A Self-Supported CuO/Cu Nanowire Electrode as Highly Efficient Sensor for COD Measurement
Abstract
:1. Introduction
2. Results and Discussion
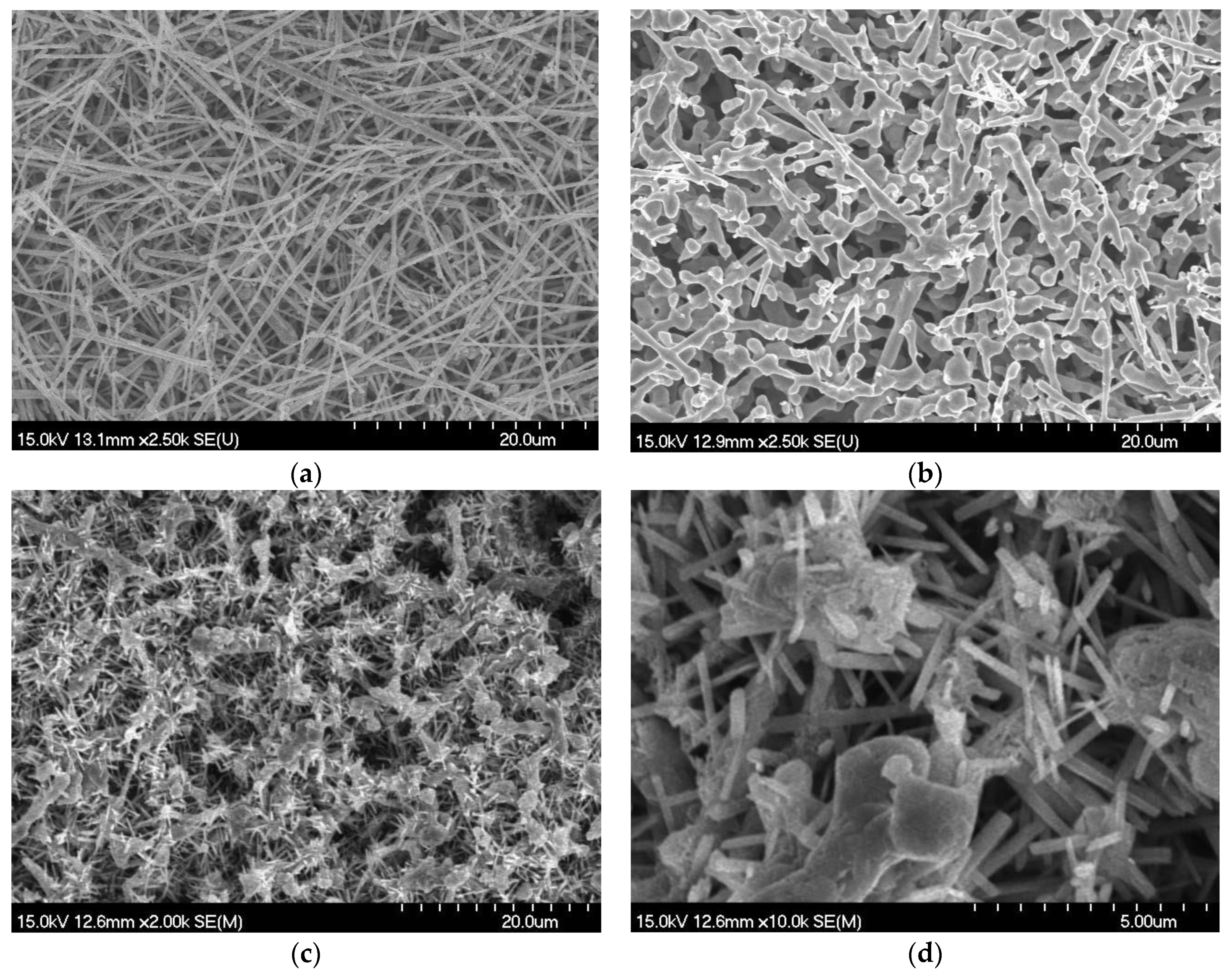
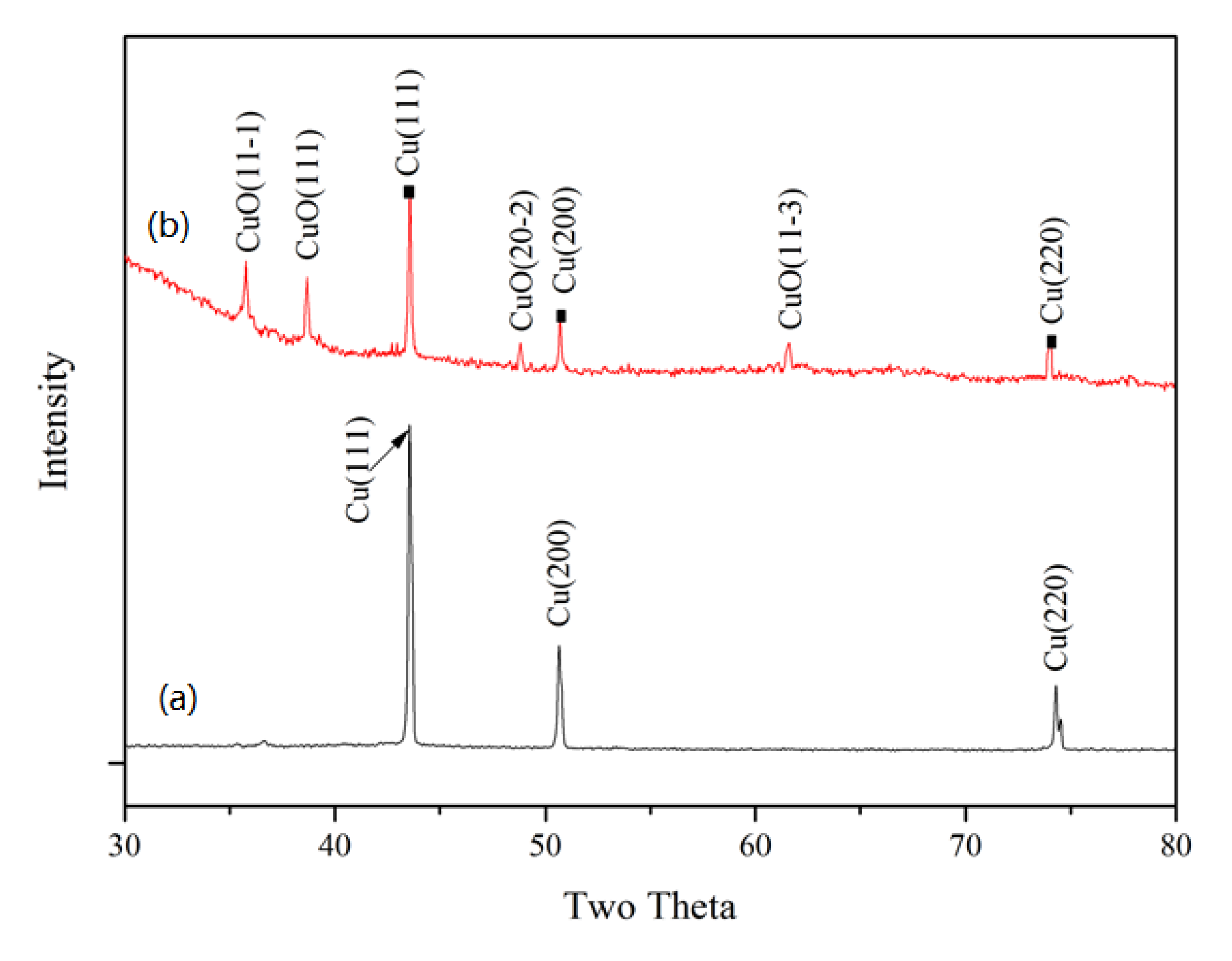
2.1. Characterizations of CuNWE and CuO/CuNWE
2.2. Electrochemical Behavior of CuNWE and CuO/CuNWE in Alkaline Medium
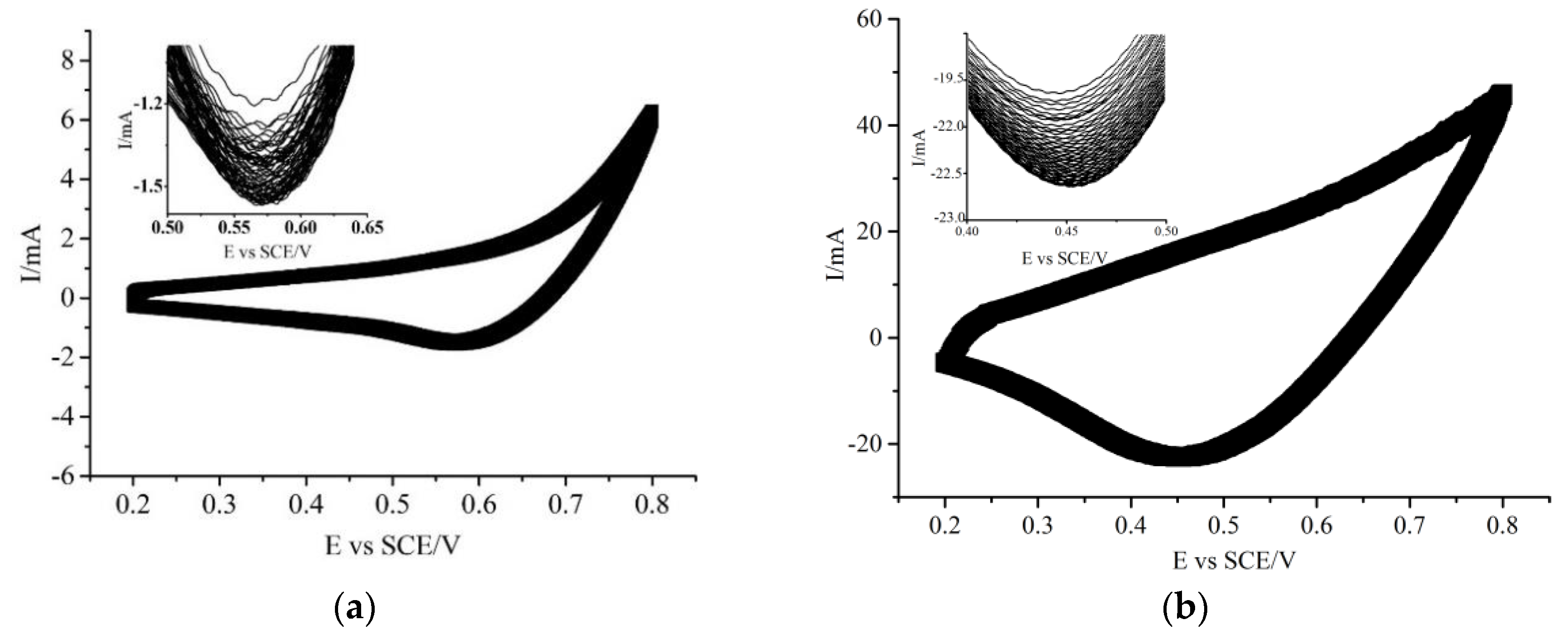
2.2.1. Electrochemical Behavior in Organics-Absent Alkaline Medium
2.2.2. Electrochemical Behavior in Organics-Present Alkaline Medium
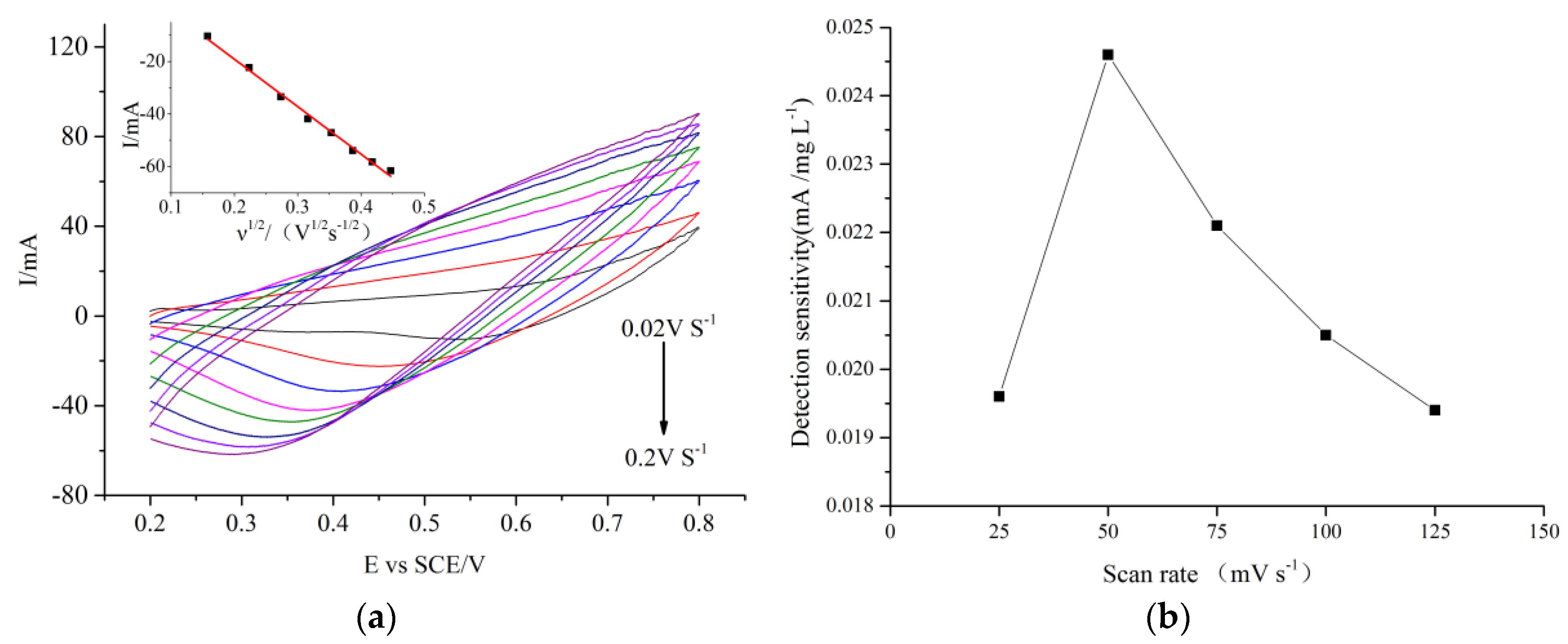
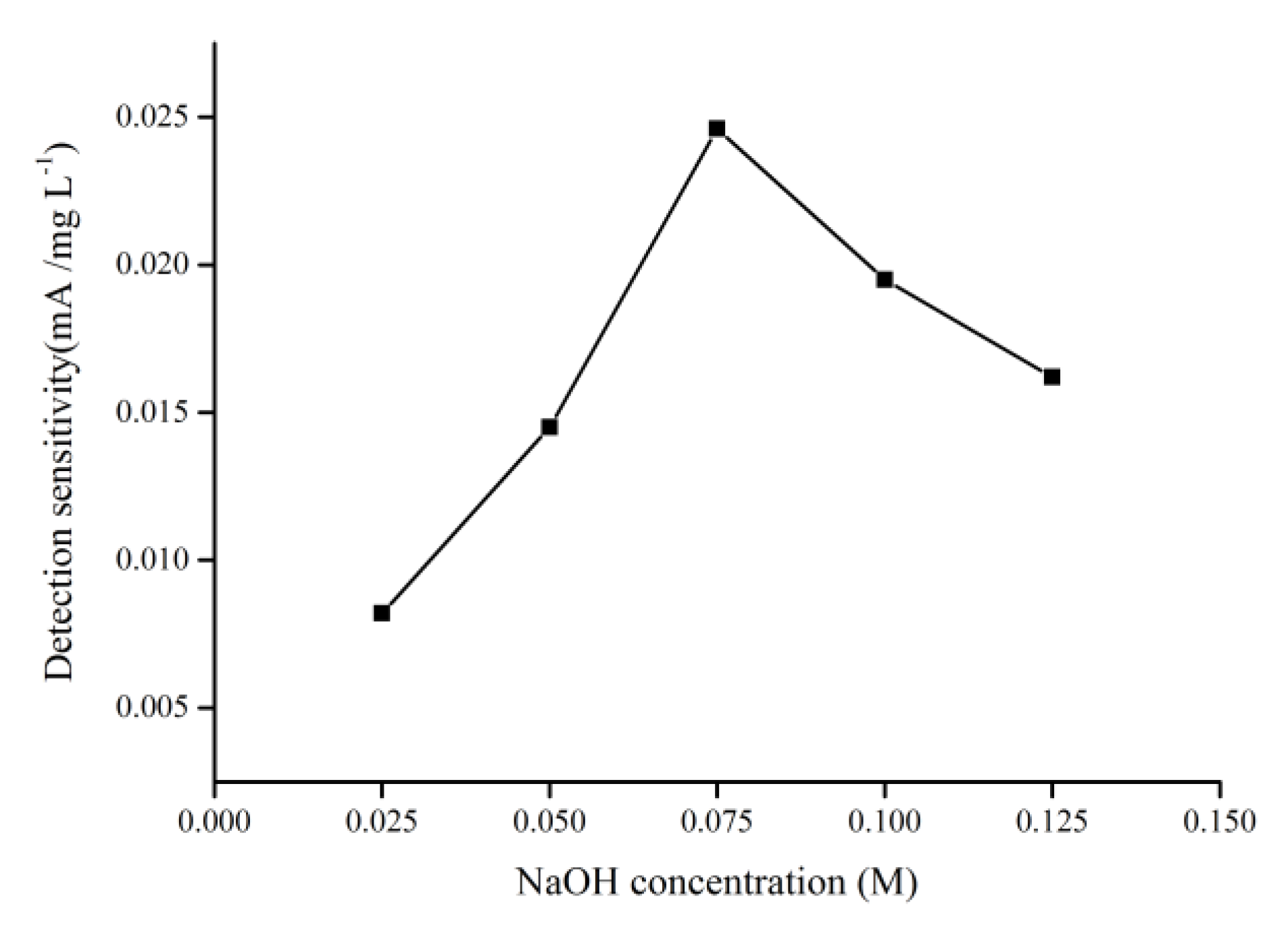
2.2.3. Optimization of Scan Rate and Alkaline Concentration for COD Measurement Using the CuO/CuNWE
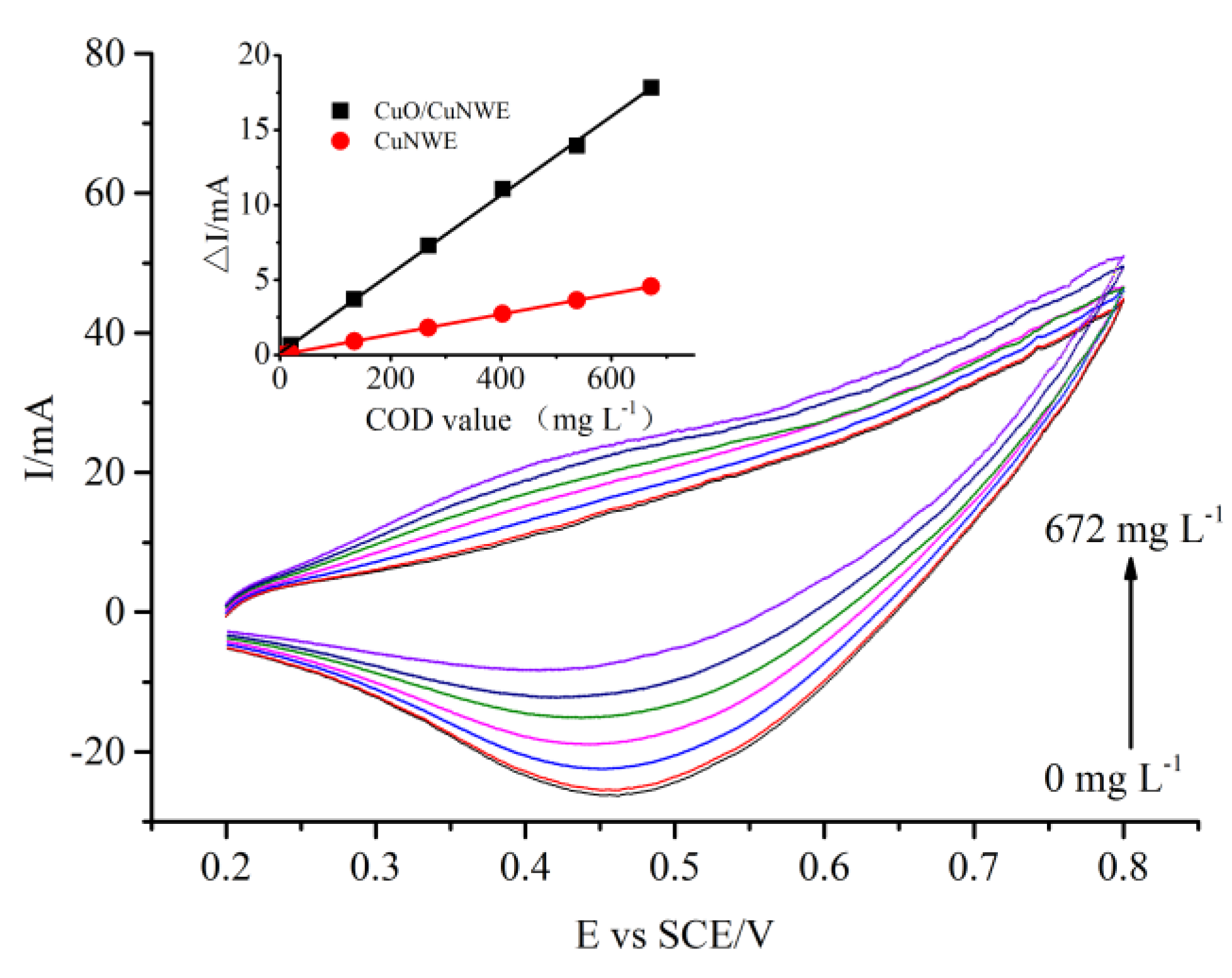
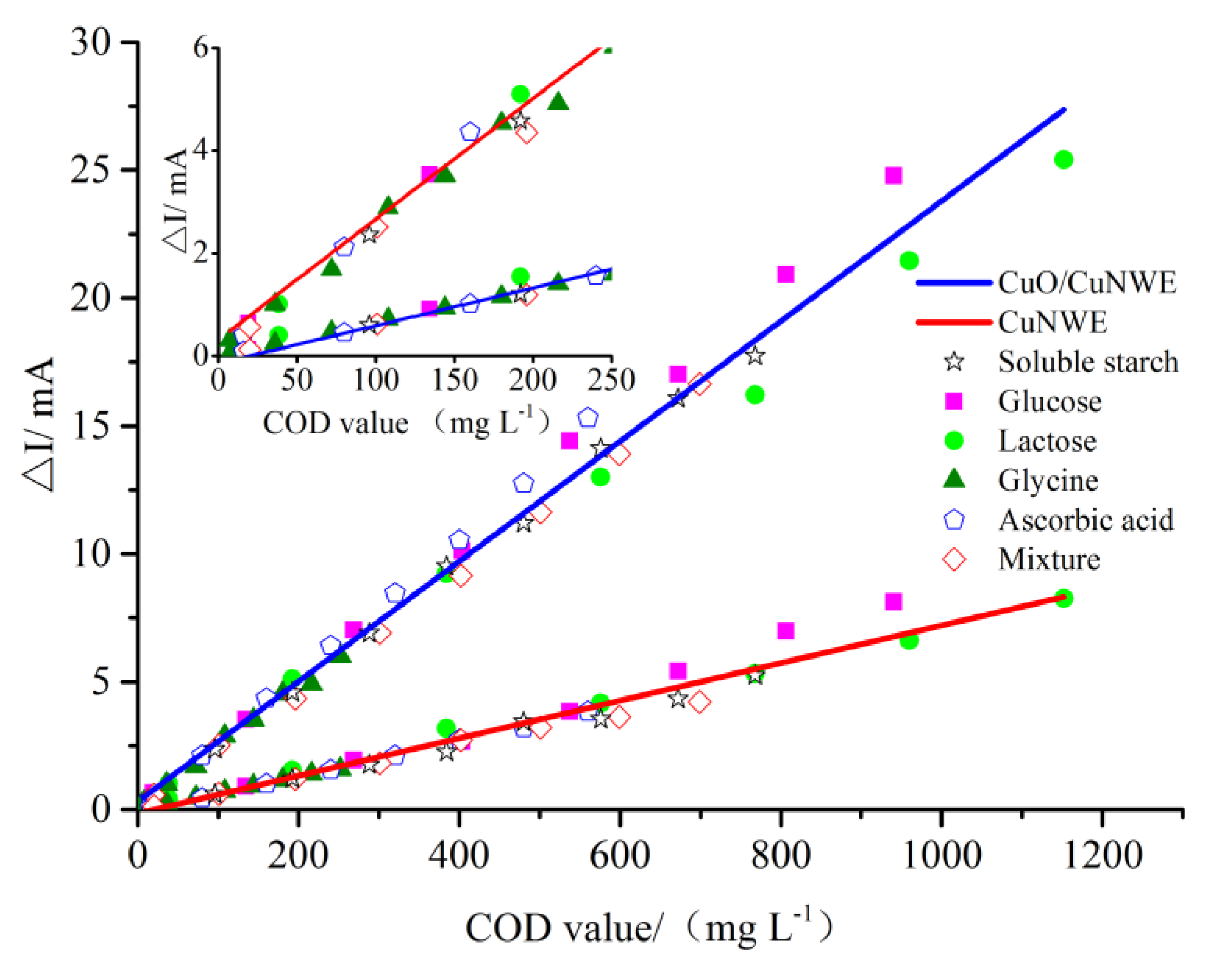
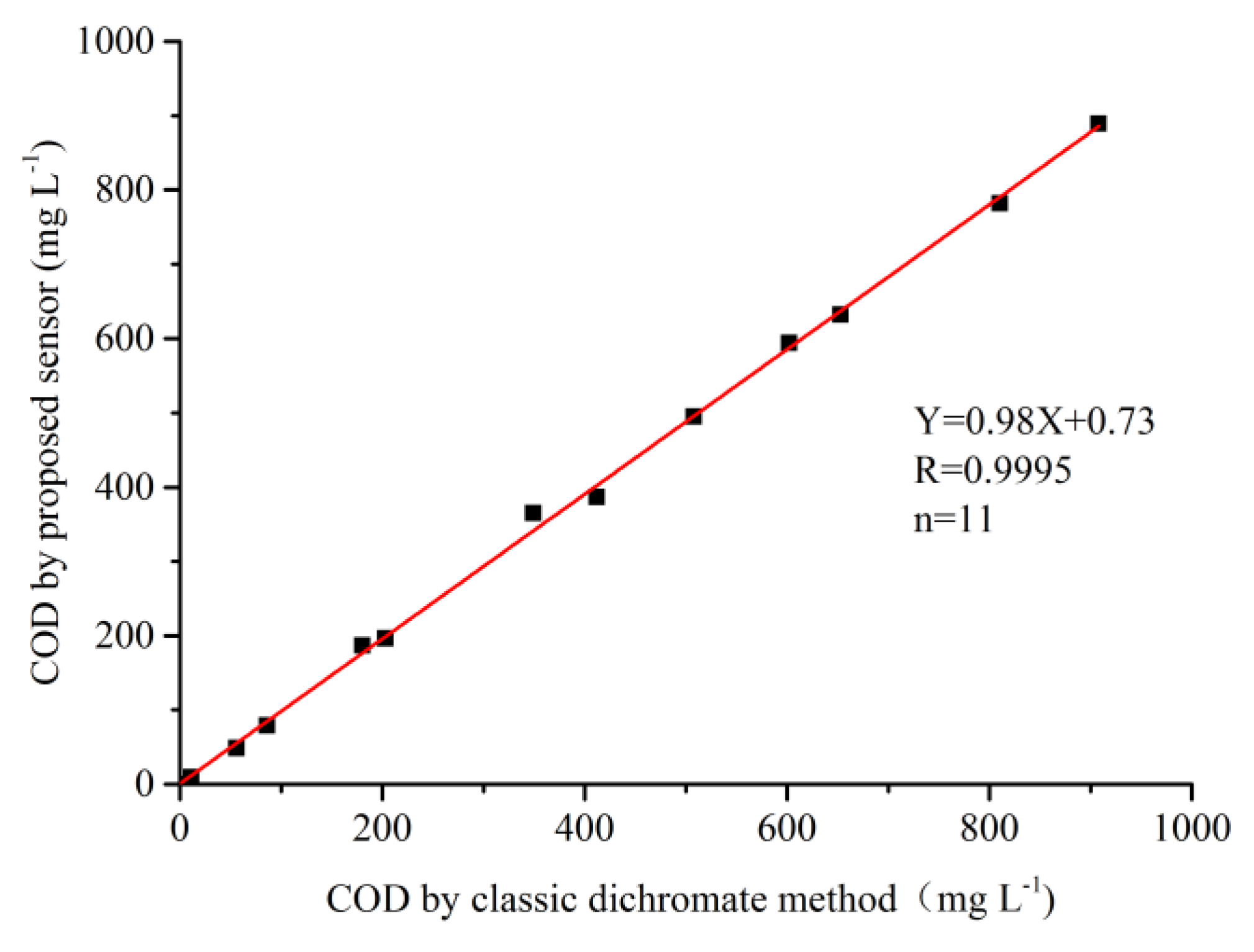
2.3. COD Measurement by CuO/CuNWE-Based Electrochemical Sensor
3. Materials and Methods
3.1. Reagents
3.2. Instrumentation
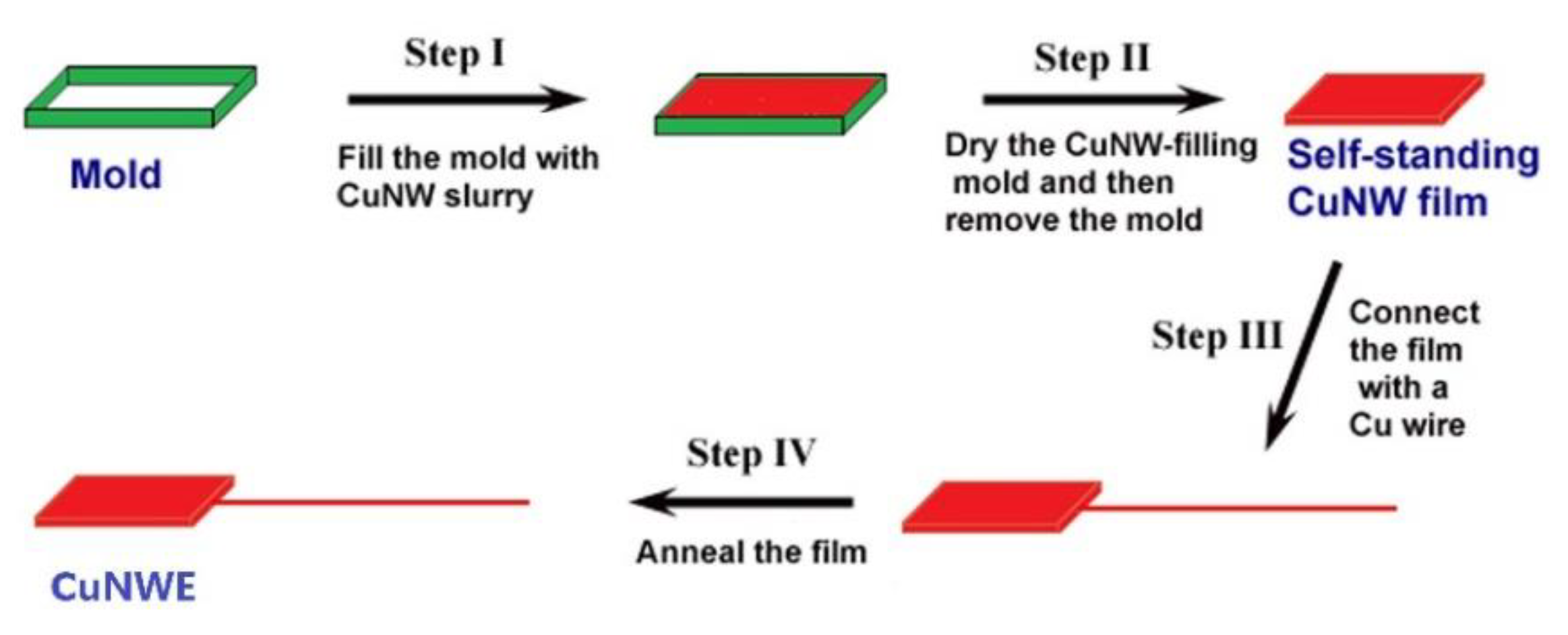
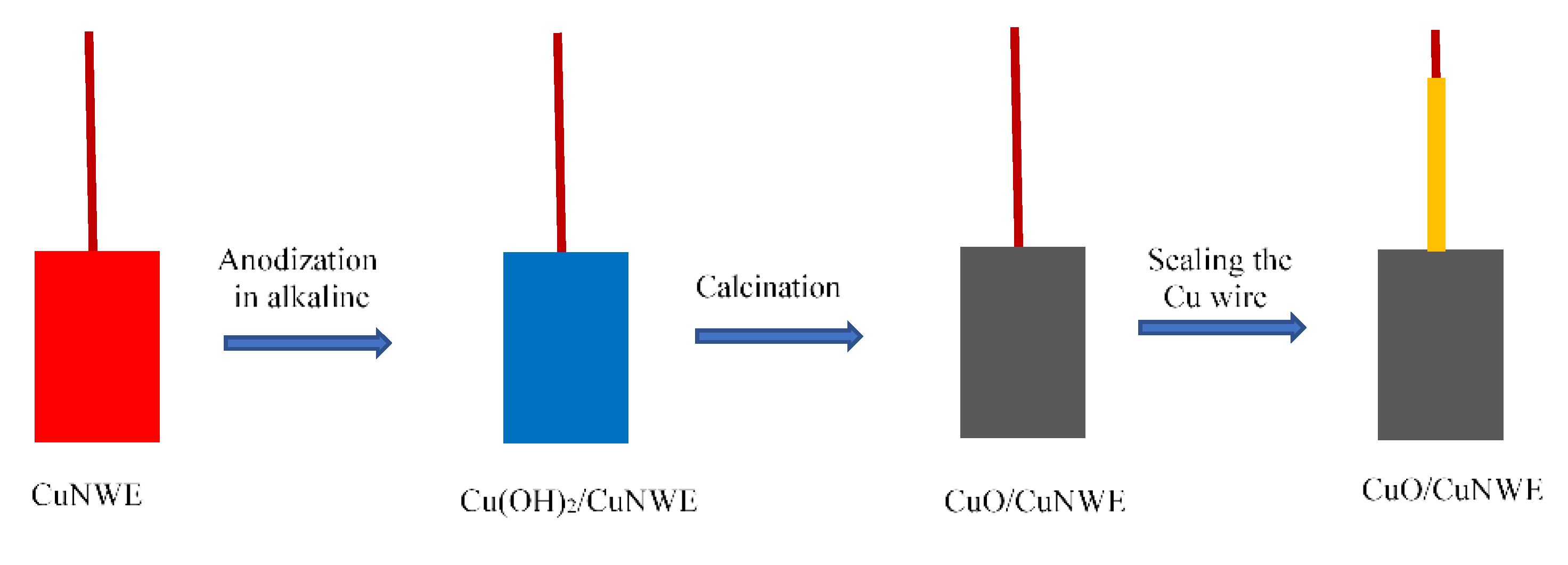
3.3. Preparation of CuO/CuNW Electrode
3.3.1. Preparation of Cu Nanowires
3.3.2. Preparation of Porous CuNWE
3.3.3. Preparation of CuO/CuNWE
3.4. Detection of COD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Luo, G.; He, L.; Xu, J.; Lyu, J. Analytical Approaches for Determining Chemical Oxygen Demand in Water Bodies: A Review. Crit. Rev. Anal. Chem. 2018, 48, 47–65. [Google Scholar] [CrossRef] [PubMed]
- El-Mekkawi, D.M.; Galal, H.R.; Abd El Wahab, R.M.; Mohamed, W.A.A. Photocatalytic activity evaluation of TiO2 nanoparticles based on COD analyses for water treatment applications: A standardization attempt. Int. J. Env. Sci. Technol. 2016, 13, 1077–1088. [Google Scholar] [CrossRef]
- Moore, W.A.; Walker, W.W. Determination of Low Chemical Oxygen Demands of Surface Waters by Dichromate Oxidation. Anal. Chem. 1956, 28, 164–167. [Google Scholar] [CrossRef]
- Ma, J. Determination of chemical oxygen demand in aqueous samples with non-electrochemical methods. Trends Env. Anal. 2017, 14, 37–43. [Google Scholar] [CrossRef]
- Zhang, Z.; Chang, X.; Chen, A. Determination of chemical oxygen demand based on photoelectrocatalysis of nanoporous TiO 2 electrodes. Sens. Actuat. B-Chem. 2016, 223, 664–670. [Google Scholar] [CrossRef]
- Wang, J.; Wu, C.; Wu, K.; Cheng, Q.; Zhou, Y. Electrochemical sensing chemical oxygen demand based on the catalytic activity of cobalt oxide film. Anal. Chim. Acta 2012, 736, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.R.; Conceição, C.D.C.; Bonifácio, V.G.; Filho, O.F.; Teixeira, M.F.S. Determination of the chemical oxygen demand (COD) using a copper electrode: A clean alternative method. J. Solid State Electrochem. 2009, 13, 665–669. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Zhou, Y.; Wu, K. A nano-copper electrochemical sensor for sensitive detection of chemical oxygen demand. Sens. Actuat. B-Chem. 2011, 153, 78–82. [Google Scholar] [CrossRef]
- Badr, I.H.A.; Hassan, H.H.; Hamed, E.; Abdel-Aziz, A.M. Sensitive and Green Method for Determination of Chemical Oxygen Demand Using a Nano-copper Based Electrochemical Sensor. Electroanal 2017, 29, 2401–2409. [Google Scholar] [CrossRef]
- Hassan, H.H.; Badr, I.H.A.; Abdel-Fatah, H.T.M.; Elfeky, E.M.S.; Abdel-Aziz, A.M. Low cost chemical oxygen demand sensor based on electrodeposited nano-copper film. Arab. J. Chem. 2018, 11, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Carchi, T.; Lapo, B.; Alvarado, J.; Espinoza-Montero, P.J.; Llorca, J.; Fernandez, L. A Nafion Film Cover to Enhance the Analytical Performance of the CuO/Cu Electrochemical Sensor for Determination of Chemical Oxygen Demand. Sensors 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jing, T.; Hao, Q.; Zhou, Y.; Mei, S. A sensitive and environmentally friendly method for determination of chemical oxygen demand using NiCu alloy electrode. Electrochim. Acta 2012, 74, 165–170. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Wang, P.; Ao, Y.; Hou, J.; Qian, J. Investigation on the application of titania nanorod arrays to the determination of chemical oxygen demand. Anal. Chim. Acta 2013, 767, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y. Electrochemical Determination of Chemical Oxygen Demand Using Ti/TiO2 Electrode. Int. J. Electrochem. Sci. 2016, 11, 9812–9821. [Google Scholar] [CrossRef]
- Ai, S.; Gao, M.; Yang, Y.; Li, J.; Jin, L. Electrocatalytic Sensor for the Determination of Chemical Oxygen Demand Using a Lead Dioxide Modified Electrode. Electroanal. 2004, 16, 404–409. [Google Scholar] [CrossRef]
- Wu, C.; Wu, K.-B. Preparation of Electrochemical Sensor Based on Morphology-Controlled Platinum Nanoparticles for Determination of Chemical Oxygen Demand. Chin. J. Anal. Chem. 2013, 41, 704–708. [Google Scholar] [CrossRef]
- Gutierrez-Capitan, M.; Baldi, A.; Gomez, R.; Garcia, V.; Jimenez-Jorquera, C.; Fernandez-Sanchez, C. Electrochemical nanocomposite-derived sensor for the analysis of chemical oxygen demand in urban wastewaters. Anal. Chem. 2015, 87, 2152–2160. [Google Scholar] [CrossRef]
- Wang, J.; Yao, N.; Li, M.; Hu, J.; Chen, J.; Hao, Q.; Wu, K.; Zhou, Y. Electrochemical tuning of the activity and structure of a copper-cobalt micro-nano film on a gold electrode, and its application to the determination of glucose and of Chemical Oxygen Demand. Mikrochim. Acta 2015, 182, 515–522. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, L.; Tang, M. A nickel nanoparticle/nafion-graphene oxide modified screen-printed electrode for amperometric determination of chemical oxygen demand. Mikrochim. Acta 2018, 185, 385. [Google Scholar] [CrossRef]
- Yu, H.; Ma, C.; Quan, X.; Chen, S.; Zhao, H. Flow Injection Analysis of Chemical Oxygen Demand (COD) by Using a Boron-Doped Diamond (BDD) Electrode. Env. Sci. Technol. 2009, 43, 1935–1939. [Google Scholar] [CrossRef]
- Kondo, T.; Tamura, Y.; Hoshino, M.; Watanabe, T.; Aikawa, T.; Yuasa, M.; Einaga, Y. Direct determination of chemical oxygen demand by anodic decomposition of organic compounds at a diamond electrode. Anal. Chem. 2014, 86, 8066–8072. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Tan, F.; Zhao, H.; Chen, S.; Quan, X. Sensitive amperometric determination of chemical oxygen demand using Ti/Sb–SnO2/PbO2 composite electrode. Sens. Actuat. B-Chem. 2011, 155, 114–119. [Google Scholar] [CrossRef]
- Mo, H.; Tang, Y.; Wang, N.; Zhang, M.; Cheng, H.; Chen, Y.; Wan, P.; Sun, Y.; Liu, S.; Wang, L. Performance improvement in chemical oxygen demand determination using carbon fiber felt/CeO2-β-PbO2 electrode deposited by cyclic voltammetry method. J. Solid State Electrochem. 2016, 20, 2179–2189. [Google Scholar] [CrossRef]
- Felix, S.; Kollu, P.; Raghupathy, B.P.C.; Jeong, S.K.; Grace, A.N. Electrocatalytic oxidation of carbohydrates and dopamine in alkaline and neutral medium using CuO nanoplatelets. J. Electroanal. Chem. 2015, 739, 1–9. [Google Scholar] [CrossRef]
- Zhang, X. Determination of 4-nitrophenol in Water using Free-Standing Cu Nanowire Electrode. Int. J. Electrochem. Sc. 2018, 5698–5708. [Google Scholar] [CrossRef]
- Liang, J.; Zheng, Y.; Liu, Z. Nanowire-based Cu electrode as electrochemical sensor for detection of nitrate in water. Sens. Actuat. B-Chem. 2016, 232, 336–344. [Google Scholar] [CrossRef]
- Zheng, Y.; Liang, J.; Chen, Y.; Liu, Z. Economical and green synthesis of Cu nanowires and their use as a catalyst for selective hydrogenation of cinnamaldehyde. Rsc Adv. 2014, 4, 41683–41689. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Q.; Liu, X.; Liu, J.; Sun, X. One-dimensional copper oxide nanotube arrays: Biosensors for glucose detection. Rsc Adv. 2014, 4, 1449–1455. [Google Scholar] [CrossRef]
- Luo, M.Z.; Baldwin, R.P. Characterization of carbohydrate oxidation at copper electrodes. J. Electroanal. Chem. 1995, 387, 87–94. [Google Scholar] [CrossRef]
- Marioli, J.M.; Kuwana, T. Electrochemical characterization of carbohydrate oxidation at copper electrodes. Electrochim. Acta 1992, 37, 1187–1197. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, L.; Manuzzi, D.; de los Monteros, H.V.; Jia, W.; Huo, D.; Hou, C.; Lei, Y. Ultrasensitive and selective non-enzymatic glucose detection using copper nanowires. Biosens Bioelectron. 2012, 31, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.D.; Bruton, G.M.; Collins, J.A. The redox properties of active sites and the importance of the latter in electrocatalysis at copper in base. Electrochim. Acta 1998, 44, 1467–1479. [Google Scholar] [CrossRef]
- You, T.; Niwa, O.; Tomita, M.; Ando, H.; Suzuki, M.; Hirono, S. Characterization and electrochemical properties of highly dispersed copper oxide/hydroxide nanoparticles in graphite-like carbon films prepared by RF sputtering method. Electrochem. Commun. 2002, 4, 468–471. [Google Scholar] [CrossRef]
- Heli, H.; Faramarzi, F.; Jabbari, A.; Parsaeid, A.; Moosavi-Movahedia, A.A. Electrooxidation and Determination of Etidronate using Copper Nanoparticles and Microparticles-modified Carbon Paste Electrodes. J. Braz. Chem. Soc. 2010, 21, 16–24. [Google Scholar] [CrossRef]
- Tian, L.; Liu, B. Fabrication of CuO nanosheets modified Cu electrode and its excellent electrocatalytic performance towards glucose. Appl. Surf. Sci. 2013, 283, 947–953. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Su, L.; Zhang, Z.; Huo, D.; Hou, C.; Lei, Y. CuO nanowires based sensitive and selective non-enzymatic glucose detection. Sens. Actuat. B-Chem. 2014, 191, 86–93. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Xin, Y.; Zhang, Z. Sensitive electrochemical nonenzymatic glucose sensing based on anodized CuO nanowires on three-dimensional porous copper foam. Sci. Rep. 2015, 5, 16115. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron Young Sci 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Water quality-Determination of the chemical oxygen demand-Dichromate method; China Environment Press: Beijing, China, 2017; Vol. HJ 828–2017.
Sample Availability: Samples of the compounds are not available from the authors. |

| Electrochemical Sensors Based on Different Electrodes | Linearity (mg L−1) | DL (mg L−1) | Sensitivity (mA /(mg L−1)) | Reference |
|---|---|---|---|---|
| Cu/CuO | 53.0–2801.4 | 20.3 | 4.717 × 10−4 | [7] |
| nano-Cu/Cu | 4.8–600 | 3.6 | 4.538 × 10−4 | [8] |
| nano-Cu/ GCE | 15–629.3 | 1.7 | 1.7 × 10−3 | [9] |
| nano-Cu/ Cu cable | 2–595 | 2.6 | 1.9 × 10−4 | [10] |
| CuO nafion /Cu | 50–1000 | 2.11 | 8.3 × 10−6 | [11] |
| NiCu | 10–1533 | 1.0 | 1.41 × 10−4 | [12] |
| CuO/AgO | 53–394 | 28 | 6.8 × 10−9 | [17] |
| Cu-Co/Au | 1.92–768 | 0.609 | 8.88 × 10−4 | [18] |
| CuO/CuNWE | 5–1153 | 2.3 | 2.46 × 10−2 | This work |
| Samples | Conventional Methods | Proposed Method RSD(%) | Relative Difference(%) | |
|---|---|---|---|---|
| CODcr (mg L−1) | COD (mg L−1) | n = 5 | ||
| The West Lake, Hangzhou | 12 | 11.2 ± 0.6 | 5.36 | −6.67 |
| The Shangtang river, Hangzhou | 26 | 24.3 ± 1.1 | 4.52 | −6.54 |
| Papermaking wastewater | 243 | 248.6 ± 11.1 | 4.47 | 2.31 |
| Pharmaceutical wastewater | 529 | 519.1 ± 14.8 | 2.86 | −1.87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Zhu, Y.; Yang, W.; Jiang, A.; Jin, X.; Zhang, Y.; Yan, L.; Zhang, G.; Liu, Z. A Self-Supported CuO/Cu Nanowire Electrode as Highly Efficient Sensor for COD Measurement. Molecules 2019, 24, 3132. https://doi.org/10.3390/molecules24173132
Huang X, Zhu Y, Yang W, Jiang A, Jin X, Zhang Y, Yan L, Zhang G, Liu Z. A Self-Supported CuO/Cu Nanowire Electrode as Highly Efficient Sensor for COD Measurement. Molecules. 2019; 24(17):3132. https://doi.org/10.3390/molecules24173132
Chicago/Turabian StyleHuang, Xinwen, Yingying Zhu, Wanquan Yang, Anhua Jiang, Xiaoqiang Jin, Yirong Zhang, Liang Yan, Geshan Zhang, and Zongjian Liu. 2019. "A Self-Supported CuO/Cu Nanowire Electrode as Highly Efficient Sensor for COD Measurement" Molecules 24, no. 17: 3132. https://doi.org/10.3390/molecules24173132
APA StyleHuang, X., Zhu, Y., Yang, W., Jiang, A., Jin, X., Zhang, Y., Yan, L., Zhang, G., & Liu, Z. (2019). A Self-Supported CuO/Cu Nanowire Electrode as Highly Efficient Sensor for COD Measurement. Molecules, 24(17), 3132. https://doi.org/10.3390/molecules24173132





