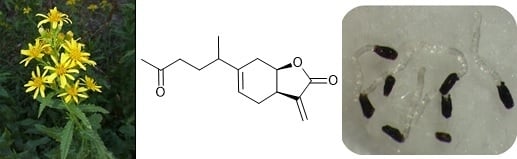
Inuloxin E, a New Seco-Eudesmanolide Isolated from Dittrichia viscosa, Stimulating Orobanche cumana Seed Germination
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Isolation of Plant Metabolites
3.3.1. Inuloxin E (1)
3.3.2. NaBH4 Reduction of Inuloxin E (1)
3.4. Preparation of Hemisynthetic Derivatives of Inuloxin D
3.4.1. 4-O-Acetyl Derivative of Inuloxin D (3)
3.4.2. 4-O-Azidopentanoyl Ester of Inuloxin D (4)
3.4.3. 4-O-Mesyl Ester of Inuloxin D (5)
3.4.4. 4-O-p-Bromobenzoylester of Inuloxin D (6)
3.5. Germination Induction Bioassays
3.6. Germination and Growth Inhibition Bioassays
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernández-Aparicio, M.; Flores, F.; Rubiales, D. Recognition of root exudates by seeds of broomrape (Orobanche and Phelipanche) species. Ann. Bot. 2009, 103, 423–431. [Google Scholar] [PubMed]
- Fernández-Aparicio, M.; Yoneyama, K.; Rubiales, D. The role of strigolactones in host specificity of Orobanche and Phelipanche seed germination. Seed Sci. Res. 2011, 21, 55–61. [Google Scholar]
- Fernández-Aparicio, M.; Masi, M.; Maddau, L.; Cimmino, A.; Evidente, M.; Rubiales, D.; Evidente, A. Induction of haustorium development by sphaeropsidones in radicles of the parasitic weeds Striga and Orobanche. A structure–activity relationship study. J. Agric. Food Chem. 2016, 64, 5188–5196. [Google Scholar] [PubMed]
- Cimmino, A.; Fernández-Aparicio, M.; Andolfi, A.; Basso, S.; Rubiales, D.; Evidente, A. Effect of fungal and plant metabolites on broomrapes (Orobanche and Phelipanche spp.) seed germination and radicle growth. J. Agric. Food Chem. 2014, 62, 10485–10492. [Google Scholar] [PubMed]
- Andolfi, A.; Zermane, N.; Cimmino, A.; Avolio, F.; Boari, A.; Vurro, M.; Evidente, A. Inuloxins A–D, phytotoxic bi-and tri-cyclic sesquiterpene lactones produced by Inula viscosa: Potential for broomrapes and field dodder management. Phytochemistry 2013, 86, 112–120. [Google Scholar] [PubMed]
- Evidente, A.; Andolfi, A.; Cimmino, A. Relationships between the stereochemistry and biological activity of fungal phytotoxins. Chirality 2011, 23, 674–693. [Google Scholar] [PubMed]
- Evidente, A.; Cimmino, A.; Andolfi, A. The effect of stereochemistry on the biological activity of natural phytotoxins, fungicides, insecticides and herbicides. Chirality 2013, 25, 59–78. [Google Scholar] [PubMed]
- Santoro, E.; Mazzeo, G.; Petrovic, A.G.; Cimmino, A.; Koshoubu, J.; Evidente, A.; Berova, N.; Superchi, S. Absolute configurations of phytotoxins seiricardine A and inuloxin A obtained by chiroptical studies. Phytochemistry 2015, 116, 359–366. [Google Scholar] [PubMed]
- Johnson, J.L.; Raghavan, V.; Cimmino, A.; Moeini, A.; Petrovic, A.G.; Santoro, E.; Superchi, S.; Berova, N.; Polavarapu, P.L. Absolute configurations of chiral molecules with multiple stereogenic centers without prior knowledge of the relative configurations: A case study of inuloxin C. Chirality 2018, 30, 1206–1214. [Google Scholar] [PubMed]
- Pretsch, E.; Bühlmann, P.; Affolter, C. Structure Determination of Organic Compounds—Tables of Spectral Data, 3rd ed.; Springer: Berlin, Germany, 1987; pp. 161–243. [Google Scholar]
- Berger, S.; Braun, S. 200 and More Basic NMR Experiments—A Practical Course, 1st ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Breitmaier, E.; Voelter, W. Carbon-13 NMR Spectroscopy; VCH: Weinheim, Germany, 1987; pp. 183–280. [Google Scholar]
- Ellestad, G.A.; Kunstmann, M.P.; Mirando, P.; Morton, G.O. Structures of fungal diterpene antibiotics LL-S491. beta and- gamma. J. Am. Chem. Soc. 1972, 94, 6206–6208. [Google Scholar]
- Steyn, P.S.; van Heerden, F.R.; Rabie, C.J. Cytochalasins E and K, toxic metabolites from Aspergillus clavatus. J. Chem. Soc. Perkin Trans. 1982, 1, 541–544. [Google Scholar]
- Evidente, A.; Sparapano, L.; Fierro, O.; Bruno, G.; Giordano, F.; Motta, A. Sphaeropsidins B and C, phytotoxic pimarane diterpenes from Sphaeropsis sapinea f. sp. cupressi and Diplodia mutila. Phytochemistry 1997, 45, 705–713. [Google Scholar]
- Evidente, A.; Maddau, L.; Scanu, B.; Andolfi, A.; Masi, M.; Motta, A.; Tuzi, A. Sphaeropsidones, phytotoxic dimedone methyl ethers produced by Diplodia cupressi: A structure− activity relationship study. J. Nat. Prod. 2011, 74, 757–763. [Google Scholar] [PubMed]
- Cala, A.; Masi, M.; Cimmino, A.; Molinillo, J.M.; Macias, F.A.; Evidente, A. (+)-epi-Epoformin, a phytotoxic fungal cyclohexenepoxide: Structure activity relationships. Molecules 2018, 23, 1529. [Google Scholar]
- Fernández-Aparicio, M.; Cimmino, A.; Evidente, A.; Rubiales, D. Inhibition of Orobanche crenata seed germination and radicle growth by allelochemicals identified in cereals. J. Agric. Food Chem. 2013, 61, 9797–9803. [Google Scholar] [PubMed]
- Grauso, L.; Cesarano, G.; Zotti, M.; Ranesi, M.; Sun, W.; Bonanomi, G.; Lanzotti, V. Exploring Dittrichia viscosa (L.) Greuter phytochemical diversity to explain its antimicrobial, nematicidal and insecticidal activity. Phytochem. Rev. 2019, 1–31. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Westwood, J.H.; Rubiales, D. Agronomic, breeding and biotechnological approaches for parasitic plant management by manipulating strigolactone levels in agricultural soils. Botany-Botanique 2011, 89, 813–826. [Google Scholar]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar]
- Zwanenburg, B.; Mwakaboko, A.S.; Kannan, C. Suicidal germination for parasitic weed control. Pest Manag. Sci. 2016, 72, 2016–2025. [Google Scholar]
Sample Availability: Samples of the compounds 1–6 are available from the authors. |





| Position | δC c | δH (J in Hz) | HMBC |
|---|---|---|---|
| 1 | 35.4 d | 2.37 m | H2-6, H-5, H2-9, H-8, Me-14 |
| 2 | 30.4 t | 2.26 m (2H) | H-3A, H-5 |
| 3 | 42.6 t | 2.56 m 2.26 m | H2-2 |
| 4 | 208.0 s | H2-3, Me-15 | |
| 5 | 120.1 d | 5.46 br dd (8.8, 5.3) | H2-6 |
| 6 | 26.6 t | 2.43 m 2.18 m | H-5, H-7, H-8 |
| 7 | 42.1 d | 3.34 m | H-5, H2-6, H-8, H2-9, H2-13 |
| 8 | 79.3 d | 4.66 ddd (11.8, 8.4, 2.7) | H2-6, H-7, H2-9 |
| 9 | 36.7 t | 2.02 ddd (13.0, 6.0, 2.7) 1.89 ddd (13.0, 11.8, 8.4) | H-1, H-7 |
| 10 | 144.5 s | H-1, H2-6, H2-9, Me-14 | |
| 11 | 139.0 s | H2-6, H-7, H-8, H2-13 | |
| 12 | 170.2 s | H-7, H2-13, H-8 | |
| 13 | 122.1 t | 6.28 d (3.2) 5.54 d (3.2) | H-7 |
| 14 | 20.9 q | 1.16 d (6.9) | H2-9, H-1, H-5 |
| 15 | 29.9 q | 2.17 s |
| Position | 2 a | 3 b | 4 c | 5 d | 6 e |
|---|---|---|---|---|---|
| δH, (J in Hz) | δH, (J in Hz) | δH, (J in Hz) | δH, (J in Hz) | δH, (J in Hz) | |
| 1 | 2.39 m | 2.36 sextet (6.6) | 2.40 m | 2.36 sextet (6.6) | 2.36 sextet (6.9) |
| 2 | 2.06 m 2.04 m | 2.06 (2H) m | 2.06 (2H) m | 2.05 (2H) m | 2.06 (2H) m |
| 3 | 1.52 m 1.48 m | 1.74 m 1.53 m | 1.79 m 1.62 m | 1.86 m 1.66 m | 1.76 m 1.65 m |
| 4 | 3.80 m | 4.84 m | 4.90 m | 4.82 m | 5.10 m |
| 5 | 5.52 dd (8.7, 4.9) | 5.47 dd (9.2, 5.2) | 5.46 dd (9.2, 5.4) | 5.54 dd (9.2, 5.2) | 5.42 dd (8.9, 5.4) |
| 6 | 2.48 m 2.21 m | 2.45 br dd (14.2, 5.2) 2.19 ddd (14.2, 9.2, 4.5) | 2.45 br dd (13.8, 5.4) 2.19 ddd (13.8, 9.2, 4.5 | 2.47 br dd (13.8, 5.2) 2.22 ddd (13.8, 9.2, 5.4) | 2.42 br dd (14.2, 5.4) 2.13 ddd (14.2, 8.9, 4.6) |
| 7 | 3.36 m | 3.38 m | 3.38 m | 3.38 m | 3.35 m |
| 8 | 4.66 ddd (11.8, 8.6, 2.9) | 4.67 ddd (11.8, 8.3, 2.7) | 4.67 ddd (11.5, 7.7, 2.4) | 4.46 ddd (11.8, 8.6, 2.5) | 4.66 ddd (11.6, 8.8, 2.5) |
| 9 | 2.01 m 1.98 m | 1.96 (2H) m | 1.96 (2H) m | 1.96 (2H) m | 2.01 m 1.93 m |
| 13 | 6.27 d (3.2) 5.55 d (3.2) | 6.28 d (2.9) 5.55 d (2.9) | 6.28 d (2.9) 5.55 d (2.9) | 6.29 d (2.9) 5.56 d (2.9) | 6.26 d (3.0) 5.51 d (3.0) |
| 14 | 1.16 d (6.9) | 1.15 d (6.6) | 1.15 d (6.7) | 1.16 d (6.9) | 1.12 d (6.9) |
| 15 | 1.24 d (6.2) | 1.25 d (6.2) | 1.25 d (6.4) | 1.46 d (6.1) | 1.35 d (6.6) |
| Broomrape Seed Germination (%) | ||||
|---|---|---|---|---|
| Inuloxin D derivative | Concentracion | O. cumana | O. minor | P. ramosa |
| 3 | 10−4 M | 57.8 | 0.0 | 0.0 |
| 10−5 M | 14.9 | 0.0 | 0.0 | |
| 10−6 M | 2.3 | 0.0 | 0.0 | |
| 10−7 M | 0.0 | 0.0 | 0.0 | |
| 4 | 10−4 M | 64.7 | 0.0 | 0.0 |
| 10−5 M | 29.4 | 0.0 | 0.0 | |
| 10−6 M | 4.2 | 0.0 | 0.0 | |
| 10−7 M | 0.0 | 0.0 | 0.0 | |
| 5 | 10−4 M | 26.4 | 0.0 | 0.0 |
| 10−5 M | 0.0 | 0.0 | 0.0 | |
| 10−6 M | 0.0 | 0.0 | 0.0 | |
| 10−7 M | 0.0 | 0.0 | 0.0 | |
| 6 | 10−4 M | 18.6 | 0.0 | 0.0 |
| 10−5 M | 3.5 | 0.0 | 0.0 | |
| 10−6 M | 0.0 | 0.0 | 0.0 | |
| 10−7 M | 0.0 | 0.0 | 0.0 | |
| GR24 | 1 | 64.0 | 80.4 | 68.1 |
| Control | 0 | 0.0 | 0.0 | 0.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, M.; Fernández-Aparicio, M.; Zatout, R.; Boari, A.; Cimmino, A.; Evidente, A. Inuloxin E, a New Seco-Eudesmanolide Isolated from Dittrichia viscosa, Stimulating Orobanche cumana Seed Germination. Molecules 2019, 24, 3479. https://doi.org/10.3390/molecules24193479
Masi M, Fernández-Aparicio M, Zatout R, Boari A, Cimmino A, Evidente A. Inuloxin E, a New Seco-Eudesmanolide Isolated from Dittrichia viscosa, Stimulating Orobanche cumana Seed Germination. Molecules. 2019; 24(19):3479. https://doi.org/10.3390/molecules24193479
Chicago/Turabian StyleMasi, Marco, Mónica Fernández-Aparicio, Roukia Zatout, Angela Boari, Alessio Cimmino, and Antonio Evidente. 2019. "Inuloxin E, a New Seco-Eudesmanolide Isolated from Dittrichia viscosa, Stimulating Orobanche cumana Seed Germination" Molecules 24, no. 19: 3479. https://doi.org/10.3390/molecules24193479
APA StyleMasi, M., Fernández-Aparicio, M., Zatout, R., Boari, A., Cimmino, A., & Evidente, A. (2019). Inuloxin E, a New Seco-Eudesmanolide Isolated from Dittrichia viscosa, Stimulating Orobanche cumana Seed Germination. Molecules, 24(19), 3479. https://doi.org/10.3390/molecules24193479