NMR-Based Metabolomics Profiling for Radical Scavenging and Anti-Aging Properties of Selected Herbs
Abstract
:1. Introduction
2. Results and Discussion
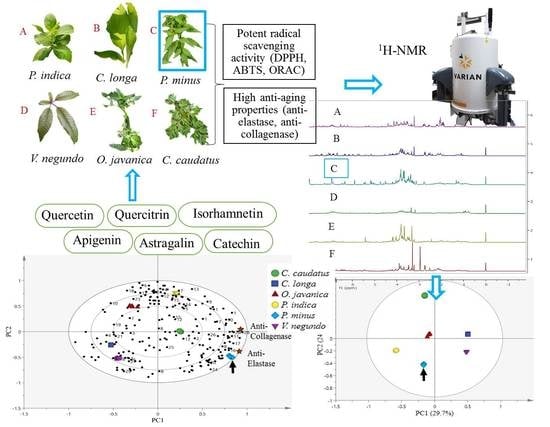
2.1. Radical Scavenging Activity of Herbs
2.2. Elastase Inhibitory Activity
2.3. Collagenase Inhibitory Activity
2.4. 1H-NMR Spectra of Herbs and Metabolites Identification
2.5. Classification of Herb Extracts by Principal Component Analysis
2.6. Correlation between Bioactivities and the Metabolites Using Partial Least-Squares Analysis (PLS)
2.7. Relative Quantification of Secondary Metabolites
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material and Sampling
3.3. Sample Preparation
3.4. Extraction
3.5. DPPH Radical Scavenging Activity
3.6. ABTS Radical Scavenging Assay
3.7. ORAC Radical Scavenging Assay
3.8. Elastase Inhibition Assay
3.9. Collagenase Inhibition Assay
3.10. Metabolite Profiling Using 1H-NMR Measurement
3.11. Bucketing of 1H-NMR Spectra
3.12. Relative Quantification of Metabolites
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raj, K.; Chanu, S.I.; Sarkar, S. Decoding complexity of aging. Cell Dev. Biol. 2012, 1, e117. [Google Scholar] [CrossRef]
- Huang, Z.M.; Yang, X.B.; Cao, W.B. Effects of Qin Ling Ke Li in the treatment of 90 patients with chronic hepatitis B. Pharm. J. Chin. Peoples Lib. Army 2001, 17, 41–44. [Google Scholar]
- Huang, Z.M.; Yang, X.B.; Cao, W.B. Modern study and clinic application of Oenanthe javanica. Pharm. J. Chin. Peoples Lib. Army 2001, 17, 266–269. [Google Scholar]
- Yang, S.A.; Jung, Y.S.; Lee, S.J.; Park, S.C.; Kim, M.J.; Lee, E.J.; Byun, H.J.; Jhee, K.H.; Lee, S.P. Hepatoprotective effects of fermented field water-dropwort (Oenanthe javanica) extract and its major constituents. Food Chem. Toxicol. 2014, 67, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Yu, Y.B.; Lee, J.H.; Hattori, M.; Lee, C.K.; Choi, J.W. Protective effect of Oenanthe javanica on the hepatic lipid peroxidation in bromobenzene-treated rats and its bioactive component. Planta Med. 1996, 62, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Q.; Huang, Z.M.; Yang, X.B.; Liu, H.Z.; Wu, G.X. In vivo and in vitro antihepatitis B virus activity of total phenolics from Oenanthe javanica. J. Ethnopharmacol. 2008, 118, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, K.H.; Lee, Y.J.; Lee, S.H.; Park, J.C.; Nam, D.H. Oenanthe javanica extract accelerates ethanol metabolism in ethanol-treated animals. BMB Rep. 2009, 42, 482–485. [Google Scholar] [CrossRef]
- Choi, H.J.; You, Y.H.; Hwang, K.T.; Lee, J.M.; Chun, J.Y.; Chung, J.W.; Shim, S.I.; Park, C.S.; Jun, W.J. Isolation and identification of compound from dropwort (Oenanthe javanica) with protective potential against oxidative stress in HepG2 cells. Food Sci. Biotechnol. 2011, 20, 1743–1746. [Google Scholar] [CrossRef]
- Muthu, C.; Ayyanar, M.; Raja, N.; Ignacimuthu, S. Medicinal plants used by traditional healers in Kancheepuram District of Tamil Nadu, India. J. Ethnobiol. Ethnomed. 2006, 2, 43. [Google Scholar] [CrossRef]
- Tiwari, O.P.; Tripathi, Y.B. Antioxidant properties of different fractions of Vitex negundo Linn. Food Chem. 2007, 100, 1170–1176. [Google Scholar] [CrossRef]
- Villaseñor, I.M.; Lamadrid, M.R.A. Comparative anti-hyperglycemic potentials of medicinal plants. J. Ethnopharmacol. 2006, 104, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, Q.Y.; Hou, T.T.; Xin, H.L.; Zheng, H.C.; Rahman, K.; Qin, L.P. Estrogen-like activities in Vitex species from China determined by a cell based proliferation assay. Pharmazie 2007, 62, 872–875. [Google Scholar] [PubMed]
- Tasduq, S.A.; Kaiser, P.J.; Gupta, B.D.; Gupta, V.K.; Johri, R.K. Negundoside, an irridiod glycoside from leaves of Vitex negundo, protects human liver cells against calcium-mediated toxicity induced by carbon tetrachloride. World J. Gastroenterol. 2008, 14, 3693–3709. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.K. Herbal Options; M/s Eastern Traders: Calcutta, India, 1996; pp. 183–215. [Google Scholar]
- Choi, M.E.; Hwang, K.J. Screening of Indonesian medicinal plants for inhibitor activity on nitric oxide production of RAW 264.7 cells and antioxidant activity. Fitoterapia 2005, 76, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.C.; Bhattacharya, P.; Biswas, R.; Bandyopadhyay, D.; Mishra, M.; Chatterjee, T.K. Hypoglycemic and antihyperglycemic activity of leaf extract of Pluchea indica Less. Orient. Pharm. Exp. Med. 2006, 6, 232–236. [Google Scholar] [CrossRef]
- Pramanik, K.C.; Biswas, R.; Mitra, A.; Bandyopadhyay, D.; Mishra, M.; Chatterjee, T.K. Tissue culture of the plant Pluchea indica (L.) Less. and evaluation of diuretic potential of its leaves. Orient. Pharm. Exp. Med. 2007, 7, 197–204. [Google Scholar] [CrossRef]
- Roslida, A.H.; Erazuliana, A.K.; Zuraini, A. Anti-inflammatory and antinoci-ceptive activities of the ethanolic extract of Pluchea indica (L) Less leaf. Pharmacol. Online 2008, 2, 349–360. [Google Scholar]
- Srisook, K.; Buapool, D.; Boonbai, R.; Simmasut, P.; Charoensuk, Y.; Srisook, E. Antioxidant and anti-inflammatory activities of hot water extract from Pluchea indica Less herbal tea. J. Med. Plant Res. 2012, 6, 4077–4081. [Google Scholar] [CrossRef]
- Burkill, I.H. A Dictionary of the Economic Products of the Malay Peninsula (Vols 1 and 2); Ministry of Agriculture, Malaysia: Kuala Lumpur, Malaysia, 1966.
- Ismail, S. Sayuran Ulam dan Penyedap Rasa; Universiti Kebangsaan Malaysia: Bangi, Malaysia, 2000. [Google Scholar]
- Shui, G.; Leong, L.P.; Shih, P.W. Rapid screening and characterization of antioxidants of Cosmos caudatus using liquid chromatography coupled with mass spectrometry. J. Chromatogr. B 2005, 827, 127–138. [Google Scholar] [CrossRef]
- Hassan, W.E.; Mahmood, M. Healing Herbs of Malaysia; Biotropics Malaysia Berhad: Selangor, Malaysia, 2006. [Google Scholar]
- Bodeker, G. Health and Beauty from the Rainforest: Malaysian Traditions of Ramuan; Didier Millet: Kuala Lumpur, Malaysia, 2009. [Google Scholar]
- Rahalison, L.; Hamburger, M.; Hostettmann, K.; Monod, M.; Frenk, E. A bioautographic agar overlay method for the detection of antifungal compounds from higher plants. Phytochem. Anal. 1991, 2, 199–203. [Google Scholar] [CrossRef]
- Rasdi, N.H.; Samah, O.A.; Ahmed, Q.U. Antimicrobial studies of Cosmos caudatus Kunth (Compositae). J. Med. Plant Res. 2010, 4, 669–673. [Google Scholar] [CrossRef]
- Loh, S.P.; Hadira, O. In vitro inhibitory potential of selected Malaysian plants against key enzymes involved in hyperglycemia and hypertension. Malays. J. Nutr. 2011, 17, 77–86. [Google Scholar] [PubMed]
- Hamidi, J.A.; Ismaili, N.H.; Ahmadi, F.B.; Lajisi, N.H. Antiviral and Cytotoxic Activities of Some Plants Used in Malaysian Indigenous Medicine. Pertanika J. Trop. Agric. Sci. 1996, 19, 129–136. [Google Scholar]
- Mackeen, M.M.; Ali, A.M.; El-Sharkawy, S.H.; Manap, M.Y.; Salleh, K.M.; Lajis, N.H.; Kawazu, K. Antimicrobial and cytotoxic properties of some Malaysian traditional vegetables (ulam). Int. J. Pharmacogn. 1997, 35, 174–178. [Google Scholar] [CrossRef]
- Sumazian, Y.; Ahmad, S.; Mansor, H.; Mahmood, M. Antioxidant activities, flavonoids, ascorbic acid and phenolic content of Malaysian vegetables. J. Med. Plant Res. 2010, 4, 881–890. [Google Scholar] [CrossRef]
- Uyub, A.M.; Nwachukwu, I.N.; Azlan, A.A.; Fariza, S.S. In-vitro antibacterial activity and cytotoxicity of selected medicinal plant extracts from Penang Island Malaysia on metronidazole-resistant-Helicobacter pylori and some pathogenic bacteria. Ethnobot. Res. Appl. 2010, 8, 95–106. [Google Scholar] [CrossRef]
- Qader, S.W.; Abdulla, M.A.; Chua, L.S.; Najim, N.; Zain, M.M.; Hamdan, S. Antioxidant, total phenolic content and cytotoxicity evaluation of selected Malaysian plants. Molecules 2011, 16, 3433–3443. [Google Scholar] [CrossRef]
- Abdullah, M.Z.; Mavaddat, M.H. Antioxidant and anticancer activities of Polygonum minus, Alpinia galanga and Etlingera elatior. Open Conf. Proc. J. 2013, 4, 203. [Google Scholar] [CrossRef]
- Almey, A.A.A.; Khan, C.A.J.; Zahir, I.S.; Suleiman, K.M.; Aisyah, M.R.; Rahim, K.K. Total phenolic content and primary antioxidant activity of methanolic and ethanolic extracts of aromatic plants leaves. Int. Food Res. J. 2010, 17, 1077–1084. [Google Scholar]
- Bunawan, H.; Talip, N.; Noor, N.M. Foliar anatomy and micromorphology of Polygonum minus Huds and their taxonomic implications. Aust. J. Crop Sci. 2011, 5, 123–127. [Google Scholar]
- Shafaei, A.; Muslim, N.S.; Nassar, Z.D.; Aisha, A.F.A.; Majid, A.M.S.A.; Ismail, Z. Antiangiogenic effect of Ficus deltoidea Jack standardised leaf extracts. Trop. J. Pharm. Res. 2014, 13, 761–768. [Google Scholar] [CrossRef]
- Gurinder, J.K.; Daljit, S.A. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement. Altern. Med. 2009, 9, 30. [Google Scholar] [CrossRef]
- Mustafa, R.A.; Hamid, A.A.; Mohamed, S.; Bakar, F.A. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. J. Food Sci. 2010, 75, C28–C35. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Natural polyphenols and diabetes: Understanding their mechanism of action. Curr. Med. Chem. 2015, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Isemura, M.; Saeki, K.; Minami, T.; Hayakawa, S.; Kimura, T.; Shoji, Y.; Sazuka, M. Inhibition of matrix metalloproteinases by tea catechins and related polyphenols. Ann. N. Y. Acad. Sci. 1999, 878, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. CMLS 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, K.S.; Han, C.S.; Yang, H.C.; Park, S.H.; Ko, K.I.; Lee, S.H.; Kim, K.H.; Lee, N.H.; Kim, J.M.; et al. Inhibitory effects of natural plants of Jeju Island on elastase and MMP-1 expression. Int. J. Cosmet. Sci. 2007, 29, 487–488. [Google Scholar] [CrossRef]
- Saragusti, A.C.; Ortega, M.G.; Cabrera, J.L.; Estrin, D.A.; Marti, M.A.; Chiabrando, G.A. Inhibitory effect of quercetin on matrix metalloproteinase 9 activity Molecular mechanism and structure–activity relationship of the flavonoid–enzyme interaction. Eur. J. Pharmacol. 2010, 644, 138–145. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based plant metabolomics: Where do we stand, where do we go? Trends Biotechnol. 2011, 29, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Khoo, L.W.; Mediani, A.; Zolkeflee, N.K.Z.; Leong, S.W.; Ismail, I.S.; Khatib, A.; Shaari, K.; Abas, F. Phytochemical diversity of Clinacanthus nutans extracts and their bioactivity correlations elucidated by NMR based metabolomics. Phytochem. Lett. 2015, 14, 123–133. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomics analysis of plants. Nat. Protoc. 2010, 5, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Choi, Y.H.; Verberne, M.; Lefeber, A.W.; Erkelens, C.; Verpoorte, R. Metabolic fingerprinting of wild type and transgenic tobacco plants by 1H NMR and multivariate analysis technique. Phytochemistry 2004, 65, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Ageing: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G. Plant Phenolics and Human Health: Biochemistry, Nutrition and Pharmacology (Vol. 1); John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Vermerris, W.; Nicholson, R. Phenolic compounds and their effects on human health. In Phenolic Compound Biochemistry; Springer: Dordrecht, The Netherlands, 2008; pp. 235–255. [Google Scholar]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Huda-Faujan, N.; Noriham, A.; Norrakiah, A.S.; Babji, A.S. Antioxidant activity of plants methanolic extracts containing phenolic compounds. Afr. J. Biotechnol. 2009, 8, 484–489. [Google Scholar]
- Urones, J.G.; Marcos, I.S.; Pérez, B.G.; Barcala, P.B. Flavonoids from Polygonum minus. Phytochemistry 1990, 29, 3687–3689. [Google Scholar] [CrossRef]
- Maizura, M.; Aminah, A.; Wan Aida, W.M. Total phenolic content and antioxidant activity of kesum (Polygonum minus), ginger (Zingiber officinale) and turmeric (Curcuma longa) extract. Int. Food Res. J. 2011, 18, 526–531. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Debelle, L.; Tamburro, A.M. Elastin: Molecular description and function. Int. J. Biochem. Cell Biol. 1999, 31, 261–272. [Google Scholar] [CrossRef]
- Fulop, T.; Khalil, A.; Larbi, A. The role of elastin peptides in modulating the immune response in aging and age-related diseases. Pathol. Biol. 2012, 60, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, A.; Oh, S.J. Age related changes of the extracellular matrix and stem cell maintenance. Prev. Med. 2012, 54, S50–S56. [Google Scholar] [CrossRef] [PubMed]
- Labat-Robert, J.; Fourtanier, A.; Boyer-Lafargue, B.; Robert, L. Age dependent increase of elastase type protease activity in mouse skin: Effect of UV-irradiation. J. Photochem. Photobiol. B Biol. 2000, 57, 113–118. [Google Scholar] [CrossRef]
- Xiao-jing, L.; Pei-dong, S.; Jing-ru, Q.; Guang-qun, C.; Cheng, Y. Elastase Inhibitory Activity of Extracts from Polygonum cuspidatum. Nat. Prod. Res. Dev. 2012, 24, 378–380. [Google Scholar]
- Kim, E.H.; Kim, J.E.; Park, S.N. Antioxidative and antiaging effects of Persicaria hydropiper L. extracts. J. Soc. Cosmet. Sci. Korea 2009, 35, 293–300. [Google Scholar]
- An, B.J.; Kwak, J.H.; Park, J.M.; Lee, J.Y.; Park, T.S.; Lee, J.T.; Son, J.H.; Jo, C.; Byun, M.W. Inhibition of enzyme activities and the anti-wrinkle effect of polyphenol isolated from the persimmon leaf (Diospyros kaki folium) on human skin. Derm. Surg. 2005, 31, 848–855. [Google Scholar] [CrossRef]
- Baylac, S.; Racine, P. Inhibition of human leukocyte elastase by natural fragrant extracts of aromatic plants. Int. J. Aromather. 1999, 14, 179–182. [Google Scholar] [CrossRef]
- Hrenn, A.; Steinbrecher, T.; Labahn, A.; Schwager, J.; Schempp, C.M.; Merfort, I. Plant phenolics inhibit neutrophil elastase. Planta Med. 2006, 72, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Mäckle, S.; Submann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Kanashiro, A.; Souza, J.G.; Kabeya, L.M.; Azzolini, A.E.C.; Lucisano-Valim, Y.M. Elastase release by stimulated neutrophils inhibited by flavonoids: Importance of the catechol group. Zeitschrift für Naturforschung C 2007, 62, 357–361. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Khalil, R.A. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharm. 2008, 75, 346–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Uyama, H.; Kobayashi, S. Inhibition effects of (+)-catechinaldehyde polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochem. Biophys. Res. Commun. 2004, 320, 256–261. [Google Scholar] [CrossRef]
- Ndlovu, G.; Fouche, G.; Tselanyane, M.; Cordier, W.; Steenkamp, V. In Vitro determination of the anti-aging potential of four southern African medicinal plants. BMC Complement. Altern. Med. 2013, 13, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Madhan, B.; Krishnamoorthy, G.; Rao, J.R.; Nair, B.U. Role of green tea polyphenols in the inhibition of collagenolytic activity by collagenase. Int. J. Biol. Macromol. 2007, 41, 16–22. [Google Scholar] [CrossRef]
- Bigg, H.F.; Clark, I.M.; Cawston, T.E. Fragments of human fibroblast collagenase: Interaction with metalloproteinase inhibitors and substrates. Biochim. Biophys. Acta-Protein Struct. Mol. Enzymol. 1994, 1208, 157–165. [Google Scholar] [CrossRef]
- Malesev, D.; Kuntić, V. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J. Serb. Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Sin, B.Y.; Kim, H.P. Inhibition of collagenase by naturally-occurring flavonoids. Arch. Pharm. Res. 2005, 28, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Mediani, A.; Abas, F.; Khatib, A.; Maulidiani, H.; Shaari, K.; Choi, Y.H.; Lajis, N.H. 1H-NMR-based metabolomics approach to understanding the drying effects on the phytochemicals in Cosmos caudatus. Food Res. Int. 2012, 49, 763–770. [Google Scholar] [CrossRef]
- Moon, J.H.; Tsushida, T.; Nakahara, K.; Terao, J. Identification of quercetin 3-O-β-d-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic. Biol. Med. 2001, 30, 1274–1285. [Google Scholar] [CrossRef]
- Qader, S.W.; Abdulla, M.A.; Chua, L.S.; Hamdan, S. Potential bioactive property of Polygonum minus Huds (kesum) review. Sci. Res. Essays 2012, 7, 90–93. [Google Scholar] [CrossRef]
- Hashim, N.H.N.; Abas, F.; Shaari, K.; Lajis, N.H. LC–DAD–ESIMS/MS characterization of antioxidant and anticholinesterase constituents present in the active fraction from Persicaria hydropiper. LWT-Food Sci Technol. 2012, 46, 468–476. [Google Scholar] [CrossRef]
- Abdullah, M.Z.; Mohd Ali, J.; Abolmaesoomi, M.; Abdul-Rahman, P.S.; Hashim, O.H. Anti-proliferative, in vitro antioxidant, and cellular antioxidant activities of the leaf extracts from Polygonum minus Huds: Effects of solvent polarity. Int. J. Food Prop. 2017, 20, S846–S862. [Google Scholar] [CrossRef]
- Son, H.S.; Kim, K.M.; Van Den Berg, F.; Hwang, G.S.; Park, W.M.; Lee, C.H.; Hong, Y.S. 1H nuclear magnetic resonance-based metabolomic characterization of wines by grape varieties and production areas. J. Agric. Food Chem. 2008, 56, 8007–8016. [Google Scholar] [CrossRef]
- Lee, S.Y.; Abas, F.; Khatib, A.; Ismail, I.S.; Shaari, K.; Zawawi, N. Metabolite profiling of Neptunia oleracea and correlation with antioxidant and α-glucosidase inhibitory activities using 1H NMR-based metabolomics. Phytochem. Lett. 2016, 16, 23–33. [Google Scholar] [CrossRef]
- Wheelock, A.M.; Wheelock, C.E. Trials and Tribulations of Omics Data Analysis: Assessing Quality of SIMCA-Based Multivariate Models Using Examples from Pulmonary Medicine. Mol. Biosyst. 2013, 9, 2589–2596. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.N.; Ku, K.M.; Kang, D.; Kim, J.S.; Park, J.H.Y.; Lee, C.H. A correlation between antioxidant activity and metabolite release during the blanching of Chrysanthemum coronarium L. Biosci. Biotechnol. Biochem. 2011, 75, 674–680. [Google Scholar] [CrossRef]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Antiinflammatory plant flavonoids and cellular action mechanisms. J. Pharm. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef]
- Alasbahi, R.; Melzig, M. The In Vitro Inhibition of Human Neutrophil Elastase Activity by some Yemeni Medicinal Plants. Sci. Pharm. 2008, 76, 471–483. [Google Scholar] [CrossRef]
- Kacem, R. Phenolic compounds from medicinal plants as Natural anti-elastase products for the therapy of pulmonary emphysema. J. Med. Plant Res. 2013, 7, 3499–3507. [Google Scholar] [CrossRef]
- Mandrone, M.; Lorenzi, B.; Venditti, A.; Guarcini, L.; Bianco, A.; Sanna, C.; Ballero, M.; Poli, F.; Antognoni, F. Antioxidant and anti-collagenase activity of Hypericum hircinum L. Ind. Crop. Prod. 2015, 76, 402–408. [Google Scholar] [CrossRef]
- Xu, G.H.; Ryoo, I.J.; Kim, Y.H.; Choo, S.J.; Yoo, I.D. Free radical scavenging and antielastase activities of flavonoids from the fruits of Thuja orientalis. Arch. Pharm. Res. 2009, 32, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Nema, N.K.; Maity, N.; Sarkar, B.; Mukherjee, P.K. Cucumis sativus fruit-potential antioxidant, anti-hyaluronidase, and anti-elastase agent. Arch. Derm. Res. 2011, 303, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Shon, M.S.; Lee, Y.; Song, J.H.; Park, T.; Lee, J.K.; Kim, M.; Park, E.; Kim, G.N. Anti-aging potential of extracts prepared from fruits and medicinal herbs cultivated in the Gyeongnam area of Korea. Prev. Nutr. Food Sci. 2014, 19, 178. [Google Scholar] [CrossRef]
- Chattuwatthana, T.; Okello, E. Anti-collagenase, anti-elastase and antioxidant activities of pueraria candollei var. mirifica root extract and coccinia grandis fruit juice extract: An in vitro study. Eur. J. Med. Plants 2015, 5, 318. [Google Scholar] [CrossRef]
- Ghimeray, A.K.; Jung, U.S.; Lee, H.Y.; Kim, Y.H.; Ryu, E.K.; Chang, M.S. In vitro antioxidant, collagenase inhibition, and in vivo anti-wrinkle effects of combined formulation containing Punica granatum, Ginkgo biloba, Ficus carica and Morus alba fruits extract. Clin. Cosmet. Investig. Dermatol. 2015, 8, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef]
- Bravo, K.; Alzate, F.; Osorio, E. Fruits of selected wild and cultivated Andean plants as sources of potential compounds with antioxidant and anti-aging activity. Ind. Crop. Prod. 2016, 85, 341–352. [Google Scholar] [CrossRef]
- Kim, D.B.; Shin, G.H.; Kim, J.M.; Kim, Y.H.; Lee, J.H.; Lee, J.S.; Song, H.J.; Choe, S.Y.; Park, I.J.; Cho, J.H.; et al. Antioxidant and anti-ageing activities of citrus-based juice mixture. Food Chem. 2016, 194, 920–927. [Google Scholar] [CrossRef]
- Kolakul, P.; Sripanidkulchai, B. Phytochemicals and anti-aging potentials of the extracts from Lagerstroemia speciosa and Lagerstroemia floribunda. Ind. Crop. Prod. 2017, 109, 707–716. [Google Scholar] [CrossRef]
- Azizan, K.A.; Baharum, S.N.; Ressom, H.W.; Noor, N.M. GC-MS analysis and PLS-DA validation of the trimethyl silyl-derivatization techniques. Am. J. Appl. Sci. 2012, 9, 1124–1136. [Google Scholar]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Trygg, J.; Wikstrom, C.; Wold, S. Multivariate and megavariate data analysis part I: Basic principles and applications. Umetrics Acad. 2006, 1, 65–67. [Google Scholar]
- Sim, G.S.; Lee, B.C.; Cho, H.S.; Lee, J.W.; Kim, J.H.; Lee, D.H.; Kim, J.H.; Pyo, H.B.; Moon, D.C.; Oh, K.W.; et al. Structure activity relationship of antioxidative property of flavonoids and inhibitory effect on matrix metalloproteinase activity in UVA-irradiated human dermal fibroblast. Arch. Pharm. Res. 2007, 30, 290. [Google Scholar] [CrossRef] [PubMed]
- Mediani, A.; Abas, F.; Khatib, A.; Tan, C.P.; Ismail, I.S.; Shaari, K.; Ismail, A.; Lajis, N.H. Relationship between metabolites composition and biological activities of Phyllanthus niruri extracts prepared by different drying methods and solvents extraction. Plant Foods Hum. Nutr. 2015, 70, 184–192. [Google Scholar] [CrossRef]
- Kong, K.W.; Mat-Junit, S.; Aminudin, N.; Ismail, A.; Abdul-Aziz, A. Antioxidant activities and polyphenolics from the shoots of Barringtonia racemosa (L.) Spreng in a polar to apolar medium system. Food Chem. 2012, 134, 324–332. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Huang, W.Y.; Majumder, K.; Wu, J. Oxygen radical absorbance capacity of peptides from egg white protein ovotransferrin and their interactions with phytochemicals. Food Chem. 2010, 123, 635–641. [Google Scholar] [CrossRef]
- Kraunsoe, J.A.E.; Claridge, T.D.W.; Lowe, G. Inhibition of human leukocyte and porcine pancreatic elastase by homologues of bovine pancreatic trypsin inhibitor. Biochemistry 1996, 35, 9090–9096. [Google Scholar] [CrossRef] [PubMed]
- Van-Wart, H.E.; Steinbrink, D.R. A continuous spectrophotometric assay for Clostridium histolyticum collagenase. Anal. Biochem. 1981, 113, 356–365. [Google Scholar] [CrossRef]
Sample Availability: Samples of the herbs (Curcurma longa, Oenanthe javanica, Vitex negundo, Pluchea indica, Cosmos caudatus and Persicaria minus) are available from the authors. |






| Metabolites | 1H-NMR Characteristics Signals | Herbs | |||||
|---|---|---|---|---|---|---|---|
| Curcuma Longa | Oenanthe Javanica | Vitex Negundo | Pluchea Indica | Cosmos Caudatus | Persicaria Minus | ||
| Quercetin | 6.83 (d, J = 8.5 Hz) 6.85 (d, J = 8.5 Hz) 6.98 (d, J = 8 Hz) | + | + | + | + | + | + |
| Quercetin 3-O-rhamnoside | 6.83 (d, J = 8.5 Hz) 6.89 (d, J = 8.5 Hz) 6.79 (d, J = 8.5 Hz) Methyl signal: 0.91 (d, J = 6.5 Hz) | + | + | − | + | + | + |
| (3) Quercetin 3-O-glucoside | 6.86 (d, J = 8.5 Hz) 6.83 (d, J = 8.5 Hz) 5.16 (d, J = 7.5 Hz) 5.04 (d, J = 8.0 Hz) Anomeric proton glucosyl 4.97 (d, J = 7.5 Hz) | + | + | − | + | + | + |
| Quercetin 3-O-glucuronide | 7.64 (s) 6.85 (d, J = 8.5 Hz) 6.87 (d, J = 8.0 Hz) 3.73 (d, J = 9.0 Hz) | − | − | + | + | + | + |
| (5) Quercetin 3-O-arabinofuranoside | 7.47 (dd, J = 8.5 Hz, 1.7 Hz) | − | − | − | − | − | + |
| Rutin | 6.95 (d, J = 8.5 Hz) 7.57 (d, J = 2.0 Hz) 6.92 (d, J = 8.5 Hz) Anomeric proton glucosyl 4.97 (d, J = 7.5 Hz) 4.99 (d, J = 7.5 Hz) | − | + | + | − | + | + |
| Myricetin derivatives | 7.05 (s) 6.51 (d, J = 2.0 Hz) 6.30 (d, J = 2.0 Hz) | − | − | − | − | − | + |
| Catechin | 4.59 (d, J = 7.5 Hz) 4.60 (d, J = 7.5 Hz) 4.61 (d, J = 7.5 Hz) 4.60 (d, J = 8.0 Hz) 3.93 (m) 2.83 (m) 2.84 (m) 2.56(dd, J = 16.5 Hz, 8.0 Hz) | + | + | + | + | + | + |
| Epicatechin | 4.30 (s) 4.99 (s) 5.00 (s) 5.01 (s) 5.03 (s) 7.08 (s) 7.10 (s) 7.11 (s) | + | + | + | + | + | + |
| Isorhamnetin | 3.84 (s) 3.85 (s) 6.21 (d, J = 8.0 Hz) 6.23 (d, J = 8.0 Hz) 6.92 (d, J = 8.5 Hz) | − | − | + | − | + | + |
| Astragalin | 6.85 (d, J = 8.5 Hz) 6.56 (d, J = 2.5 Hz) | − | − | − | + | − | + |
| Chlorogenic acid | 2.08 (m) 2.20 (m) Signal for quinic 4.04 (m) 1.88 (d, J = 12.0 Hz) 1.90 (d, J = 10.5 Hz) | − | − | − | − | + | + |
| (13) Gallic acid | 7.03 (s) | + | + | − | − | − | + |
| Coumaric acid | 7.17 (d, J = 8.0 Hz) 7.18 (d, J = 8.5 Hz) 7.06 (s) | − | + | − | − | + | + |
| Ascorbic acid | 4.54 (d, J = 7.5 Hz) 3.29 (m) | − | − | + | − | − | + |
| (16) α-glucose | 5.20 (d, J = 3.5 Hz) | − | − | + | − | + | + |
| (17) β-glucose | 4.62 (d, J = 7.5 Hz) | + | + | + | − | + | + |
| (18) Fructose | 4.20 (d, J = 9.0 Hz) 4.20 (d, J = 8.0 Hz) | + | + | + | + | − | + |
| (19) Sucrose | 5.44 (d, J = 3.5 Hz) 5.42 (d, J = 3.5 Hz) | + | + | + | + | + | + |
| (20) Fatty acid | 1.34 (m) 1.36 (m) | − | − | − | − | + | + |
| (21) Formic acid | 8.48 (s) | + | − | − | − | + | − |
| (22) Fumaric acid | 6.56 (s) | − | + | − | − | − | − |
| (23) Choline | 3.24 (s) 3.23 (s) 3.25 (s) 3.25 (s) 3.22 (s) | + | + | + | + | + | + |
| (24) Alanine | 1.51 (d, J = 7.5 Hz) 1.50 (d, J = 7.0 Hz) 1.49 (d, J = 7.0 Hz) | + | + | − | + | + | + |
| (25) Valine | 1.08 (d, J = 7.0 Hz) 1.07 (d, J = 7.0 Hz) 1.05 (d, J = 7.5 Hz) | + | + | + | + | + | + |
| (26) 3-methylxanthine | 8.02 (s) | − | − | − | − | − | + |
| (27) Serotonin | 7.28 (s) | + | − | + | − | − | − |
| (28) Apigenin | 6.95 (d, J = 8.5 Hz) | − | − | − | − | + | + |
| (29) d-Limonene | 1.72 (s) 1.89 (m) | + | − | + | − | − | − |
| Chemical Shift (ppm) | Metabolites | VIP Values | |
|---|---|---|---|
| Radical Scavenging Activity Biplot | Anti-#ging Properties Biplot | ||
| 6.78 | Quercetin 3-O-rhamnoside | 2.01017 | 2.43408 |
| 6.94 | Apigenin | 1.86661 | 1.99206 |
| 6.58 | Astragalin | 1.31507 | 1.43942 |
| 6.86 | Quercetin | 1.30401 | 1.43471 |
| 2.82 | Catechin | 1.07724 | 1.24570 |
| 6.22 | Isorhamnetin | 1.03878 | 1.23268 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussin, M.; Abdul Hamid, A.; Abas, F.; Ramli, N.S.; Jaafar, A.H.; Roowi, S.; Abdul Majid, N.; Pak Dek, M.S. NMR-Based Metabolomics Profiling for Radical Scavenging and Anti-Aging Properties of Selected Herbs. Molecules 2019, 24, 3208. https://doi.org/10.3390/molecules24173208
Hussin M, Abdul Hamid A, Abas F, Ramli NS, Jaafar AH, Roowi S, Abdul Majid N, Pak Dek MS. NMR-Based Metabolomics Profiling for Radical Scavenging and Anti-Aging Properties of Selected Herbs. Molecules. 2019; 24(17):3208. https://doi.org/10.3390/molecules24173208
Chicago/Turabian StyleHussin, Mahanom, Azizah Abdul Hamid, Faridah Abas, Nurul Shazini Ramli, Ahmad Haniff Jaafar, Suri Roowi, Nordiana Abdul Majid, and Mohd Sabri Pak Dek. 2019. "NMR-Based Metabolomics Profiling for Radical Scavenging and Anti-Aging Properties of Selected Herbs" Molecules 24, no. 17: 3208. https://doi.org/10.3390/molecules24173208