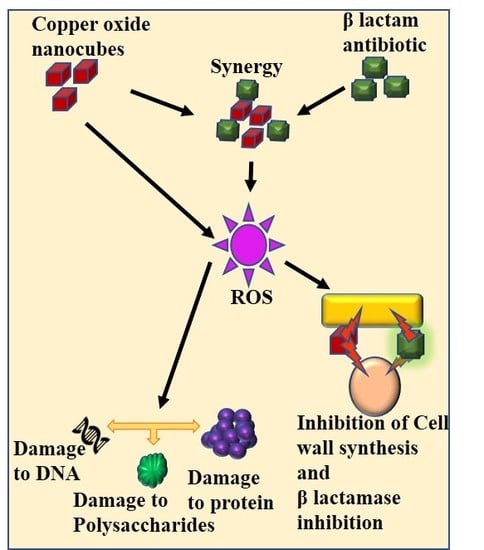
Re-Potentiation of β-Lactam Antibiotic by Synergistic Combination with Biogenic Copper Oxide Nanocubes against Biofilm Forming Multidrug-Resistant Bacteria
Abstract
1. Introduction
2. Results
2.1. Isolation, Identification, and Antibiotic Sensitivity of Bacteria from Urinary Catheter and Burn Wound
2.2. Susceptibility of Isolates to Antibiotics
2.3. Synthesis and Characterization of CuO NPs
2.4. Antibacterial Activity of CuO NPs
2.5. MIC and MBC
2.6. Synergistic Interaction between Amoxyclav and CuO NPs against Bacteria
2.7. Time-Kill Assay
2.8. Effect of Amoxyclav and CuO NPs on Biofilm Formation
2.9. Effect of Amoxyclav and CuO NPs on EPS Formation
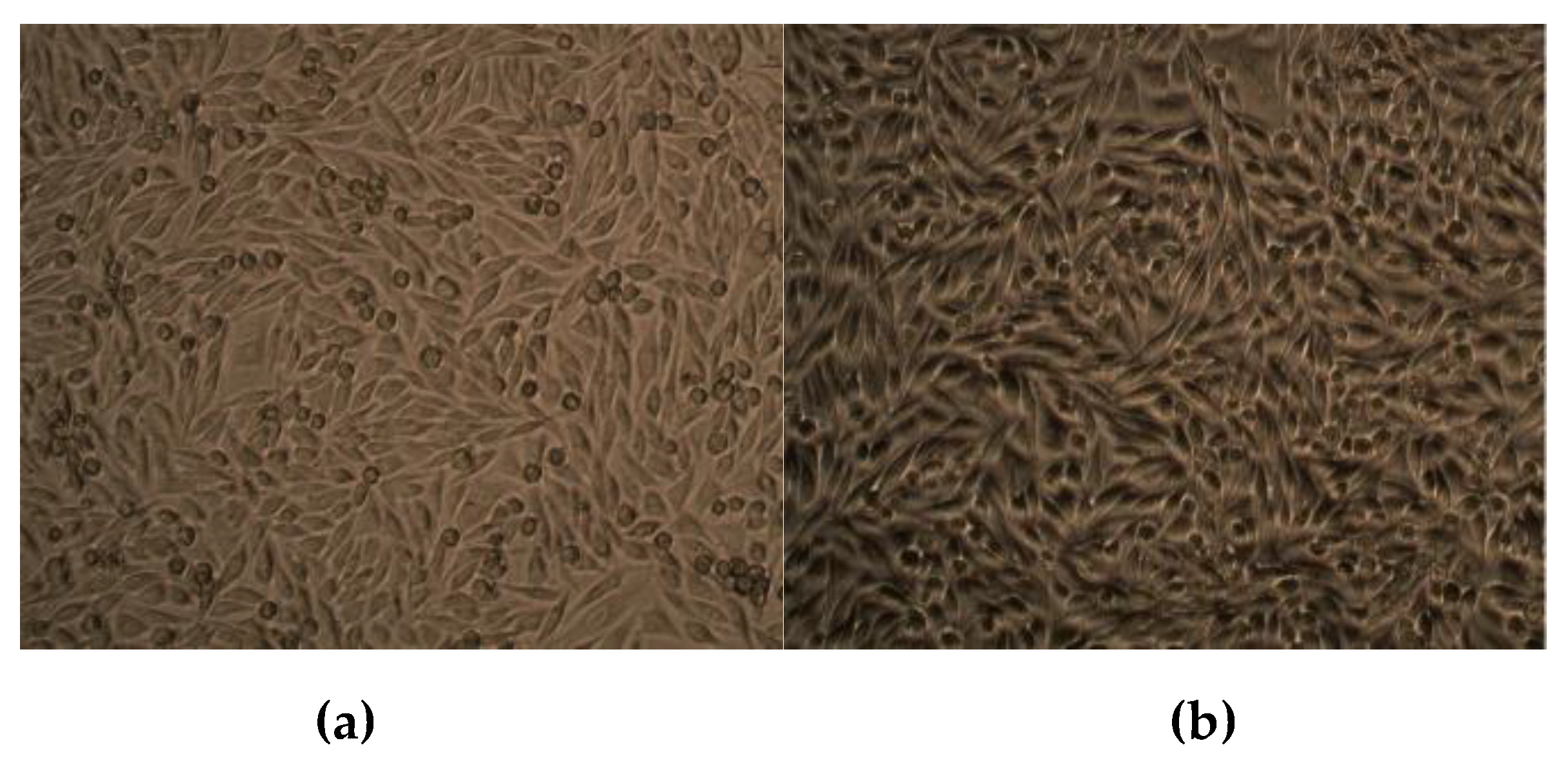
2.10. Effect of CuO NPs on Viability of Human Dermal Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Isolation and Identification of Bacteria
4.3. Susceptibility of Bacteria to Antimicrobial Agents
4.4. Synthesis and Characterization of CuO NPs
4.5. Determination of Antibacterial Activity, MIC, and MBC
4.6. Time-Kill Assay
4.7. Synergistic Interaction between Amoxyclav and CuO NPs
4.8. Effect of Amoxyclav and CuO NPs on Biofilm Formation
4.9. Effect of Amoxyclav and CuO NP on EPS Formation
5. Effect of CuO NPs on Viability of Human Dermal Fibroblasts
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernardi, S.; Continenza, M.A.; Al-Ahmad, A.; Karygianni, L.; Follo, M.; Filippi, A.; Macchiarelli, G. Streptococcus spp. and Fusobacterium nucleatum in tongue dorsum biofilm from halitosis patients: A fluorescence in situ hybridization (FISH) and confocal laser scanning microscopy (CLSM) study. New Microbiol. 2019, 42, 108–113. [Google Scholar] [PubMed]
- Seviour, T.; Derlon, N.; Dueholm, M.S.; Flemming, H.C.; Girbal-Neuhauser, E.; Horn, H.; Kjelleberg, S.; van Loosdrecht, M.C.M.; Lotti, T.; Malpei, M.F.; et al. Extracellular polymeric substances of biofilms: Suffering from an identity crisis. Water Res. 2019, 151, 1–7. [Google Scholar] [CrossRef]
- Zippel, B.; Neu, T.R. Characterization of glycoconjugates of extracellular polymeric substances in tufa-associated biofilms by using fluorescence lectin-binding analysis. Appl. Environ. Microbiol. 2011, 77, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, H.; Osaki, T.; Hojo, F.; Kamiya, S. Effect of Helicobacter pylori biofilm formation on susceptibility to amoxicillin, metronidazole and clarithomycin. Microb. Pathog. 2019. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Henly, E.L.; Dowling, J.A.R.; Maingay, J.B.; Lacey, M.M.; Smith, T.J.; Forbes, S. Biocide Exposure Induces Changes in Susceptibility, Pathogenicity, and Biofilm Formation in Uropathogenic Escherichia coli. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, A.A.; Cheng, M.P.; Sheppard, D.C.; Nguyen, D. Microbial Biofilms in Pulmonary and Critical Care Diseases. Ann. Am. Thorac. Soc. 2016, 13, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Naha, P.C.; Hwang, G.; Kim, D.; Huang, Y.; Simon-Soro, A.; Jung, H.I.; Ren, Z.; Li, Y.; Gubara, S.; et al. Topical ferumoxytol nanoparticles disrupt biofilms and prevent tooth decay in vivo via intrinsic catalytic activity. Nat. Commun. 2018, 9, 2920. [Google Scholar] [CrossRef]
- Jung, C.J.; Hsu, R.B.; Shun, C.T.; Hsu, C.C.; Chia, J.S. AtlA Mediates Extracellular DNA Release, Which Contributes to Streptococcus mutans Biofilm Formation in an Experimental Rat Model of Infective Endocarditis. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed]
- Hamilos, D.L. Biofilm Formations in Pediatric Respiratory Tract Infection Part 2: Mucosal Biofilm Formation by Respiratory Pathogens and Current and Future Therapeutic Strategies to Inhibit Biofilm Formation or Eradicate Established Biofilm. Curr. Infect. Dis. Rep. 2019, 21, 8. [Google Scholar] [CrossRef]
- Brady, R.A.; Leid, J.G.; Calhoun, J.H.; Costerton, J.W.; Shirtliff, M.E. Osteomyelitis and the role of biofilms in chonic infection. FEMS Immunol. Med. Microbiol. 2008, 52, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kiedrowski, M.R.; Gaston, J.R.; Kocak, B.R.; Coburn, S.L.; Lee, S.; Pilewski, J.M.; Myerburg, M.M.; Bomberger, J.M. Staphylococcus aureus Biofilm Growth on Cystic Fibrosis Airway Epithelial Cells Is Enhanced during Respiratory Syncytial Virus Coinfection. mSphere 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Kumar, A. The extracellular matrix of mycobacterial biofilms: Could we shorten the treatment of mycobacterial infections? Microb. Cell 2019, 6, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Kackar, S.; Suman, E.; Kotian, M.S. Bacterial and fungal biofilm formation on contact lenses and their susceptibility to lens care solutions. Indian J. Med. Microbiol. 2017, 35, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Mala, R.; Annie Aglin, A.; Ruby Celsia, A.S.; Geerthika, S.; Kiruthika, N.; VazagaPriya, C.; Srinivasa Kumar, K. Foley catheters functionalised with a synergistic combination of antibiotics and silver nanoparticles resist biofilm formation. IET Nanobiotechnol. 2017, 11, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Rieger, U.M.; Raschke, G.F.; Frei, R.; Djedovic, G.; Pierer, G.; Trampuz, A. Role of bacterial biofilms in patients after reconstructive and aesthetic breast implant surgery. J. Long-Term Eff. Med. Implants 2014, 24, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Connaughton, A.; Childs, A.; Dylewski, S.; Sabesan, V.J. Biofilm Disrupting Technology for Orthopedic Implants: What’s on the Horizon? Front. Med. (Lausanne) 2014, 1, 22. [Google Scholar] [CrossRef]
- Somogyi-Ganss, E.; Chambers, M.S.; Lewin, J.S.; Tarrand, J.J.; Hutcheson, K.A. Biofilm on the tracheoesophageal voice prosthesis: Considerations for oral decontamination. Eur. Arch. Otorhinolaryngol. 2017, 274, 405–413. [Google Scholar] [CrossRef]
- Elgharably, H.; Hussain, S.T.; Shestha, N.K.; Blackstone, E.H.; Pettersson, G.B. Current Hypotheses in Cardiac Surgery: Biofilm in Infective Endocarditis. Semin. Thorac. Cardiovasc. Surg. 2016, 28, 56–59. [Google Scholar] [CrossRef]
- Subhadra, B.; Kim, D.H.; Woo, K.; Surendran, S.; Choi, C.H. Control of Biofilm Formation in Healthcare: Recent Advances Exploiting Quorum-Sensing Interference Strategies and Multidrug Efflux Pump Inhibitors. Materials 2018, 11. [Google Scholar] [CrossRef]
- Dasgupta, M.K. Biofilms and infection in dialysis patients. Semin. Dial. 2002, 15, 338–346. [Google Scholar] [CrossRef]
- Toba, F.A.; Akashi, H.; Arrecubieta, C.; Lowy, F.D. Role of biofilm in Staphylococcus aureus and Staphylococcus epidermidis ventricular assist device driveline infections. J. Thorac. Cardiovasc. Surg. 2011, 141, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Algburi, A.; Comito, N.; Kashtanov, D.; Dicks, L.M.T.; Chikindas, M.L. Control of Biofilm Formation: Antibiotics and Beyond. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Albu, S.; Voidazan, S.; Bilca, D.; Badiu, M.; Truta, A.; Ciorea, M.; Ichim, A.; Luca, D.; Moldovan, G. Bacteriuria and asymptomatic infection in chonic patients with indwelling urinary catheter: The incidence of ESBL bacteria. Medicine 2018, 97, e11796. [Google Scholar] [CrossRef]
- Narayanan, A.; Nair, M.S.; Muyyarikkandy, M.S.; Amalaradjou, M.A. Inhibition and Inactivation of Uropathogenic Escherichia coli Biofilms on Urinary Catheters by Sodium Selenite. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, M.; Al Ahdab, S.; Jurisevic, M.; Mouselli, S. Antibiotic Resistance in Syria: A Local Problem Turns Into a Global Theat. Front. Public Health 2018, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Veiga, R.P.; Paiva, J.A. Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care 2018, 22, 233. [Google Scholar] [CrossRef]
- Wolny-Koladka, K.; Lenart-Boron, A. Antimicrobial resistance and the presence of extended-spectrum beta-lactamase genes in Escherichia coli isolated from the environment of horse riding centers. Environ. Sci. Pollut. Res. Int. 2018, 25, 21789–21800. [Google Scholar] [CrossRef] [PubMed]
- Site, N.-M.n.-A.A. Penicillin Production. Available online: https://www.news-medical.net/health/Penicillin-Production.aspx (accessed on 11 June 2019).
- Fallone, C.A.; Moss, S.F.; Malfertheiner, P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology 2019. [Google Scholar] [CrossRef]
- Adnan, S.; Paterson, D.L.; Lipman, J.; Kumar, S.; Li, J.; Rudd, M.; Roberts, J.A. Pharmacokinetics of beta-lactam antibiotics in patients with intra-abdominal disease: A structured review. Surg. Infect. 2012, 13, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Al-Sabbagh, A.; Moss, S.; Subhedar, N. Neonatal necrotising enterocolitis and perinatal exposure to co-amoxyclav. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F187. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, S.F.; Karamouzos, V.; Lefkaditi, A.; Sklavou, C.; Kolonitsiou, F.; Chistofidou, M.; Fligou, F.; Gogos, C.; Marangos, M. Triple combination therapy with high-dose ampicillin/sulbactam, high-dose tigecycline and colistin in the treatment of ventilator-associated pneumonia caused by pan-drug resistant Acinetobacter baumannii: A case series study. Infez Med. 2019, 27, 11–16. [Google Scholar]
- Pilmis, B.; Jullien, V.; Tabah, A.; Zahar, J.R.; Brun-Buisson, C. Piperacillin-tazobactam as alternative to carbapenems for ICU patients. Ann. Intensive Care 2017, 7, 113. [Google Scholar] [CrossRef]
- Shirley, M. Ceftazidime-Avibactam: A Review in the Treatment of Serious Gram-Negative Bacterial Infections. Drugs 2018, 78, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Tancawan, A.L.; Pato, M.N.; Abidin, K.Z.; Asari, A.S.; Thong, T.X.; Kochhar, P.; Muganurmath, C.; Twynholm, M.; Barker, K. Amoxicillin/Clavulanic Acid for the Treatment of Odontogenic Infections: A Randomised Study Comparing Efficacy and Tolerability versus Clindamycin. Int. J. Dent. 2015, 2015, 472470. [Google Scholar] [CrossRef]
- Chandki, R.; Banthia, P.; Banthia, R. Biofilms: A microbial home. J. Indian Soc. Periodontol. 2011, 15, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Odeh, L.H.; Talib, W.H.; Basheti, I.A. Synergistic effect of thymoquinone and melatonin against breast cancer implanted in mice. J. Cancer Res. Ther. 2018, 14, S324–S330. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Lowe, G.; Roberts, C.M.; Finlay, J.; Han, E.S.; Glackin, C.A.; Dellinger, T.H. Pterostilbene Suppresses Ovarian Cancer Growth via Induction of Apoptosis and Blockade of Cell Cycle Progression Involving Inhibition of the STAT3 Pathway. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Carrizales, M.; Velasco, K.I.; Castillo, C.; Flores, A.; Magana, M.; Martinez-Castanon, G.A.; Martinez-Gutierrez, F. In Vitro Synergism of Silver Nanoparticles with Antibiotics as an Alternative Treatment in Multiresistant Uropathogens. Antibiotics 2018, 7. [Google Scholar] [CrossRef]
- Tran, C.D.; Makuvaza, J.; Munson, E.; Bennett, B. Biocompatible Copper Oxide Nanoparticle Composites from Cellulose and Chitosan: Facile Synthesis, Unique Structure, and Antimicrobial Activity. ACS Appl. Mater. Interfaces 2017, 9, 42503–42515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; You, Z.; Xu, W.; Su, Z.; Qiu, Y.; Gao, L.; Yin, C.; Lan, L. Microwave irradiation directly excites semiconductor catalyst to produce electric current or electron-holes pairs. Sci. Rep. 2019, 9, 5470. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Ramirez, S.; Diaz, I.; Garcia, A.; Hassan, N. Easy, Quick, and Reproducible Sonochemical Synthesis of CuO Nanoparticles. Materials 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Zohora, N.; Kandjani, A.E.; Orth, A.; Brown, H.M.; Hutchinson, M.R.; Gibson, B.C. Fluorescence brightness and photostability of individual copper (I) oxide nanocubes. Sci. Rep. 2017, 7, 16905. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. Int. Sch. Res. Not. 2014, 2014, 359316. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Fayaz, A.; Balaji, K.; Kalaichelvan, P.T.; Venkatesan, R. Fungal based synthesis of silver nanoparticles--an effect of temperature on the size of particles. Colloids Surf. B Biointerfaces 2009, 74, 123–126. [Google Scholar] [CrossRef]
- Arya, A.; Gupta, K.; Chundawat, T.S.; Vaya, D. Biogenic Synthesis of Copper and Silver Nanoparticles Using Green Alga Botryococcus braunii and Its Antimicrobial Activity. Bioinorg. Chem. Appl. 2018, 2018, 7879403. [Google Scholar] [CrossRef]
- Miri, A.; Sarani, M.; Rezazade Bazaz, M.; Darroudi, M. Plant-mediated biosynthesis of silver nanoparticles using Prosopis farcta extract and its antibacterial properties. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 141, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Kassim, A.; Omuse, G.; Premji, Z.; Revathi, G. Comparison of Clinical Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing guidelines for the interpretation of antibiotic susceptibility at a University teaching hospital in Nairobi, Kenya: A cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 21. [Google Scholar] [CrossRef]
- Gunalan, S.; Sivaraj, R.; Venckatesh, R. Aloe barbadensis Miller mediated green synthesis of mono-disperse copper oxide nanoparticles: Optical properties. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 1140–1144. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Memic, A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int. J. Nanomed. 2012, 7, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- DeAlba-Montero, I.; Guajardo-Pacheco, J.; Morales-Sanchez, E.; Araujo-Martinez, R.; Loredo-Becerra, G.M.; Martinez-Castanon, G.A.; Ruiz, F.; Compean Jasso, M.E. Antimicrobial Properties of Copper Nanoparticles and Amino Acid Chelated Copper Nanoparticles Produced by Using a Soya Extract. Bioinorg. Chem. Appl. 2017, 2017, 1064918. [Google Scholar] [CrossRef] [PubMed]
- Ieven, M.; Vanden Berghe, D.A.; Mertens, F.; Vlietinck, A.; Lammens, E. Screening of higher plants for biological activities. I. Antimicrobial activity. Planta Med. 1979, 36, 311–321. [Google Scholar] [PubMed]
- Available online: www.chem.ucla.edu (accessed on 6 June 2019).
- Zaibunnisa, A.H.; Aini Marhanna, M.N.A.; Ainun Atirah, M. Characterisation and solubility study of γ-cyclodextrin and β-carotene complex. Int. Food Res. J. 2011, 18, 1061–1065. [Google Scholar]
- Sudjaroen, Y.; Haubner, R.; Wurtele, G.; Hull, W.E.; Erben, G.; Spiegelhalder, B.; Changbumrung, S.; Bartsch, H.; Owen, R.W. Isolation and structure elucidation of phenolic antioxidants from Tamarind (Tamarindus indica L.) seeds and pericarp. Food Chem. Toxicol. 2005, 43, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Topnani, N.; Kushwaha, S.; Athar, T. Wet Synthesis of Copper Oxide Nanopowder. Int. J. Green Nanotechnol. Mater. Sci. Eng. 2009, 1, M67–M73. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lay, C.L.; Shi, W.; Lee, H.K.; Yang, Y.; Li, S.; Ling, X.Y. Creating two self-assembly micro-environments to achieve supercrystals with dual structures using polyhedral nanoparticles. Nat. Commun. 2018, 9, 2769. [Google Scholar] [CrossRef]
- Karimi, M.; Zangabad, P.S.; Mehdizadeh, F.; Malekzad, H.; Ghasemi, A.; Bahami, S.; Zare, H.; Moghoofei, M.; Hekmatmanesh, A.; Hamblin, M.R. Nanocaged platforms: Modification, drug delivery and nanotoxicity. Opening synthetic cages to release the tiger. Nanoscale 2017, 9, 1356–1392. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Chen, J.; Pang, H. Copper metal-organic framework nanocrystal for plane effect nonenzymatic electro-catalytic activity of glucose. Nanoscale 2014, 6, 10989–10994. [Google Scholar] [CrossRef]
- Kolhatkar, A.G.; Chen, Y.T.; Chinwangso, P.; Nekrashevich, I.; Dannangoda, G.C.; Singh, A.; Jamison, A.C.; Zenasni, O.; Rusakova, I.A.; Martirosyan, K.S.; et al. Magnetic Sensing Potential of Fe3O4 Nanocubes Exceeds That of Fe3O4 Nanospheres. ACS Omega 2017, 2, 8010–8019. [Google Scholar] [CrossRef]
- Suresh, A.K.; Pelletier, D.A.; Doktycz, M.J. Relating nanomaterial properties and microbial toxicity. Nanoscale 2013, 5, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles. Materials 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, M.; Choudhury, B.; Yadav, R.N. Comparative study of antibiofilm activity of copper oxide and iron oxide nanoparticles against multidrug resistant biofilm forming uropathogens. Indian J. Microbiol. 2014, 54, 365–368. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; De Rijk, E.; Bonetto, A.; Wohlleben, W.; Stone, V.; Brunelli, A.; Badetti, E.; Marcomini, A.; Gosens, I.; Cassee, F.R. Toxicity of copper oxide and basic copper carbonate nanoparticles after short-term oral exposure in rats. Nanotoxicology 2019, 13, 50–72. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, G.; Valdezate, S.; Garrido, N.; Villalon, P.; Medina-Pascual, M.J.; Saez-Nieto, J.A. Identification, typing, and phylogenetic relationships of the main clinical Nocardia species in spain according to their gyrB and rpoB genes. J. Clin. Microbiol. 2013, 51, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pant, S.; Dave, V.; Tak, K.; Sadhu, V.; Reddy, K.R. Green synthesis and characterization of copper nanoparticles by Tinospora cardifolia to produce nature-friendly copper nano-coated fabric and their antimicrobial evaluation. J. Microbiol. Methods 2019, 160, 107–116. [Google Scholar] [CrossRef]
- Tippayawat, P.; Phomviyo, N.; Boueroy, P.; Chompoosor, A. Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. PeerJ 2016, 4, e2589. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.A.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In Vitro Antimicrobial Activity of Green Synthesized Silver Nanoparticles Against Selected Gram-negative Foodborne Pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Sharma, N.; Jandaik, S.; Kumar, S. Synergistic activity of doped zinc oxide nanoparticles with antibiotics: Ciprofloxacin, ampicillin, fluconazole and amphotericin B against pathogenic microorganisms. An. Acad. Bras. Cienc. 2016, 88, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. MIC and Zone Diameter Distributions and ECOFFs. Available online: http://www.eucast.org/mic_distributions_and_ecoffs/ (accessed on 14 June 2019).
- Haney, E.F.; Trimble, M.J.; Cheng, J.T.; Valle, Q.; Hancock, R.E.W. Critical Assessment of Methods to Quantify Biofilm Growth and Evaluate Antibiofilm Activity of Host Defence Peptides. Biomolecules 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Douthart, R.J.; Burnett, J.P.; Beasley, F.W.; Frank, B.H. Binding of ethidium bromide to double-stranded ribonucleic acid. Biochemistry 1973, 12, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Skladanowski, M.; Golinska, P.; Rudnicka, K.; Dahm, H.; Rai, M. Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles. Med. Microbiol. Immunol. 2016, 205, 603–613. [Google Scholar] [CrossRef]
Sample Availability: No samples of the compounds are available from the authors. |







| Diameter of Inhibition Zone (mm) | |||||||
|---|---|---|---|---|---|---|---|
| S. No | Bacteria | Amoxicillin | Amoxyclav | Cefixime | Ciprofloxacin | Gentamicin | Azithromycin |
| 1 | P. mirabilis | 5 ± 0.02 | 10 ± 0.05 | 11 ± 0.09 | 13 ± 1 | 25 ± 0.07 | 8 ± 0.04 |
| 2 | S. aureus | 3 ± 0.03 | 12 ± 0.08 | 12 ± 0.05 | 14 ± 0.06 | 28 ± 0.08 | 12 ± 0.07 |
| Concentration of CuO NPs (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| S. No. | Bacteria | 5 | 10 | 15 | 20 | 25 | 30 |
| 1 | P. mirabilis | 5 | 10 ± 0.06 | 14 ± 0.08 | 16± 0.05 | 18 ± 1 | 24 ± 1 |
| 2 | S. aureus | 8 | 14 ± 0.07 | 18 ± 0.07 | 21± 0.09 | 24 ± 1 | 29 ± 1 |
| Concentration of T. indica fruit extract (30 μg/mL) | |||||||
| 1 | P. mirabilis | 10 ± 0.06 | |||||
| 2 | S. aureus | 16 ± 0.06 | |||||
| P. mirabilis | S. aureus | ||||||
|---|---|---|---|---|---|---|---|
| S. No. | Antibacterial Activity | Amoxyclav | CuO NP | T. indica Fruit Extract | Amoxyclav | CuO NP | T. indica Fruit Extract |
| 1 | MIC (μg/mL) | 70 | 30 | 1000 | 50 | 20 | 800 |
| 2 | MBC (μg/mL) | 140 | 60 | 4000 | 100 | 40 | 3200 |
| P. mirabilis | |||||
|---|---|---|---|---|---|
| Amoxyclav (μg/mL) | CuO NP (μg/mL) | FIC of Amoxyclav | FIC of CuO NP | FICI | Interaction |
| 4.4 | 15 | 0.062 | 0.25 | 0.267 | Synergistic |
| 8.8 | 7.5 | 0.125 | 0.126 | 0.251 | Synergistic |
| 17.5 | 3.8 | 0.25 | 0.063 | 0.313 | Synergistic |
| 35 | 1.9 | 0.5 | 0.031 | 0.531 | Additive |
| S. aureus | |||||
| 1.56 | 10 | 0.03 | 0.5 | 0.503 | Additive |
| 3.15 | 5 | 0.06 | 0.25 | 0.31 | Synergistic |
| 6.25 | 2.5 | 0.12 | 0.125 | 0.225 | Synergistic |
| 12.5 | 1.25 | 0.24 | 0.06 | 0.31 | Synergistic |
| S. No. | Treatment | Viability (%) |
|---|---|---|
| 1 | Control | 100 |
| 2 | CuO NPs (30 μg/mL) | 99.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arul Selvaraj, R.C.; Rajendran, M.; Nagaiah, H.P. Re-Potentiation of β-Lactam Antibiotic by Synergistic Combination with Biogenic Copper Oxide Nanocubes against Biofilm Forming Multidrug-Resistant Bacteria. Molecules 2019, 24, 3055. https://doi.org/10.3390/molecules24173055
Arul Selvaraj RC, Rajendran M, Nagaiah HP. Re-Potentiation of β-Lactam Antibiotic by Synergistic Combination with Biogenic Copper Oxide Nanocubes against Biofilm Forming Multidrug-Resistant Bacteria. Molecules. 2019; 24(17):3055. https://doi.org/10.3390/molecules24173055
Chicago/Turabian StyleArul Selvaraj, Ruby Celsia, Mala Rajendran, and Hari Prasath Nagaiah. 2019. "Re-Potentiation of β-Lactam Antibiotic by Synergistic Combination with Biogenic Copper Oxide Nanocubes against Biofilm Forming Multidrug-Resistant Bacteria" Molecules 24, no. 17: 3055. https://doi.org/10.3390/molecules24173055
APA StyleArul Selvaraj, R. C., Rajendran, M., & Nagaiah, H. P. (2019). Re-Potentiation of β-Lactam Antibiotic by Synergistic Combination with Biogenic Copper Oxide Nanocubes against Biofilm Forming Multidrug-Resistant Bacteria. Molecules, 24(17), 3055. https://doi.org/10.3390/molecules24173055