| (-)-epicatechin | 18.3 ± 1.4 a | 14.2 ± 1.1 abc | 12.4 ± 1.9 c | 13.7 ± 1.2 bc | 10.6 ± 1.2 cde | 6.5 ± 0.4 e | 13.7 ± 0.8 bc | 16.8 ± 1 ab | 10.1 ± 0.1 cde | 6.9 ± 0.4 de | 12.8 ± 0.7 bc | 11.1 ± 0.2 cd | 10.5 ± 0.4 cde | 10.2 ± 0.1 cde | 12.4 ± 2.2 c |
| Trimer | 36.1 ± 1.7 a | 29.9 ± 0.1 c | 30.1 ± 0.1 c | 31.5 ± 0.9 bc | 29.9 ± 0.1 c | 29.3 ± 1.2 c | 33.8 ±0.9 ab | 28.5 ± 0.5 c | 30.1 ± 1.0 c | 31.6 ± 0.1 bc | 29.1 ± 0.8 c | 29.9 ± 0.5 c | 33.7 ± 0.8 ab | 29.1 ± 0.3 c | 28.7 ± 0.0 c |
| Dimer | 20.6 ± 1.1 a | 15.6 ± 0.6 cdef | 15.7 ± 0.3 cdef | 15.9 ± 0.0 cdef | 15.5 ± 0.1 def | 14.1 ± 0.6 ef | 18.3 ± 0.7 b | 15.5 ± 0.1 def | 16.2 ± 0.8 cde | 17.1 ± 0.0 bcd | 14.9 ± 0.4 ef | 15.5 ± 0.3 def | 17.6 ± 0.1 bc | 14.7 ± 0.1 ef | 14.1 ± 0.0 f |
| PHENOLIC ACIDS | |||||||||||||||
| Neochlorogenic acid | 137.2 ± 0.8 a | 53.1 ± 1.1 f | 54.9 ± 0.4 f | 66.7 ± 0.1 d | 65.4 ± 0.2 de | 61.2 ± 0.6 e | 47.8 ± 0.4 gh | 38.4 ± 0.7 i | 45.3 ± 3.6 h | 49.9 ± 0.5 fgh | 52.2 ± 2.2 fg | 69.3 ± 0.8 d | 96.7 ± 0.8 b | 82.1 ± 1.0 c | 69.2 ± 0.5 d |
| Chlorogenic acid | 33.2 ± 0.2 a | 16.6 ± 0.2 ef | 17.7 ± 0.2 e | 24.3 ± 0.3 d | 26.3 ± 0.4 c | 26.4 ± 0.1 c | 12.6 ± 0.1 g | 9.8 ± 0.4 h | 13.8 ± 0.0 g | 15.5 ± 0.7 f | 16.9 ± 0.1 e | 23.3 ± 0.2 d | 32.5 ± 0.7 a | 32.3 ± 0.3 a | 30.6 ± 0.1 b |
| 3-p-Coumaroylquinic acid | 15.1 ± 0.2 a | 6.6 ± 0.1 gh | 7.2 ± 0.2 h | 9.4 ± 0.1 f | 10.2 ± 0.2 e | 11.4 ± 0.1 cd | 6.2 ± 0.2 h | 5.1 ± 0.1 i | 6.2 ± 0.5 h | 6.8 ± 0.0 gh | 6.9 ± 0.2 gh | 8.9 ± 0.2 f | 12.5 ± 0.2 b | 11.9 ± 0.1 cd | 11.2 ± 0.0 d |
| 3-Caffeoylshikimic acid | 13.6 ± 0.1 a | 7.1 ± 0.3 def | 6.8 ± 0.1 fgh | 6.8 ± 0.1 fg | 6.1 ± 0.1 hi | 4.7 ± 0.0 j | 7.8 ± 0.1 cd | 5.8 ± 0.2 i | 6.3 ± 0.3 ghi | 7.1 ± 0.0 ef | 7.1 ± 0.3 ef | 8.4 ± 0.1 c | 10.8 ± 0.3 b | 7.7 ± 0.1 cde | 6.6 ± 0.1 fgh |
| Derivative of phenolic acid | 2.8 ± 0.3 e | 1.6 ± 0.1 gh | 1.7 ± 0.1 fg | 2.8 ± 0.1 e | 3.4 ± 0.2 cd | 5.1 ± 0.1 a | 1.3 ± 0.1 gh | 1.2 ± 0.0 h | 1.4 ± 0.0 gh | 1.6 ± 0.0 gh | 1.7 ± 0.1 fg | 2.1 ± 0.1 f | 3.1 ± 0.1 de | 3.7 ± 0.1 c | 4.2 ± 0.1 b |
| 4-O-Caffeoylquinic acid | 5.3 ± 0.4 e | 3.8 ± 0.2 cde | 3.8 ± 0.1 cde | 4.1 ± 0.1 cd | 3.9 ± 0.1 cd | 3.5 ± 0.0 def | 4.1 ± 0.1 cd | 3.1 ± 0.0 f | 3.3 ± 0.2 ef | 3.7 ± 0.1 cde | 3.6 ± 0.1 def | 3.9 ± 0.1 cde | 4.7 ± 0.1 ab | 4.2 ± 0.0 c | 4.2 ± 0.0 bc |
| Caffeoylshikimic acid | 35.5 ± 0.2 a | 16.8 ± 0.9 f | 15.8 ± 0.2 fg | 15.8 ± 0.2 fg | 14.1 ± 0.2 h | 10.9 ± 0.2 i | 19.2 ± 0.2 cd | 14.1 ± 0.3 gh | 15.4 ± 0.9 fgh | 17.1 ± 0.1 ef | 17.1 ± 0.6 f | 20.6 ± 0.1 c | 26.5 ± 0.6 b | 18.7 ± 0.1 de | 15.7 ± 0.2 fgh |
| 4-p-Coumaroylquinic acid | 5.3 ± 0.4 b | 3.2 ± 0.1 de | 3.4 ± 0.1 d | 4.6 ± 0.1 c | 5.1 ± 0.1 bc | 5.1 ± 0.1 cb | 2.9 ± 0.0 de | 2.1 ± 0.1 f | 2.7 ± 0.0 ef | 3.2 ± 0.2 de | 3.5 ± 0.1 d | 4.7 ± 0.1 c | 6.4 ± 0.2 a | 6.2 ± 0.0 a | 5.6 ± 0.1 b |
| FLAVONOIDS | |||||||||||||||
| Isorhamnetin-3-O-glucoside | 10.7 ± 0.1 a | 7.2 ± 0.1 f | 8.3 ± 0.4 cde | 8.2 ± 0.0 cde | 8.5 ± 0.3 cde | 8.7 ± 0.0 bcd | 8.9 ± 0.1 bc | 7.5 ± 0.3 ef | 7.8 ± 0.6 def | 8.5 ± 0.0 cd | 7.8 ± 0.3 def | 8.1 ± 0.1 cdef | 9.6 ± 0.3 b | 8.2 ± 0.0 cdef | 8.3 ± 0.1 cde |
| Quercetin-3-O-rutinoside | 47.4 ± 0.3 a | 29.6 ± 0.2 g | 34.1 ± 1.7 cdef | 34.7 ± 0.1 cdef | 35.2 ± 0.8 cde | 36.1 ± 0.7 cd | 36.9 ± 0.3 bc | 30.4 ± 0.8 g | 31.5 ± 2.1 fg | 34.1 ± 0.0 cdef | 31.6 ± 1.3 efg | 33.2 ± 0.6 defg | 39.7 ± 1.0 b | 34.8 ± 0.1 cdef | 35.8 ± 0.3 cd |
| Quercetin-3-O-galactoside | 7.4 ± 0.1 a | 4.7 ± 0.0 e | 5.3 ± 0.3 cde | 5.3 ± 0.0 cde | 5.6 ± 0.2 cd | 5.8 ± 0.2 bc | 5.8 ± 0.1 bc | 4.9 ± 0.1 de | 5.1 ± 0.3 cde | 5.5 ± 0.2 cde | 5.1 ± 0.4 cde | 5.2 ± 0.3 cde | 6.4 ± 0.1 b | 5.5 ± 0.1 cde | 5.8 ± 0.02 bc |
| Quercetin-3-O-xyloside | 7.4 ± 0.1 a | 5.1 ± 0.0 ghi | 5.6 ± 0.1 fgh | 5.3 ± 0.1 ghi | 5.1 ± 0.3 hi | 5.2 ± 0.2 ghi | 7.1 ± 0.2 bc | 6.4 ± 0.1 cd | 6.1 ± 0.4 def | 6.3 ± 0.1 cde | 5.4 ± 0.1 fghi | 5.2 ± 0.1 fghi | 5.8 ± 0.2 defg | 4.8 ± 0.2 i | 5.6 ± 0.0 efgh |
| Quercetin-3-O-glucoside | 0.7 ± 0.1 a | 0.5 ± 0.1 a | 0.5 ± 0.1 a | 0.5 ± 0.0 a | 0.5 ± 0.0 a | 0.5 ± 0.2 a | 0.6 ± 0.1 a | 0.5 ± 0.0 a | 0.6 ± 0.1 a | 0.6 ± 0.1 a | 0.5 ± 0.1 a | 0.6 ± 0.1 a | 0.6 ± 0.1 a | 0.5 ± 0.1 a | 0.5 ± 0.1 a |
| Derivatives of kaempferol | 1.5 ± 0.1 ab | 1.3 ± 0.1 bcd | 1.2 ± 0.0 bcde | 1.1 ± 0.0 def | 1.1 ± 0.1 cdef | 0.9 ± 0.1 fg | 1.4 ± 0.1 abc | 0.9 ± 0.0 efg | 1.1 ± 0.1 defg | 1.31 ± 0.0 bcd | 1.2 ± 0.0 bcde | 1.5 ± 0.1 ab | 1.7 ± 0.0 a | 1.1 ± 0.1 def | 0.8 ± 0.0 g |
| ANTHOCYANINS | |||||||||||||||
| Cyanidin-3-O-glucoside | 14.2 ± 0.1 a | 2.7 ± 0.1 d | 2.4 ± 0.1 def | 2.3 ± 0.1 ef | 1.4 ± 0.0 g | 0.8 ± 0.1 h | 3.2 ± 0.0 c | 2.2 ± 0.0 f | 2.5 ± 0.0 def | 2.6 ± 0.0 de | 2.4 ± 0.0 ef | 3.4 ± 0.1 c | 4.5 ± 0.21 b | 2.4 ± 0.1 def | 1.2 ± 0.1 g |
| Cyanidin-3-O-rutinoside | 26.6 ± 0.1 a | 4.7 ± 0.3 d | 4.3 ± 0.3 de | 4.1 ± 0.0 de | 2.8 ± 0.1 g | 1.6 ± 0.0 h | 5.5 ± 0.1 c | 3.5 ± 0.0 f | 4.3 ± 0.3 de | 4.1 ± 0.1 ef | 4.1 ± 0.2 ed | 5.7 ± 0.2 c | 8.3 ± 0.01 b | 4.67 ± 0.1 de | 2.6 ± 0.2 g |
| Peonidin-3-O-rutinoside | 1.6 ± 0.1 a | 0.5 ± 0.0 c | 0.4 ± 0.1 cde | 0.4 ± 0.1 cde | 0.3 ± 0.1 de | 0.2 ± 0.0 e | 0.5 ± 0.0 cd | 0.3 ± 0.1 cde | 0.4 ± 0.0 cde | 0.4 ± 0.0 cde | 0.4 ± 0.0 cde | 0.5 ± 0.0 dc | 0.8 ± 0.01 b | 0.42 ± 0.0 cde | 0.2 ± 0.1 e |
| Sum of polyphenols | 1269.2 ± 1.1 a | 1027.2 ± 3.6 de | 1014.8 ± 8.4 de | 984.9 ± 27.2 e | 841.2 ± 0.5 f | 1117 ± 5.7 bc | 1196.5 ± 33.9 ab | 1056.1 ± 8.1 cde | 879.3 ± 9.6 f | 1025.5 ± 18.4 de | 992.5 ± 7.1 e | 1062.3 ± 0.2 cde | 1120.4 ± 2.4 bc | 1101.9 ± 9.1 cd | 1268.9 ± 67.8 a |
| FRAP | 4.5 ± 0.1 a | 2.9 ± 0.1 e | 2.9 ± 0.0 de | 3.1 ± 0.3 cde | 3.2 ± 0.1 bcde | 3.6 ± 0.1 b | 3.2 ± 0.0 bcde | 3.4 ± 0.2 bcd | 3.6 ± 0.3 b | 3.5 ± 0.1 bc | 2.7 ± 0.0 e | 2.7 ± 0.06 e | 2.8 ± 0.01 e | 2.8 ± 0.1 e | 2.8 ± 0.0 e |
| TEAC ABTS | 4.6 ± 0.1 a | 2.7 ± 0.0 g | 2.9 ± 0.2 def | 3.0 ± 0.2 cdef | 3.1 ± 0.1 cde | 3.5 ± 0.0 b | 3.2 ± 0.1 bcd | 3.6 ± 0.0 b | 3.4 ± 0.1 c | 3.1 ± 0.0 cd | 2.8 ± 0.1 efg | 2.7 ± 0.01 fg | 2.7 ± 0.02 fg | 2.8 ± 0.1 fg | 2.8 ± 0.1 defg |
| CD | MVD | CD/MVD | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flavan-3-ols | Model | R2 = 0.4118 | F(2.2) = 2.400 | p < 0.294 | RMSE = 81.412 | R2 = 0.0001 | F(2.1) = 0.958 | p < 0.586 | RMSE = 126.7 | R2 = 0.7968 | F(2.2) = 8.842 | p < 0.102 | RMSE = 42.94 | ||||||
| Variables | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | |
| Constant | 2407.4 | 1509.6 | −1.59 | 0.2518 | 468.75 | 1405.3 | 0.33 | 0.7950 | 2740.5 | 478.48 | 5.73 | 0.0292 | |||||||
| Drying time | 4.11 | 1.89 | 2.818 | 1.29 | 2.18 | 0.1613 | 1.06 | 1.50 | 3.33 | 4.75 | 0.70 | 0.6104 | −0.76 | 0.24 | −1.75 | 0.55 | −3.21 | 0.0851 | |
| Temp. max | 3.94 | 1.89 | 26.442 | 12.69 | 2.08 | 0.1725 | 0.27 | 1.50 | 1.93 | 10.60 | 0.18 | 0.8856 | −0.84 | 0.24 | −19.59 | 5.53 | −3.54 | 0.0713 | |
| Polymeric procyanidins | Model | R2 = 0.4684 | F(2.2) = 2.763 | p < 0.266 | RMSE = 79.905 | R2 = 0.0001 | F(2.1) = 0.859 | p < 0.607 | RMSE = 130.5 | R2 = 0.8403 | F(2.2) = 11.52 | p < 0.080 | RMSE =3 8.02 | ||||||
| Variables | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | |
| Constant | 2770.11 | 1481.4 | −1.87 | 0.2024 | 193.84 | 1446.9 | 0.13 | 0.9152 | 2574.62 | 423.63 | 6.08 | 0.0260 | |||||||
| Drying time | 4.21 | 1.80 | 2.98 | 1.27 | 2.34 | 0.1438 | 1.12 | 1.56 | 3.53 | 4.89 | 0.72 | 0.6018 | −0.78 | 0.21 | −1.80 | 0.48 | −3.72 | 0.0651 | |
| Temp. max | 4.06 | 1.80 | 28.13 | 12.45 | 2.26 | 0.1523 | 0.37 | 1.56 | 2.61 | 10.91 | 0.24 | 0.8506 | −0.83 | 0.21 | -19.53 | 4.90 | −3.99 | 0.0575 | |
| Phenolic acids | Model | R2 = 0.9499 | F(2.2) = 38.96 | p < 0.0252 | RMSE = 2.827 | R2 = 0.7584 | F(2.1) = 5.709 | p < 0.284 | RMSE = 5.517 | R2 = 0.3695 | F(2.2) = 2.172 | p < 0.315 | RMSE = 24.57 | ||||||
| Variables | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | |
| Constant | 359.222 | 52.41 | 6.85 | 0.0206 | 110.473 | 61.17 | −1.81 | 0.3219 | 9.37 | 277.09 | 0.03 | 0.9761 | |||||||
| Drying time | −2.96 | 0.55 | −0.241 | 0.04 | −5.37 | 0.033 | 2.391 | 0.73 | 0.678 | 0.21 | 3.28 | 0.1882 | −0.656 | 0.42 | −0.5 | 0.32 | −1.58 | 0.2548 | |
| Temp. max | −2.115 | 0.55 | −1.69 | 0.44 | -3.84 | 0.0617 | 2.43 | 0.73 | 1.538 | 0.46 | 3.34 | 0.1854 | 0.347 | 0.42 | 2.675 | 3.2 | 0.84 | 0.4913 | |
| Flavonoids | Model | R2 = 0.4643 | F(2.2) = 2.734 | p < 0.268 | RMSE = 2.480 | R2 = 0.9997 | F(2.1)= 4478.8 | p < 0.011 | RMSE = 0.0826 | R2 = 0.0001 | F(2.2) = 0.645 | p < 0.608 | RMSE = 5.15 | ||||||
| Variables | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | |
| Constant | 51.465 | 45.98 | 1.12 | 0.3794 | −11.739 | 0.92 | −12.82 | 0.0496 | 38.678 | 57.37 | 0.67 | 0.5697 | |||||||
| Drying time | −0.39 | 1.8 | 0.009 | 0.04 | −0.22 | 0.849 | 2.391 | 0.03 | 0.273 | 0 | 88.15 | 0.0072 | −0.486 | 0.58 | -0.055 | 0.07 | −0.84 | 0.4878 | |
| Temp. max | 0.471 | 1.8 | 0.101 | 0.39 | 0.26 | 0.8185 | 1.838 | 0.03 | 0.468 | 0.01 | 67.76 | 0.0094 | 0.277 | 0.58 | 0.318 | 0.66 | 0.48 | 0.6789 | |
| Anthocyanins | Model | R2 = 0.9527 | F(2.2) = 41.27 | p < 0.024 | RMSE = 0.479 | R2 = 0.6262 | F(2.1) = 3.513 | p < 0.353 | RMSE = 0.8238 | R2 = 0.6569 | F(2.2) = 4.829 | p < 0.171 | RMSE = 2.091 | ||||||
| Variables | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | |
| Constant | 33.335 | 8.88 | 3.75 | 0.0643 | -6.445 | 9.13 | -0.71 | 0.6088 | −60.178 | 23.3 | −2.58 | 0.1229 | |||||||
| Drying time | −1.101 | 0.54 | 0.016 | 0.01 | −2.06 | 0.1762 | 1.922 | 0.91 | 0.065 | 0.03 | 2.12 | 0.2805 | 0.129 | 0.31 | 0.011 | 0.03 | 0.42 | 0.7143 | |
| Temp. max | −2.041 | 0.54 | 0.284 | 0.07 | −3.81 | 0.0625 | 1.209 | 0.91 | 0.092 | 0.07 | 1.33 | 0.4094 | 0.94 | 0.31 | 0.826 | 0.27 | 3.07 | 0.0919 | |
| Sum of polyphenols | Model | R2 = 0.2894 | F(2.2)= 1.815 | p < 0.355 | RMSE = 84.326 | R2 = 0.1427 | F(2.1) = 1.250 | p < 0.535 | RMSE = 120.47 | R2 = 0.8240 | F(2.2) = 10.367 | p < 0.088 | RMSE = 42.72 | ||||||
| Variables | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | |
| Constant | −1963.1 | 1563.4 | −1.26 | 0.3361 | 340.13 | 1335.7 | 0.25 | 0.8413 | 2728.46 | 476.04 | 5.73 | 0.0291 | |||||||
| Drying time | 3.96 | 2.08 | 2.55 | 1.34 | 1.90 | 0.1971 | 1.32 | 1.37 | 4.35 | 4.51 | 0.96 | 0.5118 | −0.93 | 0.22 | -2.29 | 0.54 | −4.22 | 0.0518 | |
| Temp. max | 3.88 | 2.08 | 24.57 | 13.14 | 1.87 | 0.2024 | 0.55 | 1.37 | 4.02 | 10.07 | 0.40 | 0.7581 | −0.63 | 0.22 | -15.77 | 5.50 | −2.87 | 0.1032 | |
| TEAC ABTS | Model | R2 = 0.9624 | F(2.2) = 52.15 | p < 0.019 | RMSE = 0.065 | R2 = 0.9684 | F(2.1) = 46.93 | p < 0.103 | RMSE = 0.0362 | R2 = 0.8249 | F(2.2) = 10.42 | p < 0.0876 | RMSE = 0.0224 | ||||||
| Variables | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | |
| Constant | 0.768 | 1.21 | −0.64 | 0.5903 | 7.021 | 0.4 | 17.48 | 0.0364 | 3.743 | 0.25 | 15.03 | 0.0044 | |||||||
| Drying time | 0.904 | 0.48 | 0.002 | 0 | 1.89 | 0.1992 | 2.538 | 0.26 | −0.013 | 0 | −9.63 | 0.0659 | −0.195 | 0.22 | 0 | 0 | −0.89 | 0.4665 | |
| Temp. max | 1.858 | 0.48 | 0.039 | 0.01 | 3.89 | 0.0602 | 2.448 | 0.26 | −0.028 | 0 | −9.29 | 0.0683 | −0.994 | 0.22 | -0.013 | 0 | −4.54 | 0.0452 | |
| FRAP | Model | R2 = 0.9409 | F(2.2) = 32.86 | p < 0.030 | RMSE = 0.076 | R2 = 0.5453 | F(2.1) = 2.799 | p < 0.389 | RMSE = 0.1210 | R2 = 07430 | F(2.2) = 6.781 | p < 0.129 | RMSE = 0.0176 | ||||||
| Variables | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | β | SHβ | B | SHB | T | p | |
| Constant | 1.271 | 1.41 | −0.9 | 0.463 | 4.014 | 1.34 | 2.99 | 0.2053 | 3.39 | 0.2 | 17.27 | 0.0033 | |||||||
| Drying time | 1.331 | 0.6 | 0.003 | 0 | 2.22 | 0.1562 | 1.133 | 1 | −0.005 | 0 | −1.13 | 0.4603 | −0.817 | 0.27 | −0.001 | 0 | −3.08 | 0.0912 | |
| Temp. max | 2.251 | 0.6 | 0.045 | 0.01 | 3.76 | 0.064 | 0.235 | 1 | −0.002 | 0.01 | −0.23 | 0.8533 | −0.751 | 0.27 | −0.006 | 0 | −2.83 | 0.1053 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalska, A.; Wojdyło, A.; Majerska, J.; Lech, K.; Brzezowska, J. Qualitative and Quantitative Evaluation of Heat-Induced Changes in Polyphenols and Antioxidant Capacity in Prunus domestica L. By-products. Molecules 2019, 24, 3008. https://doi.org/10.3390/molecules24163008
Michalska A, Wojdyło A, Majerska J, Lech K, Brzezowska J. Qualitative and Quantitative Evaluation of Heat-Induced Changes in Polyphenols and Antioxidant Capacity in Prunus domestica L. By-products. Molecules. 2019; 24(16):3008. https://doi.org/10.3390/molecules24163008
Chicago/Turabian StyleMichalska, Anna, Aneta Wojdyło, Joanna Majerska, Krzysztof Lech, and Jessica Brzezowska. 2019. "Qualitative and Quantitative Evaluation of Heat-Induced Changes in Polyphenols and Antioxidant Capacity in Prunus domestica L. By-products" Molecules 24, no. 16: 3008. https://doi.org/10.3390/molecules24163008
APA StyleMichalska, A., Wojdyło, A., Majerska, J., Lech, K., & Brzezowska, J. (2019). Qualitative and Quantitative Evaluation of Heat-Induced Changes in Polyphenols and Antioxidant Capacity in Prunus domestica L. By-products. Molecules, 24(16), 3008. https://doi.org/10.3390/molecules24163008