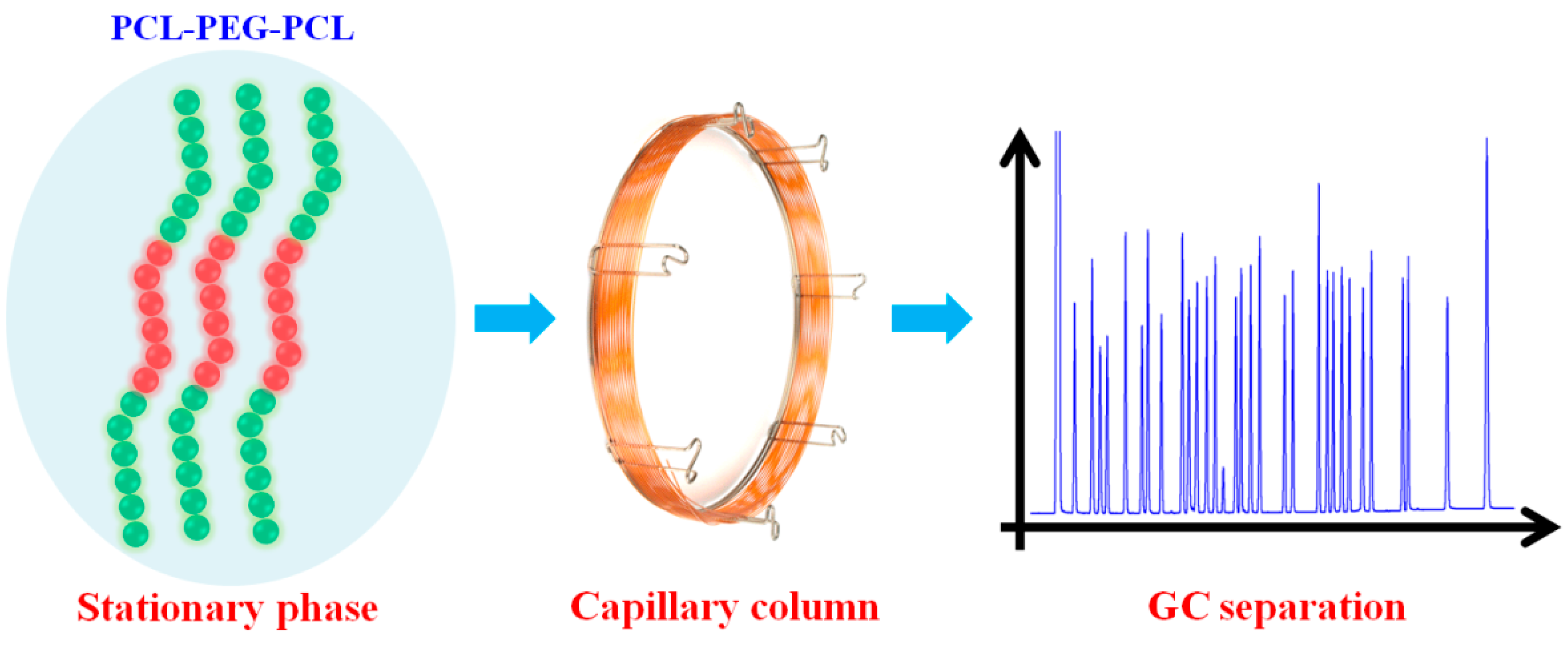
Amphiphilic Block Copolymer PCL-PEG-PCL as Stationary Phase for Capillary Gas Chromatographic Separations
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the PCL-PEG-PCL
2.2. Column Efficiency and Golay Plot
2.3. McReynolds Constants and Polarity
2.4. Separation of a Complex Mixture of 30 Analytes of Diverse Types
2.5. Separation of Positional, Structural, and cis-/trans-Isomers
2.6. Column Operating Range
2.7. Applications for Determination of Isomer Impurities in Real Samples
3. Experimental
3.1. Materials and Equipment
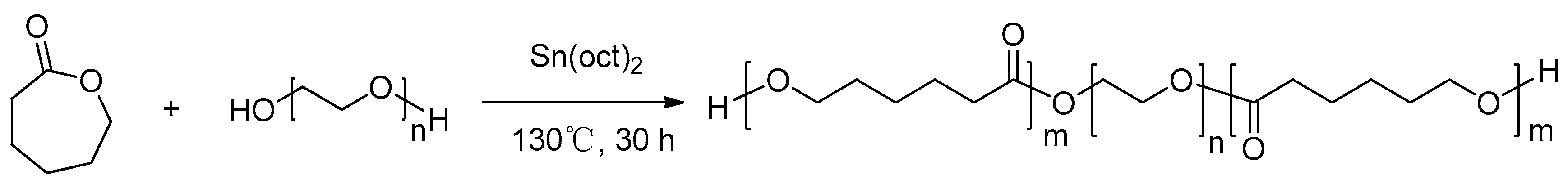
3.2. Synthesis of thePCL-PEG-PCLStationary Phase
3.3. Fabrication of the PCL-PEG-PCL Capillary Column
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Špánik, I.; Machyňáková, A. Recent applications of gas chromatography with high-resolution mass spectrometry. J. Sep. Sci. 2018, 41, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Braghiroli, D.; Vandelli, M.A.; Cannazza, G. Pharmaceutical and biomedical analysis of cannabinoids: A critical review. J. Pharmaceut. Biomed. 2018, 147, 565–579. [Google Scholar] [CrossRef]
- Russo, M.V.; Avino, P.; Perugini, L.; Notardonato, I. Extraction and GC-MS analysis of phthalate esters in food matrices: A review. RSC Adv. 2015, 5, 37023–37043. [Google Scholar] [CrossRef]
- Muscalu, A.M.; Górecki, T. Comprehensive two-dimensional gas chromatography in environmental analysis. TrAC Trends Anal. Chem. 2018, 106, 225–245. [Google Scholar] [CrossRef]
- Zhao, X.J.; Tan, K.F.; Xing, J. A flexible and convenient strategy for synthesis of ionic liquid bonded polysiloxane stationary phases. J. Chromatogr. A 2019, 1587, 197–208. [Google Scholar] [CrossRef]
- Peng, J.L.; Qi, M.L. Poly(3-hexylthiophene) stationary phase for gas chromatographic separations of aliphatic and aromatic isomers. J. Chromatogr. A 2018, 1569, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Nan, H.; Anderson, J.L. Ionic liquid stationary phases for multidimensional gas chromatography. TrAC Trends Anal. Chem. 2018, 105, 367–379. [Google Scholar] [CrossRef]
- Poole, C.F.; Lenca, N. Gas chromatography on wall-coated open-tubular columns with ionic liquid stationary phases. J. Chromatogr. A 2014, 1357, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Qin, S.J.; Qi, M.L.; Fu, R.N. Cucurbit[n]urils as a new class of stationary phases for gas chromatographic separations. J. Chromatogr. A 2014, 1334, 139–148. [Google Scholar] [CrossRef]
- Sun, T.; Qi, L.R.; Li, W.W.; Li, Y.; Shuai, X.M.; Cai, Z.Q.; Chen, H.P.; Qiao, X.G.; Ma, L.F. Amphiphilic calix [4]arenes as a highly selective gas chromatographic stationary phase for aromatic amine isomers. J. Chromatogr. A 2019, 1601, 310–318. [Google Scholar] [CrossRef]
- Sun, T.; Li, B.; Shuai, X.M.; Chen, Y.J.; Li, W.W.; Cai, Z.Q.; Qiao, X.G.; Hu, S.Q.; Ma, L.F. Performance and selectivity of lower-rim substituted calix[4]arene as a stationary phase for capillary gas chromatography. RSC Adv. 2019, 9, 21207–21214. [Google Scholar] [CrossRef]
- Sun, T.; Li, B.; Li, Y.; Zhao, X.Y.; Song, Q.Q.; Jiang, X.X.; Shuai, X.M.; Li, Y.Y.; Cai, Z.Q.; Hu, S.Q. Amphiphilic Star-Shaped Calix[4]resorcinarene as stationary phase for capillary gas chromatography. Chromatographia 2019, 1–12. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, M.; Duan, A.H.; Zhang, J.H.; Yang, R.; Yuan, L.M. Homochiral metal-organic framework used as a stationary phase for high-performance liquid chromatography. J. Sep. Sci. 2015, 38, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.M.; Zhang, M.; Fei, Z.X.; Yuan, L.M. Experimental comparison of chiral metal-organic framework used as stationary phase in chromatography. J. Chromatogr. A 2014, 1363, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Eiceman, G.A.; Gardea-Torresdey, J.; Dorman, F.; Overton, E.; Bhushan, A.; Dharmasena, H.P. Gas chromatography. Anal. Chem. 2006, 78, 3985–3996. [Google Scholar] [CrossRef] [PubMed]
- He, X.X.; Han, X.; Wang, H.; Wang, B.; Wu, B. Polysiloxanes-based stationary phases containing methoxy-substituted tetraphenyl-phenyl groups for gas chromotographic separations. RSC Adv. 2016, 6, 76514–76523. [Google Scholar] [CrossRef]
- Han, X.; Wang, H.; He, X.X.; Wang, B.; Wu, B. 7,10-Diphenylfluoranthene grafted polysiloxane as a highly selective stationary phase for gas chromatography. J. Chromatogr. A 2016, 1468, 192–199. [Google Scholar] [CrossRef]
- Liu, J.C.; Xu, L.; Bai, J.C.; Du, A.Q.; Wu, B. Ethyl carbazole-grafted polysiloxane as stationary phase for gas chromatography. Chromatographia 2019, 82, 671–682. [Google Scholar] [CrossRef]
- Rosen, T.; Goldberg, I.; Navarra, W.; Venditto, V.; Kol, M. Block-Stereoblock Copolymers of Poly(ε-Caprolactone) and Poly(Lactic Acid). Angew. Chem. Int. Edit. 2018, 130, 7309–7313. [Google Scholar] [CrossRef]
- Mezzasalma, L.; Harrisson, S.; Saba, S.; Loyer, P.; Coulembier, O.; Taton, D. Bulk organocatalytic synthetic access to statistical copolyesters from l-Lactide and ε-Caprolactone using benzoic acid. Biomacromolecules 2019, 20, 1965–1974. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Polymeric modification and its implication in drug delivery: Poly-ε-caprolactone (PCL) as a model polymer. Mol. Pharm. 2012, 9, 2365–2379. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.R.; Fonseca, F.N.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.; Guterres, S.S. Poly(ε-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Bhayo, A.M.; Musharraf, S.G.; Malik, M.I. Chromatographic characterization of amphiphilic di-and tri-block copolymers of poly(ethylene oxide) and poly(ε-caprolactone). J. Sep. Sci. 2018, 41, 3352–3359. [Google Scholar] [CrossRef] [PubMed]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Impallomeni, G.; Pistarà, V.; Cupri, S.; Graziano, A.C.E.; Cardile, V.; Ballistreri, A. New amphiphilic derivatives of poly(ethylene glycol) (PEG) as surface modifiers of colloidal drug carriers. III. Lipoamino acid conjugates with carboxy- and amino-PEG5000 polymers. Mater. Sci. Eng. C-Mater. 2015, 46, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Salehi, R.; Jelvehgari, M. Preparation and evaluation of PCL-PEG-PCL micelles as potential nanocarriers for ocular delivery of dexamethasone. Iran. J. Basic Med. Sci. 2018, 21, 153–164. [Google Scholar] [PubMed]
- Asadi, N.; Annabi, N.; Mostafavi, E.; Anzabi, M.; Khalilov, R.; Saghfi, S.; Mehrizadeh, M.; Akbarzadeh, A. Synthesis, characterization and in vitro evaluation of magnetic nanoparticles modified with PCL-PEG-PCL for controlled delivery of 5FU. Artif. Cells Nanomed. Biotechnol. 2018, 46, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.G.; Zhou, J.L.; Ding, J.L.; Xu, J.J.; Zhong, H.J.; Lei, S.J. A novel approach to prepare a tissue engineering decellularized valve scaffold with poly(ethylene glycol)-poly(ε-caprolactone). RSC Adv. 2016, 6, 14427–14438. [Google Scholar] [CrossRef]
- Liu, C.B.; Gong, C.Y.; Huang, M.J.; Wang, J.W.; Pan, Y.F.; Zhang, Y.D.; Li, G.Z.; Gou, M.L.; Wang, K.; Tu, M.J.; et al. Thermoreversible gel-sol behavior of biodegradable PCL-PEG-PCL triblock copolymer in aqueous solutions. J. Biomed. Mater. Res. B 2008, 84, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.Q.M.; Hsieh, M.F.; Chang, K.L.; Pho, Q.H.; Nguyen, V.C.; Cheng, C.Y.; Huang, C.M. Bactericidal effect of lauric acid-loaded PCL-PEG-PCL nano-sized micelles on skin commensal propionibacterium acnes. Polymers 2016, 8, 321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.C.; Qi, M.L.; Wang, J.L. Star-shaped oligothiophene-functionalized truxene materials as stationary phases for capillary gas chromatography. J. Chromatogr. A 2017, 1525, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Chen, H.; Qiao, X.G.; Ma, L.F.; Hu, S.Q.; Liu, X.M. Performance of palm fibers as stationary phase for capillary gas chromatographic separations. RSC Adv. 2018, 8, 34102–34109. [Google Scholar] [CrossRef]
- He, J.; Yu, L.N.; Huang, X.B.; Qi, M.L. Triptycene-based stationary phases for gas chromatographic separations of positional isomers. J. Chromatogr. A 2019, 1599, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F.; Poole, S.K. Separation characteristics of wall-coated open-tubular columns for gas chromatography. J. Chromatogr. A 2008, 1184, 254–280. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.D.; Poole, C.F.; Abraham, M.H. Synthesis and gas chromatographic evaluation of a high-temperature hydrogen-bond acid stationary phase. J. Chromatogr. A 1998, 805, 217–235. [Google Scholar] [CrossRef]
- Haas, R.; Schmidt, T.C.; Steinbach, K.; von Löw, E. Derivatization of aromatic amines for analysis in ammunition wastewater II: Derivatization of methyl anilines by iodination with a Sandmeyer-like reaction. Fresen. J. Anal. Chem. 1997, 359, 497–501. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhu, G.H.; Wang, S.S.; Jiang, K.Z.; Xu, J.X.; Liu, J.S.; Jiang, J.X. Simultaneous determination of isomeric substituted anilines by imidization with benzaldehyde and gas chromatography-mass spectrometry. J. Sep. Sci. 2018, 41, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.P.; Han, J.; Huan, Y.F.; Cui, Y.J.; Zhang, X.; Chen, H.W.; Gu, H.W. Desorption electrospray ionization tandem mass spectrometry for detection of 24 carcinogenic aromatic amines in textiles. Anal. Chem. 2009, 81, 6070–6079. [Google Scholar] [CrossRef]
- Mayer-Helm, B.; Rauter, W. Determination of the minimum allowable operating temperature of stationary phases in capillary columns by inverse gas chromatography. Analyst 2005, 130, 502–507. [Google Scholar]
- Yang, Y.H.; Qi, M.L.; Wang, J.L. Separation performance of a star-shaped truxene-based stationary phase functionalized with peripheral 3,4-ethylenedioxythiophene moieties for capillary gas chromatography. J. Chromatogr. A 2018, 1578, 67–75. [Google Scholar] [CrossRef]
- Peng, J.L.; Sun, T.; Wu, L.Q.; Qi, M.L.; Huang, X.B. Separation performance of dithienyl benzothiadiazole-based stationary phases for capillary gas chromatography. RSC Adv. 2017, 7, 45408–45415. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds the complex mixture of 30 analytes and isomers are available from the authors. |






| Stationary Phases | X′ | Y′ | Z′ | U′ | S′ | Average Polarity |
|---|---|---|---|---|---|---|
| I for PCL-PEG-PCL | 876 | 1009 | 908 | 1098 | 1085 | |
| I for squalane | 653 | 590 | 627 | 652 | 699 | |
| △I for PCL-PEG-PCL | 223 | 419 | 281 | 446 | 386 | 351 |
| T (°C) | e | s | a | l | c | R2 | SE | F | n |
|---|---|---|---|---|---|---|---|---|---|
| 80 | −0.001 | 1.326 | 2.239 | 0.425 | −1.968 | 0.992 | 0.052 | 594 | 29 |
| (0.007) | (0.072) | (0.087) | (0.022) | (0.093) | |||||
| 100 | −0.009 | 1.108 | 1.991 | 0.400 | −1.967 | 0.991 | 0.065 | 621 | 35 |
| (0.008) | (0.071) | (0.069) | (0.030) | (0.136) | |||||
| 120 | −0. 004 | 0.809 | 1.785 | 0.251 | −1.281 | 0.991 | 0.050 | 543 | 32 |
| (0.007) | (0.057) | (0.070) | (0.028) | (0.125) |
| Samples | Labeled Purity | Measured Purity | Isomer Impurity | Content |
|---|---|---|---|---|
| cis-decahydronaphthalene | 98% | 98.70% | trans-decahydronaphthalene | 1.27% |
| trans-decahydronaphthalene | 98% | 99.50% | cis-decahydronaphthalene | 0.45% |
| 1-pentanol | 98% | 99.44% | 3-methyl-1-butanol | 0.26% |
| 3-methyl-1-butanol | 98.5% | 98.23% | 1-pentanol | 0.04% |
| isopropylbenzene | 99% | 99.31% | n-propylbenzene | 0.10% |
| 1,2,4-trichlorobenzene | 99% | 98.41% | 1,2,3-trichlorobenzene | 0.28% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Shuai, X.; Ren, K.; Jiang, X.; Chen, Y.; Zhao, X.; Song, Q.; Hu, S.; Cai, Z. Amphiphilic Block Copolymer PCL-PEG-PCL as Stationary Phase for Capillary Gas Chromatographic Separations. Molecules 2019, 24, 3158. https://doi.org/10.3390/molecules24173158
Sun T, Shuai X, Ren K, Jiang X, Chen Y, Zhao X, Song Q, Hu S, Cai Z. Amphiphilic Block Copolymer PCL-PEG-PCL as Stationary Phase for Capillary Gas Chromatographic Separations. Molecules. 2019; 24(17):3158. https://doi.org/10.3390/molecules24173158
Chicago/Turabian StyleSun, Tao, Xiaomin Shuai, Kaixin Ren, Xingxing Jiang, Yujie Chen, Xinyu Zhao, Qianqian Song, Shaoqiang Hu, and Zhiqiang Cai. 2019. "Amphiphilic Block Copolymer PCL-PEG-PCL as Stationary Phase for Capillary Gas Chromatographic Separations" Molecules 24, no. 17: 3158. https://doi.org/10.3390/molecules24173158
APA StyleSun, T., Shuai, X., Ren, K., Jiang, X., Chen, Y., Zhao, X., Song, Q., Hu, S., & Cai, Z. (2019). Amphiphilic Block Copolymer PCL-PEG-PCL as Stationary Phase for Capillary Gas Chromatographic Separations. Molecules, 24(17), 3158. https://doi.org/10.3390/molecules24173158





