Terpenoids of the Swamp Cypress Subfamily (Taxodioideae), Cupressaceae, an Overview by GC-MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cryptomeria
2.2. Glyptostrobus
2.3. Taxodium distichum
2.4. Taxodium mucronatum
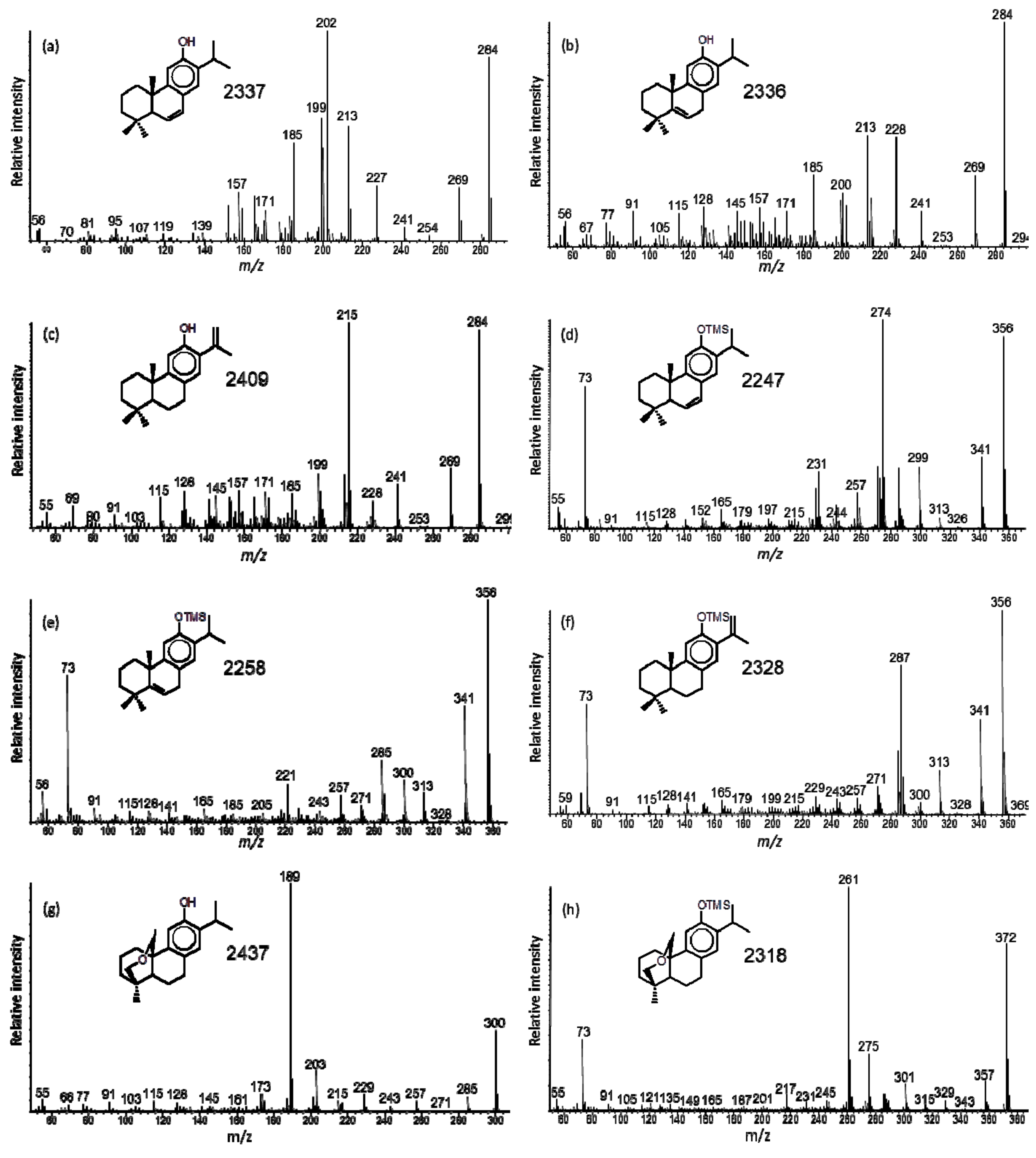
2.5. Mass Spectrometry
2.6. Key Molecular Tracers for the Taxodioideae
3. Samples and Experimental Methods
3.1. Plant Material
3.2. Extraction
3.3. Derivatization
3.4. GC-MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Simoneit, B.R.T. Diterpenoid compounds and other lipids in deep-sea sediments and their geochemical significance. Geochim. Cosmochim. Acta 1977, 41, 463–476. [Google Scholar] [CrossRef]
- Simoneit, B.R.T. The organic chemistry of marine sediments. In Chemical Oceanography, 2nd ed.; Riley, J.P., Chester, R., Eds.; Academic Press: New York, NY, USA, 1978; Volume 7, pp. 233–311. [Google Scholar]
- Johns, R.B. Biological Markers in the Sedimentary Record; Elsevier: Amsterdam, The Netherlands, 1986; p. 364. [Google Scholar]
- Simoneit, B.R.T. Organic matter of the troposphere—V: Application of molecular marker analysis to biogenic emissions into the troposphere for source reconciliations. J. Atmos. Chem. 1989, 8, 251–275. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Mazurek, M.A. Organic matter of the troposphere—II. Natural background of biogenic lipid matter in aerosols over the rural western United States. Atmos. Environ. 1982, 16, 2139–2159. [Google Scholar] [CrossRef]
- Simoneit, B.R.T. A review of biomarker compounds as source indicators and tracers for air pollution. Environ. Sci. Pollut. Res. 1999, 6, 153–163. [Google Scholar] [CrossRef]
- Otto, A.; White, J.D.; Simoneit, B.R.T. Natural product terpenoids in Eocene and Miocene conifer fossils. Science 2002, 297, 1543–1545. [Google Scholar] [CrossRef]
- Otto, A.; Simoneit, B.R.T.; Wilde, V.; Kunzmann, L.; Püttmann, W. Terpenoid composition of three fossil resins from Cretaceous and Tertiary conifers. Rev. Palaeobot. Palynol. 2002, 120, 203–215. [Google Scholar] [CrossRef]
- Otto, A.; Simoneit, B.R.T.; Wilde, V. Terpenoids as chemosystematic markers in selected fossil and extant species of pine (Pinus, Pinaceae). Bot. J. Linn. Soc. 2007, 154, 129–140. [Google Scholar] [CrossRef]
- Evershed, R.P. Organic residue analysis in archaeology: The archaeological biomarker revolution. Archaeometry 2008, 50, 895–924. [Google Scholar] [CrossRef]
- Medeiros, P.M.; Simoneit, B.R.T. Multi-biomarker characterization of sedimentary organic carbon in small rivers draining the northwestern United States. Org. Geochem. 2008, 39, 52–74. [Google Scholar] [CrossRef]
- Menor-Salván, C.; Najarro, M.; Velasco, F.; Rosales, I.; Tornos, F.; Simoneit, B.R.T. Terpenoids in extracts of Lower Cretaceous ambers from the Basque-Cantabrian basin (El Soplao, Cantabria, Spain): Paleochemotaxonomic aspects. Org. Geochem. 2010, 41, 1089–1103. [Google Scholar] [CrossRef]
- Schnell, G.; Schaeffer, P.; Tardiron, H.; Motsch, E.; Connan, J.; Ertlen, D.; Schwarz, D.; Schneider, N.; Adam, P. Contrasting diagenetic pathways of higher plant triterpenoids in buried wood as a function of tree species. Org. Geochem. 2014, 66, 107–123. [Google Scholar] [CrossRef]
- Stefanova, M.; Simoneit, B.R.T.; Marinov, S.P.; Zdravkov, A.; Kortenski, J. Novel polar biomarkers of the Miocene Maritza-East lignite, Bulgaria. Org. Geochem. 2016, 96, 1–10. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Cox, R.C.; Oros, D.R.; Otto, A. Terpenoid compositions of resins from Callitris species (Cupressaceae). Molecules 2018, 23, 3384. [Google Scholar] [CrossRef]
- González, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef]
- Otto, A.; Wilde, V. Sesqui-, di-, and triterpenoids as chemosystematic markers in extant conifers—A review. Bot. Rev. 2001, 67, 141–238. [Google Scholar]
- Hickey, L.J. The paleobotany and stratigraphy of the Golden Valley Formation (Late Paleocene—Early Eocene) of western North Dakota. Geol. Soc. Am. Mem. 1977, 150, 181. [Google Scholar]
- Mai, D.H.; Walther, H. Die oligozänen und untermiozänen Floren NW-Sachsens und des Bitterfelder Raumes. Abh. Staatl. Mus. Mineral. Geol. Dresd. 1991, 38, 1–230. [Google Scholar]
- LePage, B.A. The taxonomy and biogeographic history of Glyptostrobus Endlicher (Cupressaceae). Bull. Peabody Mus. Nat. Hist. 2007, 48, 359–426. [Google Scholar] [CrossRef]
- Eckenwalder, J.E. Re-evaluation of Curessaceae and Taxodiaceae: A proposed merger. Madroño 2009, 23, 237–256. [Google Scholar]
- Ding, W.N.; Kunzmann, L.; Su, T.; Huang, J.; Zhou, Z.K. A new fossil species of Cryptomeria (Cupressaceae) from the Rupelian of the Lühe Basin, Yunnan, East Asia: Implications for palaeogeography and palaeoecology. Rev. Palaeobot. Palynol. 2018, 248, 41–51. [Google Scholar] [CrossRef]
- Dehmer, J. Petrological and organic geochemical investigation of recent peats with known environments of deposition. Int. J. Coal Geol. 1995, 28, 111–138. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Karim, A.; Marcks, C. Tumor inhibitors. XLVIII. Taxodione and taxodone, two novel diterpenoid quinone methide tumor inhibitors from Taxodium distichum. J. Org. Chem. 1969, 34, 3912–3918. [Google Scholar] [CrossRef]
- Shibuya, T. Cryptoquinonemethides D and E, C30-terpene quinone methides, from Cryptomeria japonica. Phytochemistry 1992, 31, 4289–4294. [Google Scholar] [CrossRef]
- Su, W.C.; Fang, J.M.; Cheng, Y.S. Hexacarbocyclic triterpenes from the leaves of Cryptomeria japonica. Phytochemistry 1993, 34, 779–782. [Google Scholar] [CrossRef]
- Su, W.C.; Fang, J.M.; Cheng, Y.S. Abietanes and kauranes from leaves of Cryptomeria japonica. Phytochemistry 1994, 35, 1279–1284. [Google Scholar] [CrossRef]
- Su, W.C.; Fang, J.M.; Cheng, Y.S. Labdanes from Cryptomeria japonica. Phytochemistry 1994, 37, 1109–1114. [Google Scholar]
- Su, W.C.; Fang, J.M.; Cheng, Y.S. Diterpenoids from leaves of Cryptomeria japonica. Phytochemistry 1996, 41, 255–261. [Google Scholar]
- Su, Z.; Yuan, W.; Wang, P.; Li, S. Ethnobotany, phytochemistry, and biological activities of Taxodium Rich. Pharmaceut. Crops 2013, 4, 1–14. [Google Scholar]
- Yamamoto, H.; Hirao, T.; Wakayama, K.-I.; Chida, T. Abietane diterpenes from cones of Taxodium distichum Rich. Bull. Fac. Edu. Ibaraki Univ. Nat. Sci. 2003, 52, 31–39. [Google Scholar]
- Kusumoto, N.; Murayama, T.; Kawai, Y.; Ashitani, T.; Ogiyama, K.; Takahashi, K. Taxodal, a novel irregular abietane-type diterpene from the cones of Taxodium distichum. Tetrahedron Lett. 2008, 49, 4845–4847. [Google Scholar] [CrossRef]
- Kusumoto, N.; Ashitani, T.; Hayasaka, Y.; Murayama, T.; Ogiyama, K.; Takahashi, K. Antitermitic activities of abietane-type diterpenes from Taxodium distichum cones. J. Chem. Ecol. 2009, 35, 635–642. [Google Scholar] [CrossRef]
- Kusumoto, N.; Ashitani, T.; Murayama, T.; Ogiyama, K.; Takahashi, K. Antifungal abietane-type diterpenes from the cones of Taxodium distichum Rich. J. Chem. Ecol. 2010, 36, 1381–1386. [Google Scholar] [CrossRef]
- Li, W.H.; Chang, S.T.; Chang, S.C.; Chang, H.T. Isolation of antibacterial diterpenoids from Cryptomeria japonica bark. Nat. Prod. Res. 2008, 22, 1085–1093. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, W.M. New sesquiterpene and triterpene from the fruits of Cryptomeria fortunei. J Asian Nat. Prod. Res. 2010, 12, 382–387. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Yin, R.T.; Jia, R.R.; Yang, E.H.; Xu, H.M.; Tan, N.H. A new diterpene from Glyptostrobus pensilis. Fitoterapia 2010, 81, 1202–1204. [Google Scholar] [CrossRef]
- Naman, C.B.; Gromovsky, A.D.; Vela, C.M.; Fletcher, J.N.; Gupta, G.; Varikuti, S.; Zhu, X.; Zywot, E.M.; Chai, H.; Werbovetz, K.A.; et al. Antileishmanial and cytotoxic activity of some highly oxidized abietane diterpenoids from the bald cypress, Taxodium distichum. J. Nat. Prod. 2016, 79, 598–606. [Google Scholar] [CrossRef]
- Chang, C.I.; Chen, C.C.; Wang, S.Y.; Chao, C.Y.; Chao, L.K.; Chen, J.J.; Ko, H.H.; Chen, C.C.; Kuo, Y.H. Three new abietane-type diterpenoids from the bark of Cryptomeria japonica. Helv. Chim. Acta 2016, 99, 710–715. [Google Scholar] [CrossRef]
- Chang, C.I.; Chen, C.C.; Wang, S.Y.; Chang, H.S.; Chao, L.K.; Chen, J.J.; Chen, C.C.; Kuo, Y.H. Three new abietane-type diterpenes from the bark of Cryptomeria japonica. Phytochem. Lett. 2017, 19, 46–49. [Google Scholar] [CrossRef]
- Chang, C.I.; Wang, S.Y.; Wu, M.D.; Cheng, M.J.; Ko, H.H.; Chang, H.S.; Chen, J.J.; Chen, C.C.; Kuo, Y.H. Two new sesquaterpenoids from the bark of Cryptomeria japonica. Phytochem. Lett. 2017, 22, 56–60. [Google Scholar] [CrossRef]
- Chang, C.I.; Lee, T.H.; Li, Y.J.; Chao, C.Y.; You, B.J.; Cheng, M.J.; Wang, S.Y.; Chen, C.C.; Kuo, Y.H. New 7-oxoabietane-type diterpenoids from bark of Cryptomeria japonica and their xanthine oxidase inhibitor activity. Phytochem. Lett. 2018, 27, 69–73. [Google Scholar] [CrossRef]
- Lu, Y.; Hautevelle, Y.; Michels, R. Determination of the molecular signature of fossil conifers by experimental palaeochemotaxonomy—Part 1: The Araucariaceae family. Biogeosciences 2013, 10, 1943–1962. [Google Scholar] [CrossRef]
- Erdtman, H.; Norin, T. The chemistry of the order Cupressales. Prog. Chem. Org. Nat. Prod. 1966, 24, 207–287. [Google Scholar]
- Hegnauer, R. Chemotaxonomie der Pflanzen: Eine Übersicht über die Verbreitung und die Systematische Bedeutung der Pflanzenstoffe. In Band, I: Thallophyten, Bryophyten, Pteridophyten, Gymnospermen; Birkhäuser Verlag: Basel, Switzerland, 1962. [Google Scholar]
- Hegnauer, R. Chemotaxonomie der Pflanzen: Eine Übersicht über die Verbreitung und die Systematische Bedeutung der Pflanzenstoffe. In Band VII: Addendum; Birkhäuser Verlag: Basel, Switzerland, 1986. [Google Scholar]
- Gadek, P.A.; Alpers, D.L.; Heslewood, M.M.; Quinn, C.J. Relationships within Cupressaceae sensu lato: A combined morphological and molecular analysis. Am. J. Bot. 2000, 87, 1044–1057. [Google Scholar] [CrossRef]
- Chang, C.I.; Chen, C.C.; Chen, C.R.; Wu, M.D.; Cheng, M.J.; Sung, P.J.; Kuo, Y.H. Bioactive dimeric abietanoid peroxides from the bark of Cryptomeria japonica. Molecules 2019, 24, 2178. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.R.; Escamilla, E.M.; Calderón, J.; Rodríguez, B. 8β-Hydroxypimar-15-en-19-oic acid from Taxodium mucronatum. Phytochemistry 1984, 23, 1329–1330. [Google Scholar] [CrossRef]
- Shieh, B.; Iizuka, Y.; Matsubara, Y. Monoterpenoid and sesquiterpenoid constituents of the essential oil of sugi (Cryptomeria japonica D. Don.). Agric. Biol. Chem. 1981, 45, 1493–1495. [Google Scholar] [CrossRef]
- Vernin, G.; Metzger, J.; Mondon, J.P.; Pieribattesti, J.C. GC-MS analysis of the leaf oil of Cryptomeria japonica D. Don from Reunion Island. J. Essent. Oil Res. 1991, 3, 197–207. [Google Scholar] [CrossRef]
- Yao, S.; Tang, C.P.; Ke, C.Q.; Ye, Y. Abietane diterpenoids from the bark of Cryptomeria fortunei. J. Nat. Prod. 2008, 71, 1242–1246. [Google Scholar] [CrossRef]
- Tulloch, A.P.; Bergter, L. Epicuticular wax of Juniperus scopulorum. Phytochemistry 1981, 20, 2711–2716. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Otto, A.; Kusumoto, N.; Basinger, J.F. Biomarker compositions of Metasequoia and Glyptostrobus (Cupressaceae) fossils from the Eocene Buchanan Lake Formation, Axel Heiberg Island, Nunavut, Canada reflect diagenesis from terpenoids of their related extant species. Rev. Paleobot. Palynol. 2016, 235, 81–93. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4.1st ed.; Allured Publishing: Carol Stream, IL, USA, 2017; ISBN 978-1-932633-21-4. [Google Scholar]
- Cox, R.E.; Yamamoto, S.; Otto, A.; Simoneit, B.R.T. Oxygenated di- and tricyclic diterpenoids of southern hemisphere conifers. Biochem. Syst. Ecol. 2007, 35, 342–362. [Google Scholar] [CrossRef]
- Hasegawa, S.; Kojima, T.; Hirose, Y. Terpenoids from the seed of Chamaecyparis pisifera: The structure of six diterpenoids. Phytochemistry 1985, 24, 1545–1551. [Google Scholar] [CrossRef]
- Wang, S.Y.; Wu, J.H.; Shyur, L.F.; Kuo, Y.H.; Chang, S.T. Antioxidant activity of abietane-type diterpenes from heartwood of Taiwania cryptomerioides Hayata. Holzforschung 2002, 56, 487–492. [Google Scholar] [CrossRef]
- Fraga, B.M.; Díaz, C.E.; Guadaño, A.; González-Coloma, A. Diterpenes from Salvia broussonetii transformed roots and their insecticidal activity. J. Agric. Food Chem. 2005, 53, 5200–5206. [Google Scholar] [CrossRef]
- Barrero, A.F.; Herrador, M.M.; Arteaga, P.; Arteaga, J.F.; Arteaga, A.F. Communic acids: Occurrence, properties and use as chirons for the synthesis of bioactive compounds. Molecules 2012, 17, 1448–1467. [Google Scholar] [CrossRef]
- Carman, R.M.; Craig, W.J.; Shaw, I.M. Diterpenoids XXXI. Three new resin acids. Aust. J. Chem. 1973, 26, 209–214. [Google Scholar] [CrossRef]
- Fang, J.M.; Lang, C.I.; Chen, W.L.; Cheng, Y.S. Diterpenoid acids from the leaves of Armand pine. Phytochemistry 1991, 30, 2793–2795. [Google Scholar] [CrossRef]
- Dai, D.N.; Thai, T.H. Chemical composition of the woods oil of Glyptostrobus pensilis (Staunton ex D. Don) K. Koch from Vietnam. Tap Chi Sinh Hoc 2012, 34, 204–206. [Google Scholar] [CrossRef]
- Otto, A.; Simoneit, B.R.T.; Lesiak, M.; Wilde, V.; Worobiec, G. Resin and wax biomarkers preserved in Miocene Cupressaceae s.l. from Bełchatów and Lipnica Wielka, Poland. Acta Palaeobot. 2001, 41, 195–206. [Google Scholar]
- Otto, A.; Simoneit, B.R.T.; Rember, W.C. Resin compounds preserved in the seed cones of three fossil conifer species from the Miocene Clarkia flora, Emerald Creek, Idaho, USA and from related extant species. Rev. Palaeobot. Palynol. 2003, 26, 225–241. [Google Scholar] [CrossRef]
- Hirao, T.; Nakano, Y.; Yamamoto, H. Four new 6,7-dioxoabietane diterpenes from cones of Taxodium distichum Rich. Bull. Coll. Edu. Ibaraki Univ. Nat. Sci. 2008, 57, 71–76. [Google Scholar]
- Otto, A.; Simoneit, B.R.T. Chemosystematics and diagenesis of terpenoids in fossil conifer species and sediment from the Eocene Zeitz formation, Saxony, Germany. Geochim. Cosmochim. Acta 2001, 65, 3505–3527. [Google Scholar] [CrossRef]
- Otto, A.; Walther, H.; Püttmann, W. Sesqui- and diterpenoid biomarkers preserved in Taxodium-rich Oligocene oxbow lake clays, Weisselster basin, Germany. Org. Geochem. 1997, 26, 105–115. [Google Scholar] [CrossRef]
- Otto, A.; Simoneit, B.R.T.; Rember, W.C. Conifer and angiosperm biomarkers in clay sediments and fossil plants from the Miocene Clarkia formation, Idaho, USA. Org. Geochem. 2005, 36, 907–922. [Google Scholar] [CrossRef]
- Marynowski, L.; Otto, A.; Zatón, M.; Philippe, M.; Simoneit, B.R.T. Biomolecules preserved in ca. 160 million year old fossil conifer wood. Naturwissenschaften 2007, 94, 228–236. [Google Scholar] [CrossRef]
- Tsujimura, M.; Goto, M.; Tsuji, M.; Yamaji, Y.; Ashitani, T.; Kimura, K.I.; Ohira, T.; Kofujita, H. Isolation of diterpenoids from sugi wood-drying byproducts and their bioactivities. J. Wood Sci. 2019, 65, 19. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.H.; Tao, L.X.; Ke, C.Q.; Tang, C.; Zhang, H.Y.; Ye, Y.; Lin, L.G.; Yao, S. Taxodikaloids A and B, two dimeric abietane-type diterpenoids from Taxodium ascendens possessing an oxazoline ring linkage. Org. Lett. 2017, 19, 556–559. [Google Scholar] [CrossRef]
- Shibuya, T. Structures and natural synthesis of terpene quinone methides from Cryptomeria japonica. Symp. Chem. Terps Essen. Oils Arom. (TEAC) Utsunomiya 1995, 39, 1–4. [Google Scholar]
- Enzell, C.R.; Ryhage, R. Mass spectrometric studies of diterpenes. 5. Aromatic diterpenes. Arkiv Kemi 1967, 27, 213–229. [Google Scholar]
- Asano, N.; Yamamoto, H. Isolation and analysis using gas chromatography-mass spectrometry of terpenes from leaf and cones of Chamaecyparis obtusa. Bull. Fac. Edu. Ibaraki Univ. Nat. Sci. 1994, 43, 61–73. [Google Scholar]
- Hsu, K.C.; Fang, J.M.; Cheng, Y.S. Diterpenes from pericarps of Chamaecyparis formosensis. J. Nat. Prod. 1995, 58, 1592–1595. [Google Scholar] [CrossRef]
- Rybicki, M.; Marynowski, L.; Misz-Kennan, M.; Simoneit, B.R.T. Molecular tracers preserved in Lower Jurassic “Blanowice brown coals” from southern Poland at the onset of coalification: Organic geochemical and petrological characteristics. Org. Geochem. 2016, 102, 77–92. [Google Scholar] [CrossRef]
- Nytoft, H.P.; Kildahl-Andersen, G.; Lindström, S.; Rise, F.; Bechtel, A.; Mitrović, D.; Đoković, N.; Životić, D.; Stojanović, K.A. Dihydroicetexanes in sediments and crude oils: Possible markers for Cupressoideae. Org. Geochem. 2019, 129, 14–23. [Google Scholar] [CrossRef]
- Kovats, E. Gas-chromatogaphische Charakterisierung organischer Verbindungen. Teil 1: Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
Sample Availability: The samples of the compounds are not available from the authors. |


| Botanical Name | Common Name | Sample Type | Sampling Location | Number of Analyses |
|---|---|---|---|---|
| Cryptomeria japonica | Sugi cedar | Resin on trunk, branchlet | Narita Temple, Tokyo Metro, Japan | 7 |
| Glyptostrobus pensilis | Chinese swamp cypress | Resin on trunk, cone, shoot | Botanical Institute, Chinese Academy of Sciences, Guangzhou, China | 22 |
| Resin on trunk, cone, shoot | Boise, ID, USA | |||
| Taxodium distichum | Swamp cypress | Resin on trunk, shoot | Cypress National Park, FL, USA | 22 |
| Resin on trunk, shoot | Botanical Institute, Chinese Academy of Sciences, Guangzhou, China | |||
| Resin on trunk, shoot | Lithia Park, Ashland, OR, USA | |||
| Taxodium mucronatum | Montezuma cypress | Resin on trunk, cone | Tule Tree, Oaxaca, Mexico | 14 |
| Resin on trunk, cone | Botanical Garden, Los Angeles, CA, USA |
| Number (Appendix A) | Name | Formula 1 | MW | Relative Abundance 2 | ID 3 | References | |||
|---|---|---|---|---|---|---|---|---|---|
| Crytomeria japonica | Glyptostr. pensilis | Taxodium distichum | Taxodium mucronatum | ||||||
| ALIPHATICS | |||||||||
| n1 | n-Nonacosan-10-ol | C29H60O | 424 | 6 | 15 | 8 | L | [53] | |
| n2 | n-Nonacosan-10-one | C29H58O | 422 | 8 | L | [54] | |||
| SESQUITERPENOIDS | |||||||||
| s1 | Cuparene | C15H22 | 202 | 3 | S | [55] | |||
| s2 | Widdrene (thujopsene) | C15H24 | 204 | 20 | S | [55] | |||
| s3 | Mayurone | C14H20O | 204 | 5 | L | [55] | |||
| s4 | Widdrol (thujopsol) | C15H24O | 220 | 4 | S | [55] | |||
| DITERPENOIDS | |||||||||
| Abietanes | |||||||||
| a1 | 6,7-Dehydroferruginol | C20H28O | 284 | 18 | 20 | 55 | 25 | L | [31,56] |
| a2 | Ferruginol | C20H30O | 286 | 40 | 80 | 80 | 100 | S | [34] |
| a3 | 6,7-Dehydropisiferol | C20H28O2 | 300 | 48 | I | [54] | |||
| a4 | Sugiol (7-ketoferruginol) | C20H28O2 | 300 | 10 | 30 | 30 | 10 | S | [54] |
| a5 | 19(20)-Oxoferruginol | C20H28O2 | 300 | 26 | I | ||||
| a6 | 11-Hydroxy-12-oxoabieta-7,9(11),13-triene (or 6-deoxotaxodione) | C20H28O2 | 300 | 8 | 9 | 21 | S,L,I | [55] | |
| a7 | Pisiferol | C20H30O2 | 302 | 60 | L, I | [7,54] | |||
| a8 | abeo-Pisiferol | C20H30O2 | 302 | 75 | L | [57] | |||
| a9 | 11-Hydroxyferruginol | C20H30O2 | 302 | 12 | 23 | L | [58] | ||
| a10 | Taxodione, R=H | C20H26O3 | 314 | 50 | 10 | S,L | [24] | ||
| a11 | Salvinolone | C20H26O3 | 314 | 50 | 8 | 1 | S | [33] | |
| a12 | 6,7-Dehydroroyleanone | C20H26O3 | 314 | 6 | 4 | S | [34] | ||
| a13 | Royleanone | C20H28O3 | 316 | 10 | 8 | S,L | [24] | ||
| a14 | Taxodone | C20H28O3 | 316 | 100 | 12 | S,L | [24] | ||
| a15 | 11-Hydroxysugiol | C20H28O3 | 316 | 60 | L | [27,31] | |||
| a16 | abeo-Carnosol (demethyl salvicanol) | C20H30O3 | 318 | 100 | L | [59] | |||
| a17 | 6-Hydroxysalvinolone (14-deoxycoleon U) | C20H26O4 | 330 | 60 | 74 | S | [33,54] | ||
| a18 | Taxoquinone | C20H28O4 | 332 | 27 | 40 | L | [24] | ||
| a19 | 7-Hydroxytaxodone | C20H28O4 | 332 | 24 | 70 | I | [54] | ||
| a20 | 7-epi-Taxoquinone (horminone) | C20H28O4 | 332 | 12 | I | [24] | |||
| a21 | 6α,11-Dihydroxysugiol (5,6-dihydro-6β-hydroxysalvinolone) | C20H28O4 | 332 | 35 | 8 | L, I | [31] | ||
| a22 | 6β,11-Dihydroxysugiol (5,6-dihydro-6β-hydroxysalvinolone) | C20H28O4 | 332 | 16 | I | ||||
| a23 | 6α-Hydroxytaxoquinone | C20H28O5 | 348 | 55 | 6 | S,I | [54] | ||
| a24 | 6β-Hydroxytaxoquinone | C20H28O5 | 348 | 70 | 11 | S,I | [54] | ||
| a10 | Taxodione acetate, R=Ac | C22H28O4 | 356 | 12 | 14 | I | [54] | ||
| a25 | 7-Acetoxy-6,7-dehydroroyleanone | C22H28O5 | 372 | 5 | 20 | S | [54] | ||
| a26 | 7α-p-Cymenylferruginol | C30H42O | 418 | 2 | 1 | 15 | 5 | L | [36] |
| a27 | Chamaecydin | C30H40O3 | 448 | 42 | 1 | 40 | 25 | S | [26,54] |
| a28 | Iso-chamaecydin | C30H40O3 | 448 | 2 | 2 | 8 | 4 | L | [26,54] |
| a29 | 6β-Hydroxychamaecydin | C30H40O4 | 464 | 2 | 3 | 3 | 1 | S | [26,54] |
| Pimaranes | |||||||||
| p1 | Sandaracopimaric acid | C20H30O2 | 302 | 35 | 18 | 10 | S | ||
| p2 | Isopimaric acid | C20H30O2 | 302 | 100 | S | ||||
| Labdanes | |||||||||
| L1 | Manool | C20H34O | 290 | 12 | 5 | 15 | S | ||
| L2 | E,Z-Communic acids | C20H30O2 | 302 | 11 | L | [55] | |||
| L3 | Iso-communic acid | C20H30O2 | 302 | 4 | L | [60] | |||
| L4 | Imbricataloic acid | C20H32O3 | 320 | 5 | S | ||||
| L5 | 13-epi-Cupressic acid | C20H32O3 | 320 | 75 | L | [28,61] | |||
| L6 | Iso-cupressic acid | C20H32O3 | 320 | 20 | L | [62] | |||
| L7 | Imbricatoloic acid | C20H34O3 | 322 | 48 | S | [28,62] | |||
| L8 | 13,14-Dihydroagathic acid | C20H32O4 | 336 | 9 | S | [54] | |||
| TRITERPENOIDS | |||||||||
| T1 | 24-Methylenecycloartan-3-one | C31H50O | 438 | 1 | I | [54] | |||
| T2 | 24-Ethylenecycloartan-3-one | C32H52O | 452 | 0.4 | I | [54] | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simoneit, B.R.T.; Otto, A.; Oros, D.R.; Kusumoto, N. Terpenoids of the Swamp Cypress Subfamily (Taxodioideae), Cupressaceae, an Overview by GC-MS. Molecules 2019, 24, 3036. https://doi.org/10.3390/molecules24173036
Simoneit BRT, Otto A, Oros DR, Kusumoto N. Terpenoids of the Swamp Cypress Subfamily (Taxodioideae), Cupressaceae, an Overview by GC-MS. Molecules. 2019; 24(17):3036. https://doi.org/10.3390/molecules24173036
Chicago/Turabian StyleSimoneit, Bernd R. T., Angelika Otto, Daniel R. Oros, and Norihisa Kusumoto. 2019. "Terpenoids of the Swamp Cypress Subfamily (Taxodioideae), Cupressaceae, an Overview by GC-MS" Molecules 24, no. 17: 3036. https://doi.org/10.3390/molecules24173036
APA StyleSimoneit, B. R. T., Otto, A., Oros, D. R., & Kusumoto, N. (2019). Terpenoids of the Swamp Cypress Subfamily (Taxodioideae), Cupressaceae, an Overview by GC-MS. Molecules, 24(17), 3036. https://doi.org/10.3390/molecules24173036







