Proximate Composition and Nutritional Profile of Rainbow Trout (Oncorhynchus mykiss) Heads and Skipjack tuna (Katsuwonus Pelamis) Heads
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
2.2. Amino Acid Profile
2.3. Fatty Acid Profile
2.4. Determination of Carnosine and Anserine
2.4.1. Method Performance
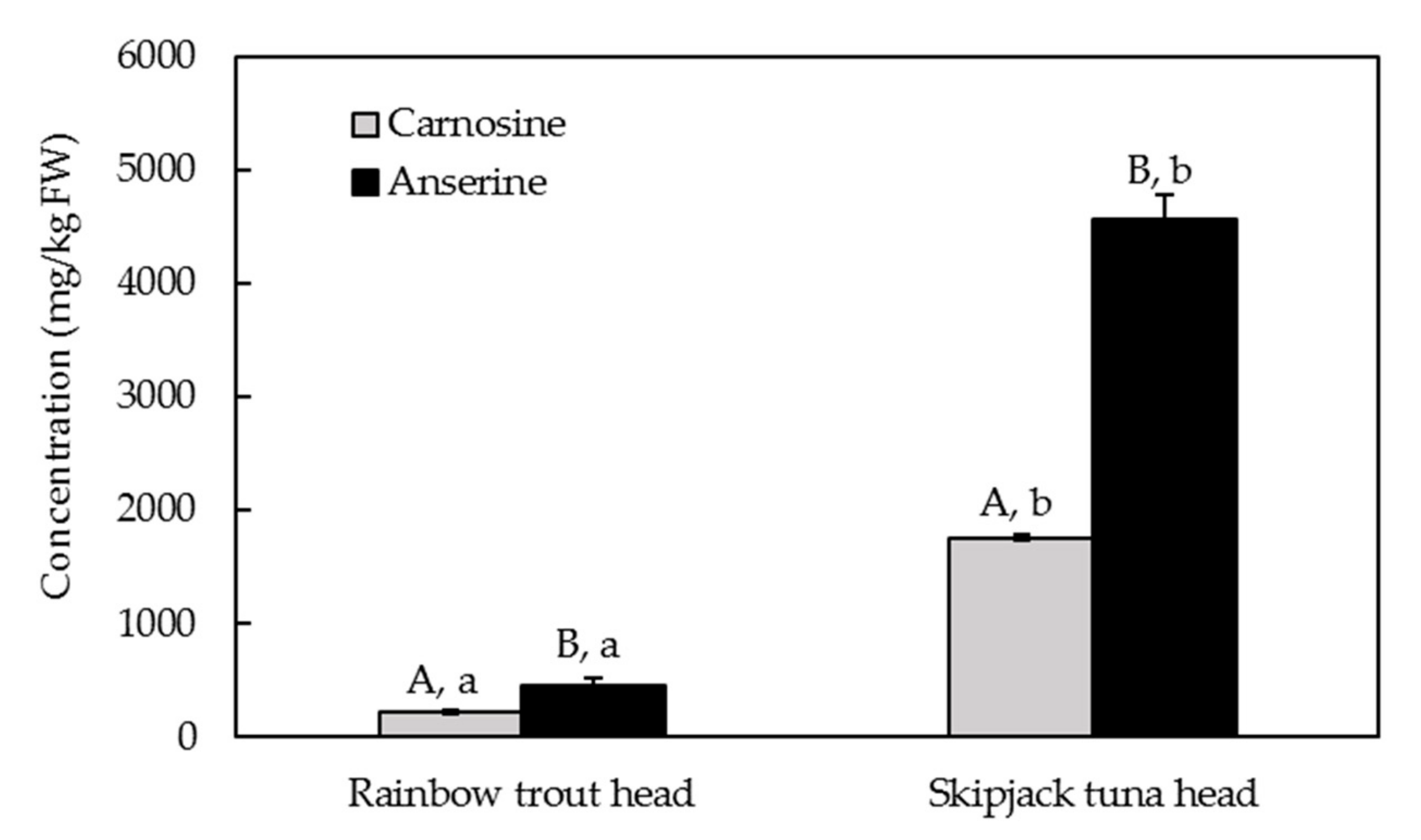
2.4.2. Quantification of Carnosine and Anserine Contents in Rainbow Trout Heads and Skipjack Tuna Heads
3. Materials and Methods
3.1. Chemicals
3.2. Samples
3.3. Determination of Physicochemical Properties
3.4. Determination of Amino Acid Profile
3.5. Determination of Fatty Acid Profile
3.6. Determination of Carnosine and Anserine Content
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Bruno, S.F.; Kudre, T.G.; Bhaskar, N. Impact of pretreatment-assisted enzymatic extraction on recovery, physicochemical and rheological properties of oil from Labeo rohita head. J. Food Process. Eng. 2019, 42, e12990. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Yasemi, M.; Ahmadi Gavlighi, H.; Xu, X. Hydrolysates from rainbow trout (Oncorhynchus mykiss) processing by-product with different pretreatments: Antioxidant activity and their effect on lipid and protein oxidation of raw fish emulsion. Lwt-Food Sci. Technol. 2019, 108, 120–128. [Google Scholar] [CrossRef]
- Gbogouri, G.A.; Linder, M.; Fanni, J.; Parmentier, M. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J. Food Sci. 2004, 69, C615–C622. [Google Scholar] [CrossRef]
- Sathivel, S.; Bechtel, P.J.; Babbitt, J.; Prinyawiwatkul, W.; Negulescu, I.I.; Reppond, K.D. Properties of protein powders from arrowtooth flounder (Atheresthes stomias) and herring (Clupea harengus) byproducts. J. Agric. Food Chem. 2004, 52, 5040–5046. [Google Scholar] [CrossRef] [PubMed]
- Haddar, A.; Bougatef, A.; Balti, R.; Souissi, N.; Koched, W.; Nasri, M. Physicochemical and functional properties of gelatin from tuna (Thunnus thynnus) head bones. J. Food Nutr. Res. 2011, 50, 150–159. [Google Scholar]
- Panpipat, W.; Chaijan, M. Functional properties of pH-shifted protein isolates from bigeye snapper (Priacanthus tayenus) head by-product. Int. J. Food Prop. 2017, 20, 596–610. [Google Scholar] [CrossRef]
- Abdollahi, M.; Undeland, I. Structural, functional, and sensorial properties of protein isolate produced from salmon, cod, and herring by-products. Food Bioprocess. Technol. 2018, 11, 1733–1749. [Google Scholar] [CrossRef] [Green Version]
- Glowacz-Rozynska, A.; Tynek, M.; Malinowska-Panczyk, E.; Martysiak-Zurowska, D.; Pawlowicz, R.; Kolodziejska, I. Comparison of oil yield and quality obtained by different extraction procedures from salmon (Salmo salar) processing byproducts. Eur. J. Lipid Sci. Technol. 2016, 118, 1759–1767. [Google Scholar] [CrossRef]
- De Oliveira, D.A.S.B.; Licodiedoff, S.; Furigo, A.; Ninow, J.L.; Bork, J.A.; Podestá, R.; Block, J.M.; Waszczynskyj, N. Enzymatic extraction of oil from yellowfin tuna (Thunnus albacares) by-products: A comparison with other extraction methods. Int. J. Food Sci. Technol. 2017, 52, 699–705. [Google Scholar] [CrossRef]
- Das, A.K.; Anjaneyulu, A.S.R.; Biswas, S. Effect of carnosine preblending on the quality of ground buffalo meat. Food Chem. 2006, 97, 531–538. [Google Scholar] [CrossRef]
- Anderson, E.J.; Vistoli, G.; Katunga, L.A.; Funai, K.; Regazzoni, L.; Monroe, T.B.; Gilardoni, E.; Cannizzaro, L.; Colzani, M.; De Maddis, D.; et al. A carnosine analog mitigates metabolic disorders of obesity by reducing carbonyl stress. J. Clin. Invest. 2018, 128, 5280–5293. [Google Scholar] [CrossRef] [PubMed]
- Berezhnoy, D.S.; Stvolinsky, S.L.; Lopachev, A.V.; Devyatov, A.A.; Lopacheva, O.M.; Kulikova, O.I.; Abaimov, D.A.; Fedorova, T.N. Carnosine as an effective neuroprotector in brain pathology and potential neuromodulator in normal conditions. Amino Acids 2019, 51, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Posa, D.K.; Kumar, V.; Hoetker, D.; Kumar, A.; Ganesan, S.; Riggs, D.W.; Bhatnagar, A.; Wempe, M.F.; Baba, S.P. Carnosine protects cardiac myocytes against lipid peroxidation products. Amino Acids 2019, 51, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Pampalone, M.; Frazziano, G.; Grasso, G.; Rizzarelli, E.; Ricordi, C.; Casu, A.; Iannolo, G.; Conaldi, P.G. Carnosine protects pancreatic beta cells and islets against oxidative stress damage. Mol. Cell. Endocrinol. 2018, 474, 105–118. [Google Scholar] [CrossRef]
- Han, Y.; Gao, B.; Zhao, S.; Wang, M.; Jian, L.; Han, L.; Liu, X. Simultaneous Detection of Carnosine and Anserine by UHPLC-MS/MS and Its Application on Biomarker Analysis for Differentiation of Meat and Bone Meal. Molecules 2019, 24, 217. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, A.; Perna, A.; Gambacorta, E. Comparison of antioxidant compounds in pig meat from Italian autochthonous pig Suino Nero Lucano and a modern crossbred pig before and after cooking. Food Chem. 2019, 292, 108–112. [Google Scholar] [CrossRef]
- Liu, S.; Wang, G.; Xiao, Z.; Pu, Y.; Ge, C.; Liao, G. H-1-NMR-based water-soluble low molecular weight compound characterization and free fatty acid composition of five kinds of Yunnan dry-cured hams. Lwt-Food Sci. Technol. 2019, 108, 174–182. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, M.; Wang, W.; Wu, H.; Fu, Z.; Lin, L. Determination of carnosine in Black-Bone Silky Fowl (Gallus gallus domesticus Brisson) and common chicken by HPLC. Eur. Food Res. Technol. 2007, 226, 311–314. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Medana, C.; Visentin, S.; Giancotti, V.; Zunino, V.; Meineri, G. Determination of carnosine, anserine, homocarnosine, pentosidine and thiobarbituric acid reactive substances contents in meat from different animal species. Food Chem. 2011, 126, 1939–1947. [Google Scholar] [CrossRef]
- Shumilina, E.; Slizyte, R.; Mozuraityte, R.; Dykyy, A.; Stein, T.A.; Dikiy, A. Quality changes of salmon by-products during storage: Assessment and quantification by NMR. Food Chem. 2016, 211, 803–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, S.; Antunes, S.C.; Nunes, B.; Correia, A.T. Histopathological effects of the antibiotic erythromycin on the freshwater fish species Oncorhynchus mykiss. Ecotoxicol. Env. Saf. 2019, 181, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kiyofuji, H.; Aoki, Y.; Kinoshita, J.; Okamoto, S.; Masujima, M.; Matsumoto, T.; Fujioka, K.; Ogata, R.; Nakao, T.; Sugimoto, N.; et al. Northward migration dynamics of skipjack tuna (Katsuwonus pelamis) associated with the lower thermal limit in the western Pacific Ocean. Prog. Oceanogr. 2019, 175, 55–67. [Google Scholar] [CrossRef]
- He, S.; Franco, C.; Zhang, W. Characterisation of processing wastes of Atlantic Salmon (Salmo salar) and Yellowtail Kingfish (Seriola lalandi) harvested in Australia. Int. J. Food Sci. Technol. 2011, 46, 1898–1904. [Google Scholar] [CrossRef]
- Oliveira, D.; Bernardi, D.; Drummond, F.; Dieterich, F.; Boscolo, W.; Leivas, C.; Kiatkoski, E.; Waszczynskyj, N. Potential Use of Tuna (Thunnus albacares) by-product: Production of Antioxidant Peptides and Recovery of Unsaturated Fatty Acids from Tuna Head. Int. J. Food Eng. 2017, 13, 20150365. [Google Scholar] [CrossRef]
- Wu, T.H.; Nigg, J.D.; Stine, J.J.; Bechtel, P.J. Nutritional and Chemical Composition of By-Product Fractions Produced from Wet Reduction of Individual Red Salmon (Oncorhynchus nerka) Heads and Viscera. J. Aquat. Food Prod. Technol. 2011, 20, 183–195. [Google Scholar] [CrossRef]
- Batista, I.; Ramos, C.; Coutinho, J.; Bandarra, N.M.; Nunes, M.L. Characterization of protein hydrolysates and lipids obtained from black scabbardfish (Aphanopus carbo) by-products and antioxidative activity of the hydrolysates produced. Process. Biochem. 2010, 45, 18–24. [Google Scholar] [CrossRef]
- Fernandes, T.J.R.; Alves, R.C.; Souza, T.; Silva, J.M.G.; Castro-Cunha, M.; Valente, L.M.P.; Oliveira, M.B.P.P. Lipid content and fatty acid profile of Senegalese sole (Solea senegalensis Kaup, 1858) juveniles as affected by feed containing different amounts of plant protein sources. Food Chem. 2012, 134, 1337–1342. [Google Scholar] [CrossRef]
- Prego, R.; Pazos, M.; Medina, I.; Aubourg, S.P. Comparative chemical composition of different muscle zones in angler (Lophius piscatorius). J. Food Compos. Anal. 2012, 28, 81–87. [Google Scholar] [CrossRef]
- Sathivel, S.; Smiley, S.; Prinyawiwatkul, W.; Bechtel, P.J. Functional and nutritional properties of red salmon (Oncorhynchus nerka) enzymatic hydrolysates. J. Food Sci. 2005, 70, C401–C406. [Google Scholar] [CrossRef]
- Gbogouri, G.A.; Linder, M.; Fanni, J.; Parmentier, M. Analysis of lipids extracted from salmon (Salmo salar) heads by commercial proteolytic enzymes. Eur. J. Lipid Sci. Technol. 2006, 108, 766–775. [Google Scholar] [CrossRef]
- He, C.; Cao, J.; Jiang, X.; Wen, C.; Bai, X.; Li, C. Fatty Acid Profiles of Triacylglycerols and Phospholipids of Sea-Cage Cultured Trachinotus blochii: A Comparative Study of Head, Viscera, Skin, Bone, and Muscle. J. Food Sci. 2019, 84, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.; Eilertsen, K.-E.; Elvevoll, E.O. Health benefits of marine foods and ingredients. Biotechnol. Adv. 2011, 29, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, H.W.; Cooksy, K.D.; Ellis, B.; Anderson, J.M. The separation of o-phthalaldehyde derivatives of amino acids by reversed-phase chromatography on octylsilica columns. Anal. Biochem. 1986, 153, 189–198. [Google Scholar] [CrossRef]
- Al-Farga, A.; Zhang, H.; Siddeeg, A.; Shamoon, M.V.M.; Chamba, M.; Al-Hajj, N. Proximate composition, functional properties, amino acid, mineral and vitamin contents of a novel food: Alhydwan (Boerhavia elegana Choisy) seed flour. Food Chem. 2016, 211, 268–273. [Google Scholar] [CrossRef] [PubMed]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists′ Society, 6th ed.; AOCS: Champaign, IL, USA, 2009. [Google Scholar]
- Ye, Z.; Li, R.; Cao, C.; Xu, Y.-J.; Cao, P.; Li, Q.; Liu, Y. Fatty acid profiles of typical dietary lipids after gastrointestinal digestion and absorbtion: A combination study between in-vitro and in-vivo. Food Chem. 2019, 280, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.R.; Kirkland, J.J.; Dolan, J.W. Introduction to Modern Liquid Chromatography, 3rd ed.; John Willy & Sons Inc.: Hoboken, NJ, USA, 2010; pp. 510–512. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

| Parameters | Rainbow Trout Heads | Skipjack Tuna Heads |
|---|---|---|
| Moisture | 62.4 ± 0.7 a | 75.6 ± 0.5 b |
| Ash | 1.91 ± 0.06 a | 3.88 ± 0.08 b |
| Protein | 29 ± 1 b | 18 ± 3 a |
| Lipid | 6.0 ± 0.3 b | 4.8 ± 0.5 a |
| Amino Acids | Rainbow Trout Heads | Skipjack Tuna Heads |
|---|---|---|
| Aspartic acid | 10.3± 0.8 a | 10.9 ± 0.6 a |
| Glutamic acid | 15.6 ± 0.8 a | 16.3 ± 0.9 a |
| Serine | 3.3 ± 0.1 a | 3.7 ± 0.3 a |
| Histidine | 7.3 ± 0.2 b | 2.9 ± 0.2 a |
| Glycine | 4.8 ± 0.2 a | 6.2 ± 0.2 b |
| Threonine | 3.8 ± 0.1 a | 3.9 ± 0.2 a |
| Arginine | 6.2 ± 0.3 a | 6.3 ± 0.1 a |
| Alanine | 6.0 ± 0.4 a | 6.6 ± 0.2 a |
| Tyrosine | 2.4 ± 0.1 a | 2.4 ± 0.1 a |
| Cysteine | 0.38 ± 0.04 a | 0.24 ± 0.11 a |
| Valine | 6.2 ± 0.3 a | 6.1 ± 0.2 a |
| Methionine | 2.7 ± 0.1 a | 2.6 ± 0.3 a |
| Phenylalanine | 4.2 ± 0.4 a | 4.4 ± 0.6 a |
| Isoleucine | 5.6 ± 0.3 a | 5.4 ± 0.3 a |
| Leucine | 8.4 ± 0.4 a | 8.2 ± 0.2 a |
| Lysine | 10.2± 0.7 a | 9.5 ± 0.4 a |
| Proline | 2.7 ± 0.3 a | 4.5 ± 0.2 b |
| ∑ EAAs | 41 | 40 |
| Fatty Acids | Rainbow Trout Heads | Skipjack Tuna Heads |
|---|---|---|
| C12:0 | 0.03 ± 0.01 a | 0.09 ± 0.00 b |
| C14:0 | 1.65 ± 0.02 a | 4.70 ± 0.03 b |
| C15:0 | 0.20 ± 0.01 a | 1.38 ± 0.03 b |
| C16:0 | 15.4 ± 0.2 a | 36 ± 1 b |
| C17:0 | 0.22 ± 0.03 a | 1.69 ± 0.06 b |
| C18:0 | 4.6 ± 0.1 a | 10.0 ± 0.2 b |
| C20:0 | 0.23 ± 0.04 a | 0.60 ± 0.03 b |
| C22:0 | 0.49 ± 0.02 b | 0.37 ± 0.02 a |
| ∑ SFAs | 22.8 | 54.5 |
| C16:1 | 3.13 ± 0.05 a | 6.8 ± 0.3 b |
| C18:1 | 32 ± 1 b | 23.9 ± 0.8 a |
| C20:1 n-9 | 1.7 ± 0.1 a | 1.9 ± 0.1 a |
| C22:1 n-9 | 1.74 ± 0.08 b | 0.26 ± 0.01 a |
| ∑ MUFAs | 38.6 | 32.8 |
| C18:2 n-6 | 29.1 ± 0.6 b | 0.98 ± 0.01 a |
| C18:3 n-6 | 0.35 ± 0.06 b | 0.11 ± 0.03 a |
| C18:3 n-3 | 3.1 ± 0.2 b | 0.48 ± 0.05 a |
| C20:2 n-6 | 1.20 ± 0.03 b | 0.35 ± 0.02 a |
| C20:3 n-3 | 0.27 ± 0.03 b | 0.10 ±0.01 a |
| C20:3 n-6 | 0.49 ± 0.04 b | 0.08 ± 0.00 a |
| C20:4 n-6 | 0.37 ± 0.01 a | 1.56 ± 0.04 b |
| C20:5 n-3 (EPA) | 0.91 ± 0.06 a | 1.28 ±0.20 b |
| C22:3 n-3 | 0.06 ± 0.01 a | 0.20 ± 0.06 b |
| C22:4 n-3 | 0.24 ± 0.05 b | 0.10 ± 0.00 a |
| C22:4 n-3 | 0.07 ± 0.00 a | 0.80 ± 0.02 b |
| C22:5 n-3 | 0.30 ± 0.07 a | 0.37 ± 0.03 a |
| C22:6 n-3 (DHA) | 2.2 ± 0.1 a | 6.3 ± 0.1 b |
| ∑ PUFAs | 38.6 | 12.7 |
| Carnosine | Anserine | |
|---|---|---|
| Regression equation * | Y = 1303.9X + 1838.2 | Y = 3002.9X − 1190.7 |
| R2 | 0.9999 | 1 |
| RSDr (%, n = 10) | 0.62 | 0.51 |
| Recovery (%), fortified with 0.5 mg/g | 106.1 | 106.0 |
| Recovery (%), fortified with 1.0 mg/g | 103.6 | 92.7 |
| Recovery (%), fortified with 1.5 mg/g | 97.8 | 92.1 |
| LOQ (mg/kg) | 3.12 | 9.46 |
| LOD (mg/kg) | 1.07 | 3.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Liu, Y.; Jiang, W.; Yan, X. Proximate Composition and Nutritional Profile of Rainbow Trout (Oncorhynchus mykiss) Heads and Skipjack tuna (Katsuwonus Pelamis) Heads. Molecules 2019, 24, 3189. https://doi.org/10.3390/molecules24173189
Li W, Liu Y, Jiang W, Yan X. Proximate Composition and Nutritional Profile of Rainbow Trout (Oncorhynchus mykiss) Heads and Skipjack tuna (Katsuwonus Pelamis) Heads. Molecules. 2019; 24(17):3189. https://doi.org/10.3390/molecules24173189
Chicago/Turabian StyleLi, Weinan, Yu Liu, Wei Jiang, and Xiaojun Yan. 2019. "Proximate Composition and Nutritional Profile of Rainbow Trout (Oncorhynchus mykiss) Heads and Skipjack tuna (Katsuwonus Pelamis) Heads" Molecules 24, no. 17: 3189. https://doi.org/10.3390/molecules24173189
APA StyleLi, W., Liu, Y., Jiang, W., & Yan, X. (2019). Proximate Composition and Nutritional Profile of Rainbow Trout (Oncorhynchus mykiss) Heads and Skipjack tuna (Katsuwonus Pelamis) Heads. Molecules, 24(17), 3189. https://doi.org/10.3390/molecules24173189







