Comparative Theoretical Studies on a Series of Novel Energetic Salts Composed of 4,8-Dihydrodifurazano[3,4-b,e]pyrazine-based Anions and Ammonium-based Cations
Abstract
:1. Introduction
2. Results and Discussions
2.1. Crystal Density
2.2. Heats of Formation
2.3. Energetic Properties
2.4. Impact Sensitivity
2.5. Gibbs Free Energies of Formation
2.6. Synthesis of Triaminoguanidinium 4,8-Dihydrodifurazano[3,4-b,e]pyrazine
3. Computational Methods
3.1. Calculations of Density
3.2. Calculations of Heats of Formation
3.3. Calculations of Energetic Properties
3.4. Calculations of Impact Sensitivity
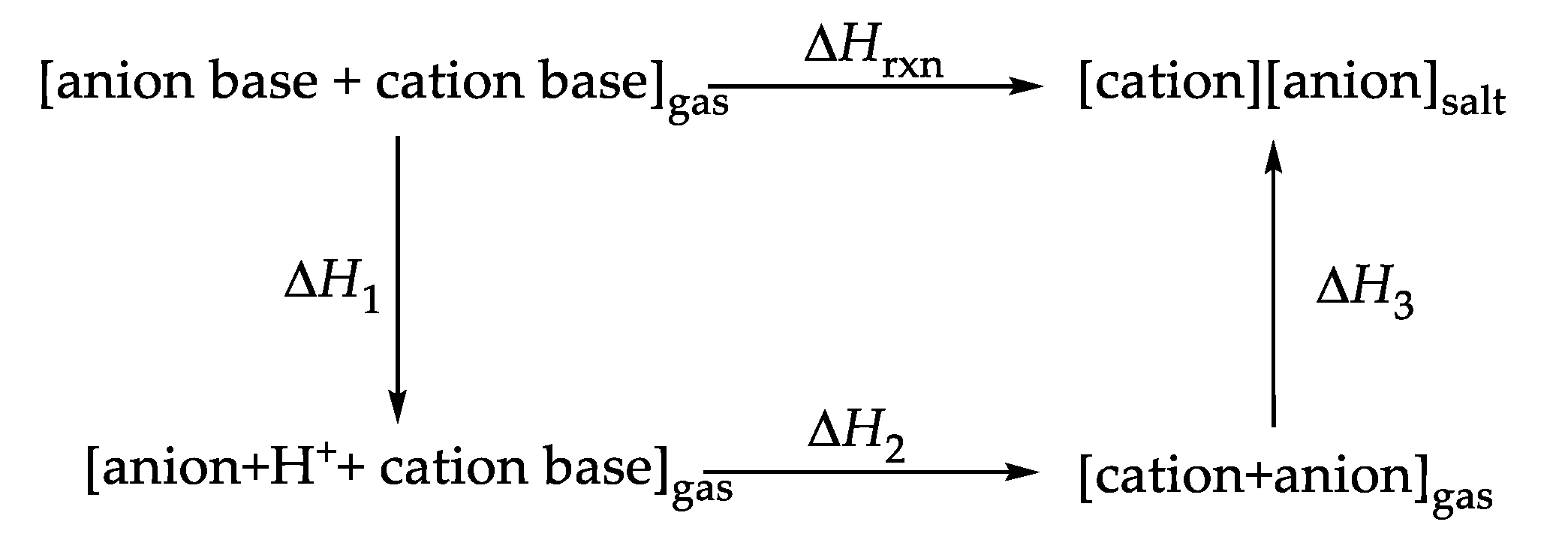
3.5. Calculations of Gibbs Free Energies of Formation
4. Materials and Methods
4.1. General Methods
4.2. Synthesis and Characterization of Energetic Materials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stinecipher, M.; Lee, K.Y.; Hiskey, M. New high-nitrogen energetic materials for gas generators in space ordnance. Off. Sci. Tech. Inf. Tech. Rep. 1995, 2857. [Google Scholar]
- Venugopal, T.; Ping, Y.; Zhang, J.H.; Parrish, D.A.; Shreeve, J.M. 1,2,3-Triazolo[4,5,-e]furazano[3,4,-b]pyrazine 6-oxide–a fused heterocycle with a roving hydrogen forms a new class of insensitive energetic materials. Chemistry 2014, 20, 542–548. [Google Scholar]
- Zhang, J.; Shreeve, J.M. Nitroaminofurazans with azo and azoxy linkages: A comparative study of structural, electronic, physicochemical, and energetic properties. J. Phys. Chem. C 2015, 119, 12887–12895. [Google Scholar] [CrossRef]
- Snyder, C.J.; Wells, L.A.; Chavez, D.E.; Imler, G.H.; Parrish, D.A. Polycyclic N-oxides: High performing, low sensitivity energetic materials. Chem. Commun. 2019, 55, 2461–2464. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tian, J.; Qi, X.; Wang, K.; Jin, Y.; Wang, B.; Zhang, Q. Synthesis of 4,8-dinitraminodifurazano[3,4-b,e]pyrazine derived nitrogen-rich salts as potential energetic materials. Chem. Select 2018, 3, 849–854. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Xiong, H.L.; Lin, Q.H.; Cheng, G.B.; Yang, H.W. Synthesis and characterization of 4,5-bis(tetrazol-5-yl)-1,2,3-triazole and its energetic salts. Chin. J. Explos. Propellants 2017, 40, 41–46. [Google Scholar]
- Peng, M.; Yong, P.; Jiang, J.; Zhu, S. A novel energetic perchlorate amine salt: Synthesis, properties, and density functional theory calculation. J. Energ. Mater. 2017, 35, 1–15. [Google Scholar]
- Zhang, X.; Gong, X. Theoretical studies on the energetic salts of substituted 3,3′-amino-N,N′-azo-1,2,4-triazoles: The role of functional groups. J. Chem. Eng. Data 2015, 60, 2869–2878. [Google Scholar] [CrossRef]
- Yin, P.; Shreeve, J.M. From N-nitro to N-nitroamino: Preparation of high-performance energetic materials by introducing nitrogen-containing ions. Angew. Chem. Int. Ed. Engl. 2016, 54, 14513–14517. [Google Scholar] [CrossRef]
- Liu, W.; Lin, Q.H.; Yang, Y.Z.; Zhang, X.J.; Li, Y.C.; Lin, Z.H.; Pang, S.P. Energetic salts based on an oxygen-containing cation: 2,4-diamino-1,3,5-triazine-6-one. Chem–Asian J 2014, 9, 479–486. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Du, Z.M.; Han, Z.Y.; Zhang, Y.H.; He, C.L. Nitrogen-rich energetic salts: Both cations and anions contain tetrazole rings. J. Energ. Mater. 2016, 34, 183–196. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, W.; Xiao, H. Comparative theoretical studies of energetic substituted carbon- and nitrogen-bridged difurazans. J. Phys. Chem. A 2010, 114, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Eberspächer, M.; Klapötke, T.M. Nitrogen-rich salts based on the energetic 5,5′-(hydrazine-1,2-diyl)bis[1h-tetrazolide] anion. Helv. Chim. Acta 2010, 92, 977–996. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Computational analysis of polyazoles and their N-oxides. Struct. Chem. 2017, 28, 1045–1063. [Google Scholar] [CrossRef]
- Ma, Q.; Lu, Z.; Liao, L.; Huang, J.; Liu, D.; Li, J.; Fan, G. 5,6-Di(2-fluoro-2,2-dinitroethoxy)furazano [3,4-b]pyrazine: A high performance melt-cast energetic material and its polycrystalline properties. Rsc Adv. 2017, 7, 38844–38852. [Google Scholar] [CrossRef]
- Zheng, C.; Chu, Y.; Xu, L.; Wang, F.; Lei, W.; Xia, M.; Gong, X. Theoretical studies on a new furazan compound bis[4-nitramino-furazanyl-3-azoxy]azofurazan (ADNAAF). J. Mol. Model. 2016, 22, 1–9. [Google Scholar] [CrossRef]
- Guillou, S.; Jacob, G.; Terrier, F.; Goumont, R. An unexpected synthesis of 7-azidofurazano[3,4-b,e]tetrazolopyrazine. Tetrahedron 2009, 65, 8891–8895. [Google Scholar] [CrossRef]
- Xiang, F.; Wu, Q.; Zhu, W.; Xiao, H. Comparativetheoretical studies on energetic ionicsalts composed of heterocycle-functionalized nitraminofurazanate-basedanions and triaminoguanidinium cation. J. Chem. Eng. Data 2014, 59, 295–306. [Google Scholar] [CrossRef]
- Wang, R.; Guo, Y.; Zeng, Z.; Twamley, B.; Shreeve, J.M. Furazan-functionalized tetrazolate-based salts: A new family of insensitive energetic materials. Chemistry 2010, 15, 2625–2634. [Google Scholar] [CrossRef]
- Xiang, F.; Wu, Q.; Zhu, W.; Xiao, H. A comparative theoretical study of heterocycle-functionalized tetrazolate- and tetrazolate-1-oxide-based dianionic salts. Can. J. Chem. 2013, 91, 1233–1242. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Qi, X.J.; Song, S.W.; Wang, K.C.; Jin, Y.H.; Liu, T.L.; Zhang, Q.H. Design and synthesis of polycyclic DFP-based low-sensitivity energetic materials with excellent thermal stability. Chin. J. Energ. Mater. 2018, 26, 901–909. [Google Scholar]
- Starchenkov, I.B.; Andrianov, V.G.; Mishnev, A.F. Chemistry of furazano[3,4-b]pyrazine. 1. Synthesis and thermodynamic appraisal of 4,8-dihydrodifurazano[3,4-b,e]pyrazine and its derivatives. Chem. Heterocycl. Compd. 1997, 33, 216–228. [Google Scholar] [CrossRef]
- Liu, N.; Lian, P.; Lai, W.P.; Li, H.; Wang, B.Z. Synthesis, characterization and performance of difurazanopyrazine derivatives. Chin. J. Explos. Propellants 2014, 22, 473–477. [Google Scholar]
- Sheremetev, A.B.; Yudin, I.L. Advances in the chemistry of furazano[3,4-b]pyrazines and their analogues. Russ. Chem. Rev. 2003, 72, 87–100. [Google Scholar] [CrossRef]
- Huynh, M.H.V.; Hiskey, M.A.; Chavez, D.E.; Gilardi, R.D. Preparation, characterization, and properties of 7-nitrotetrazolo[1,5-f]furazano[4,5-b]pyridine 1-oxide. J. Energ. Mater. 2005, 23, 99–106. [Google Scholar] [CrossRef]
- Shreeve, J.N.; Tang, Y.; Dharavath, S.; Imler, G.; Parrish, D. Nitramino- and dinitromethyl-substituted 1,2,4-triazole derivatives as high-performance energetic materials. Chem. Eur. J. 2017, 23. [Google Scholar]
- Zhang, X.; Zhu, W.; Tao, W.; Zhang, C.; Xiao, H. Densities, heats of formation, energetic properties, and thermodynamics of formation of energetic nitrogen-rich salts containing substituted protonated and methylated tetrazole cations: A computational study. J. Phys. Chem. C 2010, 114, 13142–13152. [Google Scholar] [CrossRef]
- Wang, K.; Fu, X.L.; Tang, Q.F.; Li, H.; Shu, Y.J.; Li, J.Q.; Pang, W.P. Theoretical investigations on novel energetic salts composed of 4-nitro-7-(4-nitro-1,2,3-triazol-1-olate)-furazano[3,4-d]pyridazine-based anions and ammonium-based cations. Comput. Mater. Sci. 2018, 146, 230–239. [Google Scholar] [CrossRef]
- Long, L.; Zhang, Y.; Li, Z.; Zhang, S. Nitrogen-rich energetic 4-R-5-nitro-1,2,3-triazolate salts (R = –CH3, –NH2, –N3, –NO2 and –NHNO2) as high performance energetic materials. J. Mater. Chem. A 2015, 3, 14768–14778. [Google Scholar]
- Li, Y.N.; Liu, N.; Lian, P.; Ge, Z.X.; Wang, B.Z. Synthesis and properties for two N-amino derivatives of 4,8-dihydrodifurazano[3,4-b,e]pyrazine. Chin. J. Energ. Mater. 2014, 22, 129–131. [Google Scholar]
- Kamlet, M.J.; Jacobs, S.J. Chemistry of detonations. I. A simple method for calculating detonation properties of C–H–N–O explosives. J. Chem. Phys. 2003, 48, 23–35. [Google Scholar] [CrossRef]
- Shen, C.; Wang, P.; Lu, M. Molecular design and property prediction for a series of novel dicyclic cyclotrimethylene trinitramines (RDX) derivatized as high energy density materials. J. Phys. Chem. A 2015, 119, 8250–8255. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Li, H.; Guo, W.; Geng, X.; Wang, J. Nano cyclotetramethylene tetranitramine particles prepared by a green recrystallization process. Propell. Explos. Pyrotech. 2014, 39, 701–706. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. High performance, low sensitivity: Conflicting or compatible? Propell. Explos. Pyrotech. 2016, 41, 414–425. [Google Scholar] [CrossRef]
- Frem, D. The specific impulse as an important parameter for predicting chemical high explosives Performance. Z Anorg. Allg. Chem. 2018, 644, 1781–1789. [Google Scholar] [CrossRef]
- Keshavarz, M.H.; Pouretedal, H.R.; Semnani, A. Novel correlation for predicting impact sensitivity of nitroheterocyclic energetic molecules. J. Hazard. Mater. 2007, 141, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Lane, P.; Murray, J.S. Sensitivities of ionic explosives. Mol. Phys. 2017, 115, 497–509. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, J.J.; Zhu, W.H. A comparison of high-level theoretical methods to predict the heats of formation of azo compounds. J. Mol. Struct. 2010, 956, 55–60. [Google Scholar] [CrossRef]
- Jursic, B.S. Computing the heat of formation for cubane and tetrahedrane with density functional theory and complete basis set ab initio methods. J. Mol. Struct. 2000, 499, 137–140. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Head, G.M.; Pople, J.A.; Frisch, M.J. A direct MP2 gradient method. Chem. Phys. Lett. 1988, 153, 503–513. [Google Scholar] [CrossRef]
- Xiang, F.; Zhu, W.; Xiao, H. Theoretical studies of energetic nitrogen-rich ionic salts composed of substituted 5-nitroiminotetrazolate anions and various cations. J. Mol. Model. 2013, 19, 3103–3118. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Shreeve, J.M. Rapid and accurate estimation of densities of room-temperature ionic liquids and salts. J. Phys. Chem. A 2007, 111, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Rice, B.M.; Hare, J.J.; Byrd, E.F. Accurate predictions of crystal densities using quantum mechanical molecular volumes. J. Phys. Chem. A 2007, 111, 10874–10879. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Martinez, J.; Murray, J.S.; Concha, M.C.; Toro-Labbé, A. An electrostatic interaction correction for improved crystal density prediction. Mol. Phys. 2009, 107, 2095–2101. [Google Scholar] [CrossRef]
- Bouhmaida, N.; Ghermani, N.E. Elusive contribution of the experimental surface molecular electrostatic potential and promolecule approximation in the empirical estimate of the crystal density. J. Chem. Phys. 2005, 122, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhu, W.; Xiao, H. Structure-property relationships of energetic nitrogen-rich salts composed of triaminoguanidinium or ammonium cation and tetrazole-based anions. J. Mol. Graph. Model. 2013, 40, 54–63. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Tudelat, D.; Glasser, L. Lattice potential energy estimation for complex ionic salts from density measurements. Inorg. Chem. 2002, 41, 2364–2369. [Google Scholar] [CrossRef]
- Zhu, W.; Yan, Q.; Li, J.; Cheng, B.; Shao, Y.; Xia, X.; Xiao, H. Prediction of the properties and thermodynamics of formation for energetic nitrogen-rich salts composed of triaminoguanidiniumcation and 5-nitroiminotetrazolate-based anions. J. Comput. Chem. 2012, 33, 1781–1789. [Google Scholar] [CrossRef]
- Byrd, E.F.C.; Rice, B.M. Improved prediction of heats of formation of energetic materials using quantum mechanical calculations. J. Phys. Chem. A 2006, 110, 1005–1013. [Google Scholar] [CrossRef]
- Rayne, S.; Forest, K. Estimated gas-phase standard state enthalpies of formation for organic compounds using the Gaussian-4 (G4) and W1BD theoretical methods. J. Chem. Eng. Data 2010, 55, 5359–5364. [Google Scholar] [CrossRef]
- Byrd, E.F.C.; Rice, B.M. A comparison of methods to predict solid phase heats of formation of molecular energetic salts. J. Phys. Chem. A 2009, 113, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, W.; Xiao, H. Theoretical studies on heats of formation, detonation properties, and bond dissociation energies of monofurazan derivatives. Int. J. Quantum Chem. 2010, 110, 1549–1558. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Dickinson, C. Chemistry of detonations. III. Evaluation of the simplified calculational method for Chapman-Jouguet detonation pressures on the basis of available experimental information. J. Chem. Phys. 1968, 48, 43–50. [Google Scholar] [CrossRef]
- Frem, D. Simple correlations for the estimation of propellants specific impulse and the gurney velocity of high explosives. Combus. Sci. Technol. 2016, 188, 77–81. [Google Scholar] [CrossRef]
- Michalchuk, A.A.L.; Trestman, M.; Rudić, S.; Portius, P.; Fincham, P.T.; Pulham, C.R.; Morrison, C.A. Predicting the reactivity of energetic materials: An ab initio multi-phonon approach. J. Mater. Chem. A 2019. [CrossRef]
- Keshavarz, M.H. A new general correlation for predicting impact sensitivity of energetic compounds. Propell. Explos. Pyrotech. 2013, 38, 754–760. [Google Scholar] [CrossRef]
- Keshavarz, M.H. Prediction of impact sensitivity of nitroaliphatic, nitroaliphatic containing other functional groups and nitrate explosives. J. Hazard. Mater. 2007, 148, 648–652. [Google Scholar] [CrossRef]
- Glasser, L.; Jenkins, H.D.B. Standard absolute entropies, S°298, from volume or density: Part II. organic liquids and solids. Thermochim. Acta 2004, 414, 125–130. [Google Scholar] [CrossRef]
- Liu, N.; Wang, B.Z.; Li, H.; Li, Y.N.; Lai, W.P. Synthesis and thermal performance of 4H,8H-difurazano[3,4-b:3′,4′-e]pyrazine. Chin. J. Explos. Propellants 2014, 37, 12–16. [Google Scholar]
- National Military Standard of China. Experimental Methods of Sensitivity and Safety GJB/772A-97; COSTIND: Beijing, China, 1997. [Google Scholar]
Sample Availability: Samples of the compound triaminoguanidinium 4,8-dihydrodifurazano[3,4-b,e]pyrazine are available from the authors. |

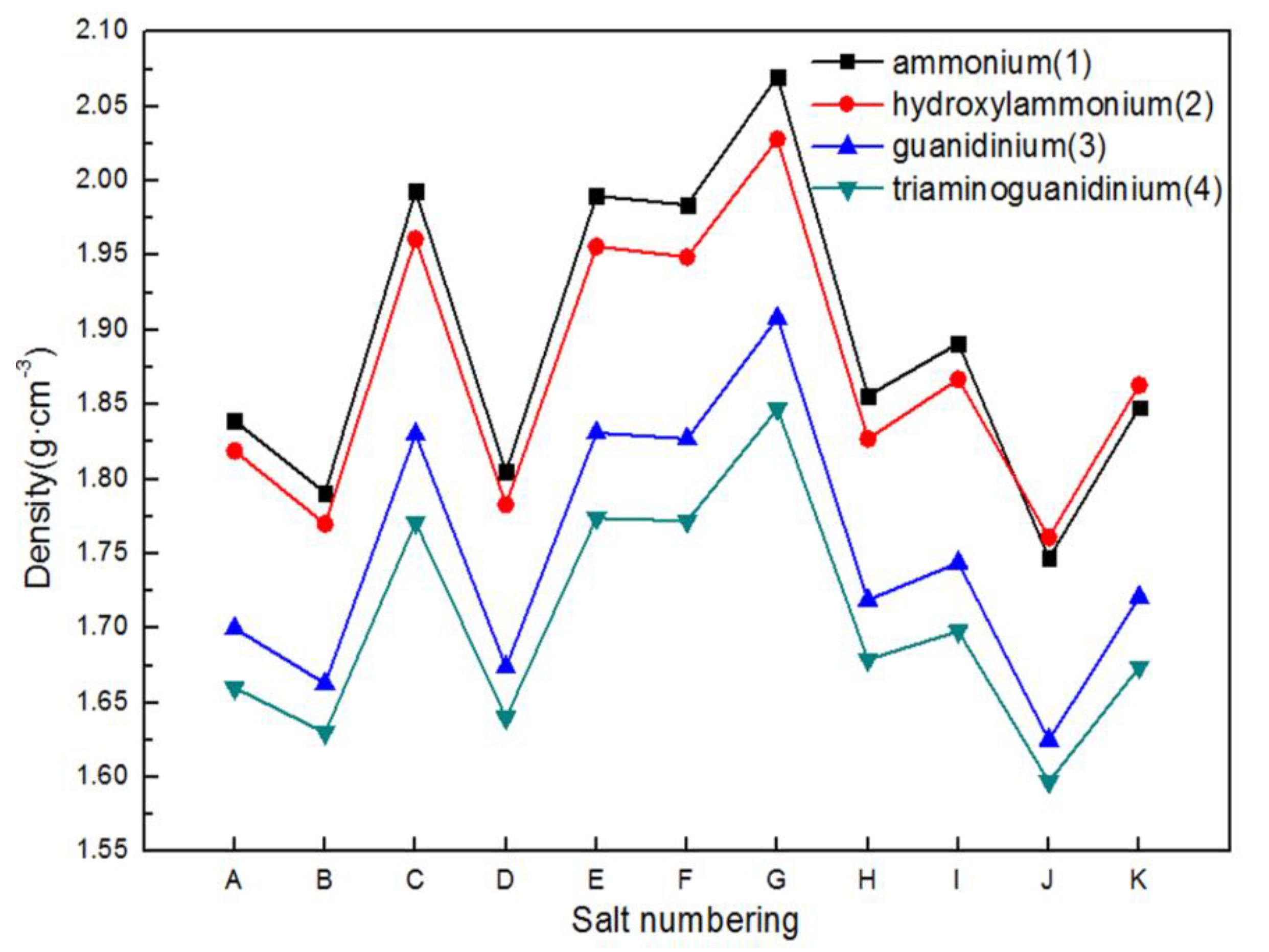
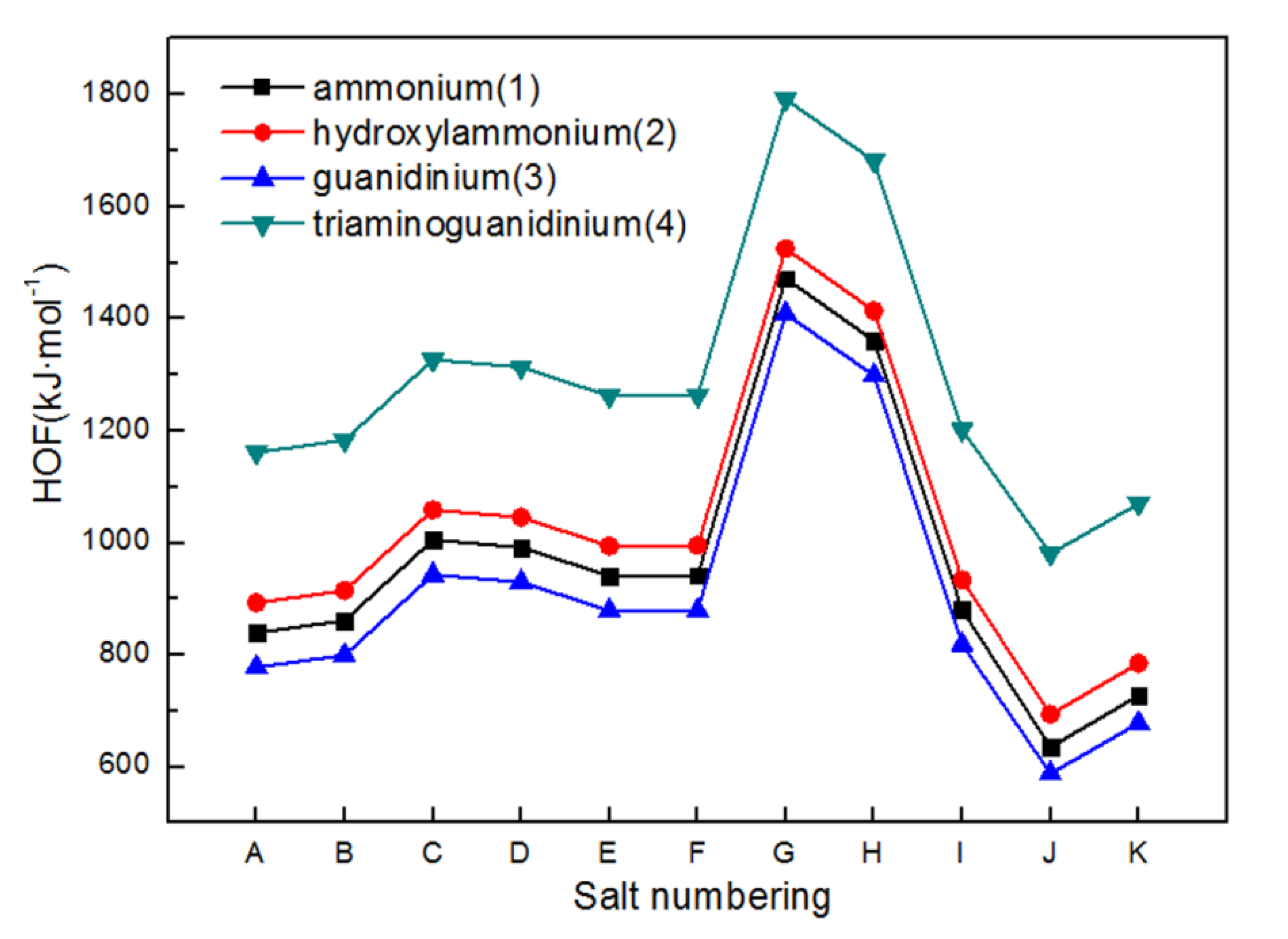

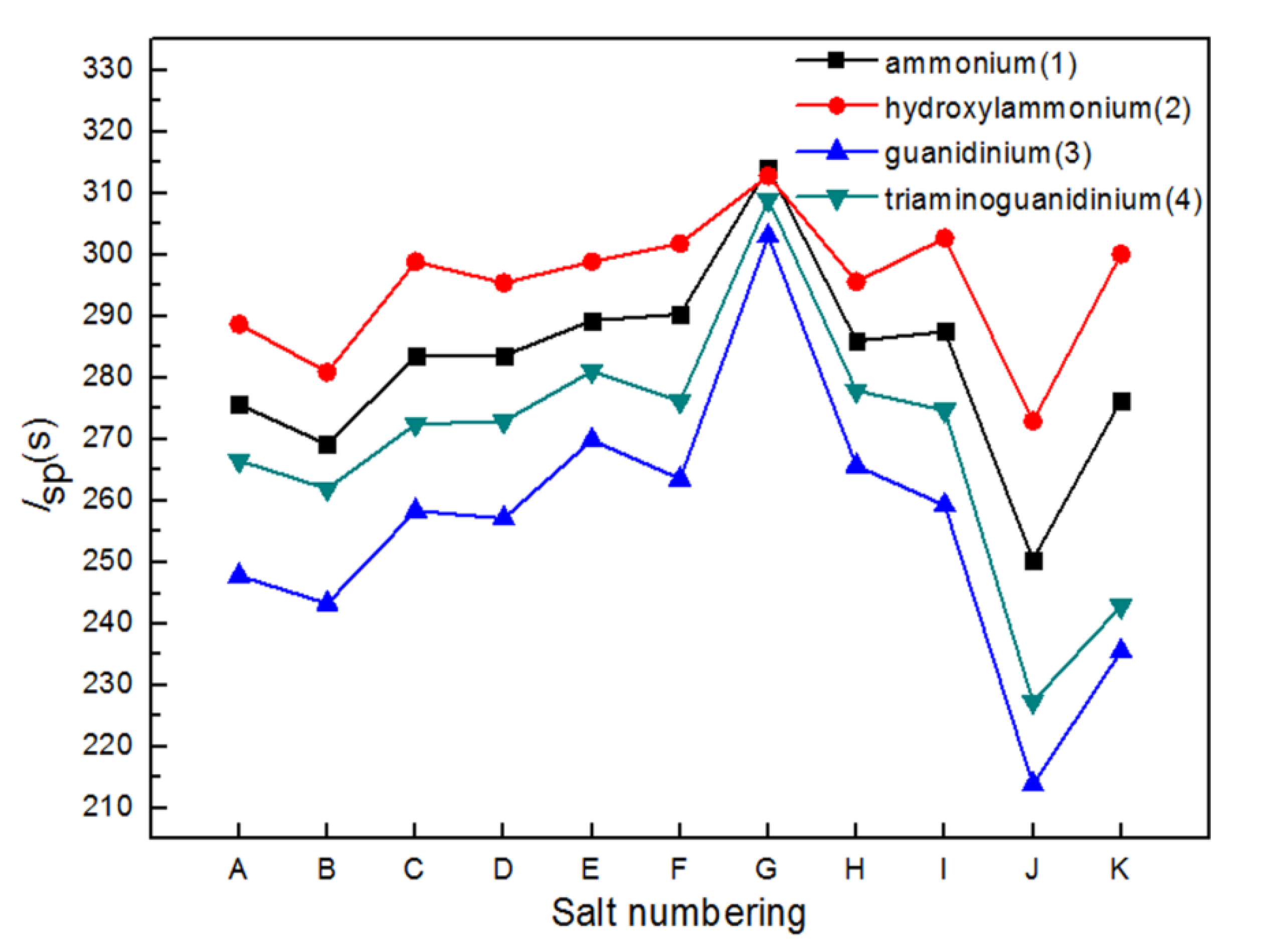

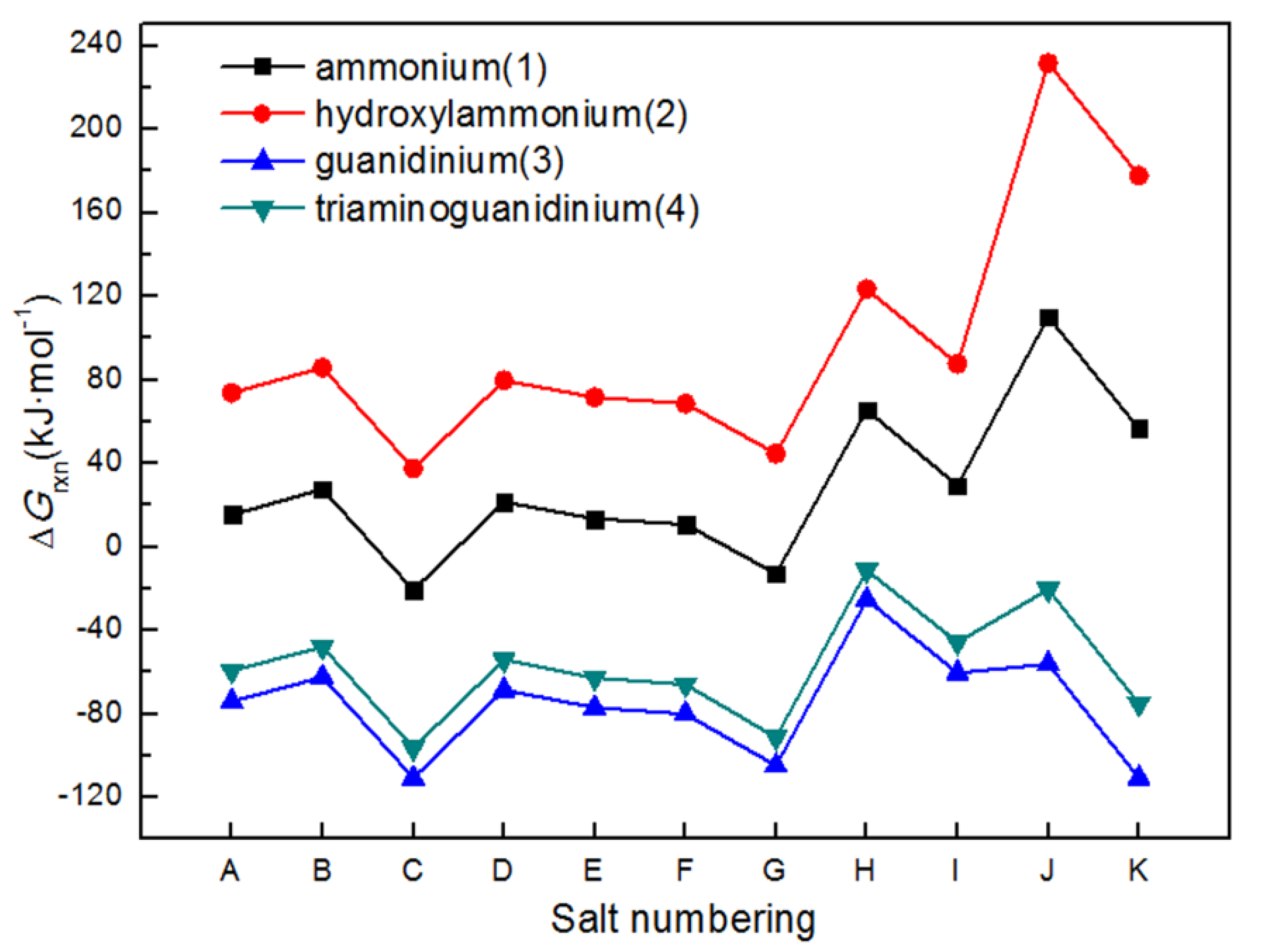


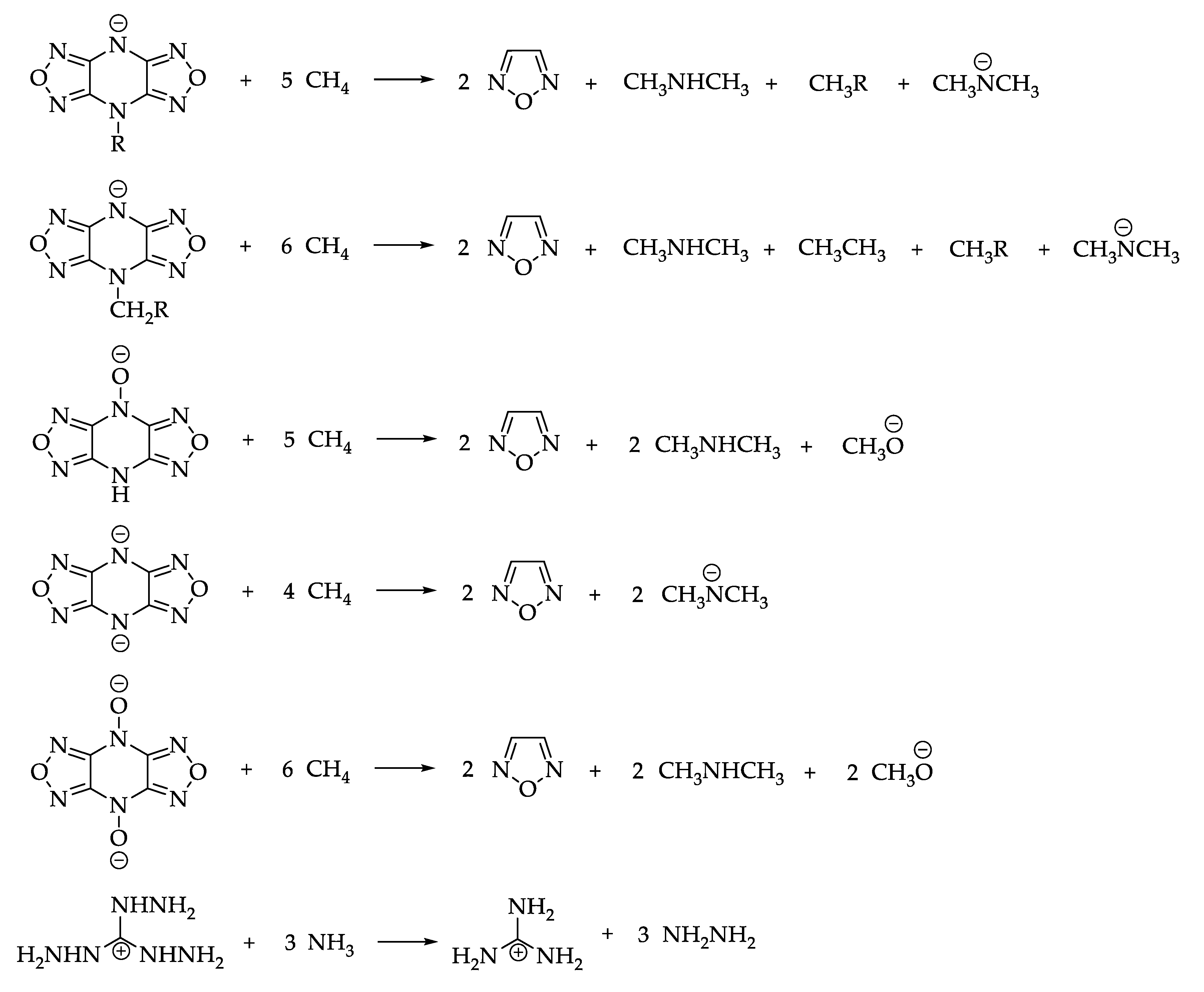
| Salts | ρ (g·cm−3) | ΔHf (kJ·mol−1) | Q (J·g−1) | D (km·s−1) | P (GPa) | Isp (s) | OB (%) | H50 (cm) | ΔGrxn (kJ·mol−1) |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 1.839 | 839.34 | 7224.4 | 8.70 | 34.69 | 275.69 | −74.32 | 31.04 | 15.33 |
| A2 | 1.819 | 892.94 | 8014.4 | 8.88 | 35.84 | 288.74 | −60.30 | 21.13 | 73.82 |
| A3 | 1.700 | 777.85 | 5602.5 | 7.88 | 27.10 | 247.82 | −81.78 | 65.79 | −73.96 |
| A4 | 1.660 | 1161.66 | 6088.9 | 8.31 | 29.70 | 266.56 | −77.04 | 92.83 | −59.20 |
| B1 | 1.791 | 861.46 | 6822.6 | 8.47 | 32.32 | 269.16 | −93.40 | 75.11 | 27.67 |
| B2 | 1.770 | 914.95 | 7696.0 | 8.56 | 32.77 | 280.93 | −78.87 | 49.08 | 85.88 |
| B3 | 1.663 | 799.61 | 5364.9 | 7.69 | 25.49 | 243.31 | −97.07 | 130.44 | −62.32 |
| B4 | 1.630 | 1183.19 | 5864.2 | 8.12 | 28.04 | 261.99 | −90.14 | 162.36 | −47.87 |
| C1 | 1.993 | 1005.48 | 8252.6 | 9.69 | 45.03 | 283.53 | −42.11 | 24.98 | −20.68 |
| C2 | 1.961 | 1058.87 | 8736.4 | 9.82 | 45.80 | 298.89 | −32.79 | 18.14 | 37.54 |
| C3 | 1.830 | 943.42 | 6905.6 | 8.66 | 34.27 | 258.38 | −53.33 | 50.38 | −110.66 |
| C4 | 1.771 | 1326.92 | 7278.4 | 8.83 | 34.91 | 272.51 | −53.33 | 71.56 | −96.22 |
| D1 | 1.805 | 991.81 | 7446.4 | 8.88 | 35.70 | 283.61 | −72.73 | 38.42 | 21.53 |
| D2 | 1.783 | 1045.30 | 8269.2 | 8.92 | 35.74 | 295.46 | −59.81 | 26.45 | 79.76 |
| D3 | 1.674 | 929.97 | 5885.6 | 8.04 | 27.98 | 257.14 | −80.00 | 74.85 | −68.42 |
| D4 | 1.640 | 1313.56 | 6300.9 | 8.41 | 30.20 | 272.95 | −75.79 | 101.63 | −53.95 |
| E1 | 1.990 | 941.20 | 7934.8 | 9.45 | 42.02 | 289.33 | −64.52 | 24.88 | 13.59 |
| E2 | 1.956 | 994.52 | 8401.1 | 9.58 | 42.75 | 298.91 | −54.55 | 18.87 | 71.61 |
| E3 | 1.831 | 878.88 | 6726.2 | 8.50 | 32.41 | 269.88 | −71.72 | 46.01 | −76.90 |
| E4 | 1.774 | 1262.19 | 7168.7 | 8.62 | 32.71 | 281.07 | −69.25 | 63.72 | −62.70 |
| F1 | 1.984 | 942.08 | 7983.9 | 9.52 | 43.36 | 290.39 | −49.61 | 20.85 | 10.76 |
| F2 | 1.949 | 995.36 | 8430.0 | 9.62 | 43.87 | 301.83 | −40.88 | 16.14 | 68.68 |
| F3 | 1.827 | 879.64 | 6808.0 | 8.58 | 33.59 | 263.56 | −58.67 | 38.72 | −79.98 |
| F4 | 1.772 | 1262.86 | 7160.3 | 8.74 | 34.21 | 276.18 | −57.97 | 54.33 | −65.89 |
| G1 | 2.070 | 1471.80 | 9441.4 | 10.50 | 54.02 | 314.17 | −19.28 | 14.29 | −12.80 |
| G2 | 2.028 | 1524.88 | 9725.2 | 10.49 | 53.25 | 312.85 | −13.79 | 11.89 | 44.67 |
| G3 | 1.908 | 1408.79 | 8332.8 | 9.54 | 42.61 | 303.05 | −29.95 | 24.51 | −104.67 |
| G4 | 1.847 | 1791.64 | 8512.0 | 9.50 | 41.47 | 309.00 | −32.46 | 34.02 | −91.08 |
| H1 | 1.856 | 1361.04 | 7745.3 | 9.00 | 37.30 | 286.03 | −73.95 | 25.40 | 65.59 |
| H2 | 1.827 | 1414.35 | 8419.0 | 8.99 | 36.86 | 295.66 | −62.99 | 19.04 | 123.45 |
| H3 | 1.719 | 1298.68 | 6360.7 | 8.23 | 29.79 | 265.72 | −80.00 | 47.88 | −25.26 |
| H4 | 1.679 | 1681.93 | 6658.1 | 8.53 | 31.50 | 277.95 | −76.31 | 66.60 | −11.21 |
| I1 | 1.891 | 880.26 | 7950.7 | 9.10 | 38.58 | 287.56 | −60.30 | 21.13 | 29.35 |
| I2 | 1.867 | 933.78 | 8522.8 | 9.31 | 40.04 | 302.73 | −48.37 | 15.24 | 87.73 |
| I3 | 1.744 | 818.54 | 6402.3 | 8.20 | 29.82 | 259.29 | −69.71 | 45.57 | −60.22 |
| I4 | 1.698 | 1202.22 | 6735.2 | 8.57 | 32.01 | 274.76 | −67.13 | 66.82 | −45.60 |
| J1 | 1.747 | 635.33 | 5590.5 | 8.29 | 30.31 | 250.45 | −80.00 | 35.91 | 110.15 |
| J2 | 1.761 | 693.94 | 7155.8 | 8.63 | 33.06 | 272.99 | −55.17 | 25.06 | 231.71 |
| J3 | 1.625 | 588.26 | 3771.2 | 7.21 | 21.91 | 213.91 | −90.14 | 81.06 | −56.11 |
| J4 | 1.597 | 980.69 | 3911.9 | 7.57 | 23.91 | 227.36 | −81.28 | 119.94 | −20.34 |
| K1 | 1.848 | 727.65 | 7301.0 | 9.03 | 37.45 | 276.35 | −55.17 | 12.32 | 56.69 |
| K2 | 1.863 | 785.27 | 8124.3 | 9.50 | 41.66 | 300.13 | −36.36 | 9.42 | 177.74 |
| K3 | 1.721 | 678.36 | 5204.0 | 8.03 | 28.39 | 235.55 | −70.89 | 27.74 | −110.66 |
| K4 | 1.674 | 1070.13 | 5014.3 | 8.25 | 29.40 | 242.91 | −67.00 | 42.99 | −75.12 |
| RDX | 1.80 | 70.3 | 6656.7 | 8.87 (8.75 a) | 34.73 (34.70 a) | 290.52 | −21.62 | 26.0 b | — |
| HMX | 1.91 | 116.12 | 6836.2 | 9.28 (9.10 a) | 39.19 (39.00 a) | 291.24 | −21.62 | 29.0 b | — |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, B.; Liu, N.; Wang, B.; Lu, X.; Mo, H. Comparative Theoretical Studies on a Series of Novel Energetic Salts Composed of 4,8-Dihydrodifurazano[3,4-b,e]pyrazine-based Anions and Ammonium-based Cations. Molecules 2019, 24, 3213. https://doi.org/10.3390/molecules24183213
Duan B, Liu N, Wang B, Lu X, Mo H. Comparative Theoretical Studies on a Series of Novel Energetic Salts Composed of 4,8-Dihydrodifurazano[3,4-b,e]pyrazine-based Anions and Ammonium-based Cations. Molecules. 2019; 24(18):3213. https://doi.org/10.3390/molecules24183213
Chicago/Turabian StyleDuan, Binghui, Ning Liu, Bozhou Wang, Xianming Lu, and Hongchang Mo. 2019. "Comparative Theoretical Studies on a Series of Novel Energetic Salts Composed of 4,8-Dihydrodifurazano[3,4-b,e]pyrazine-based Anions and Ammonium-based Cations" Molecules 24, no. 18: 3213. https://doi.org/10.3390/molecules24183213







