28-Hydroxy-3-oxoolean-12-en-29-oic Acid, a Triterpene Acid from Celastrus Orbiculatus Extract, Inhibits the Migration and Invasion of Human Gastric Cancer Cells In Vitro
Abstract
1. Introduction
2. Results
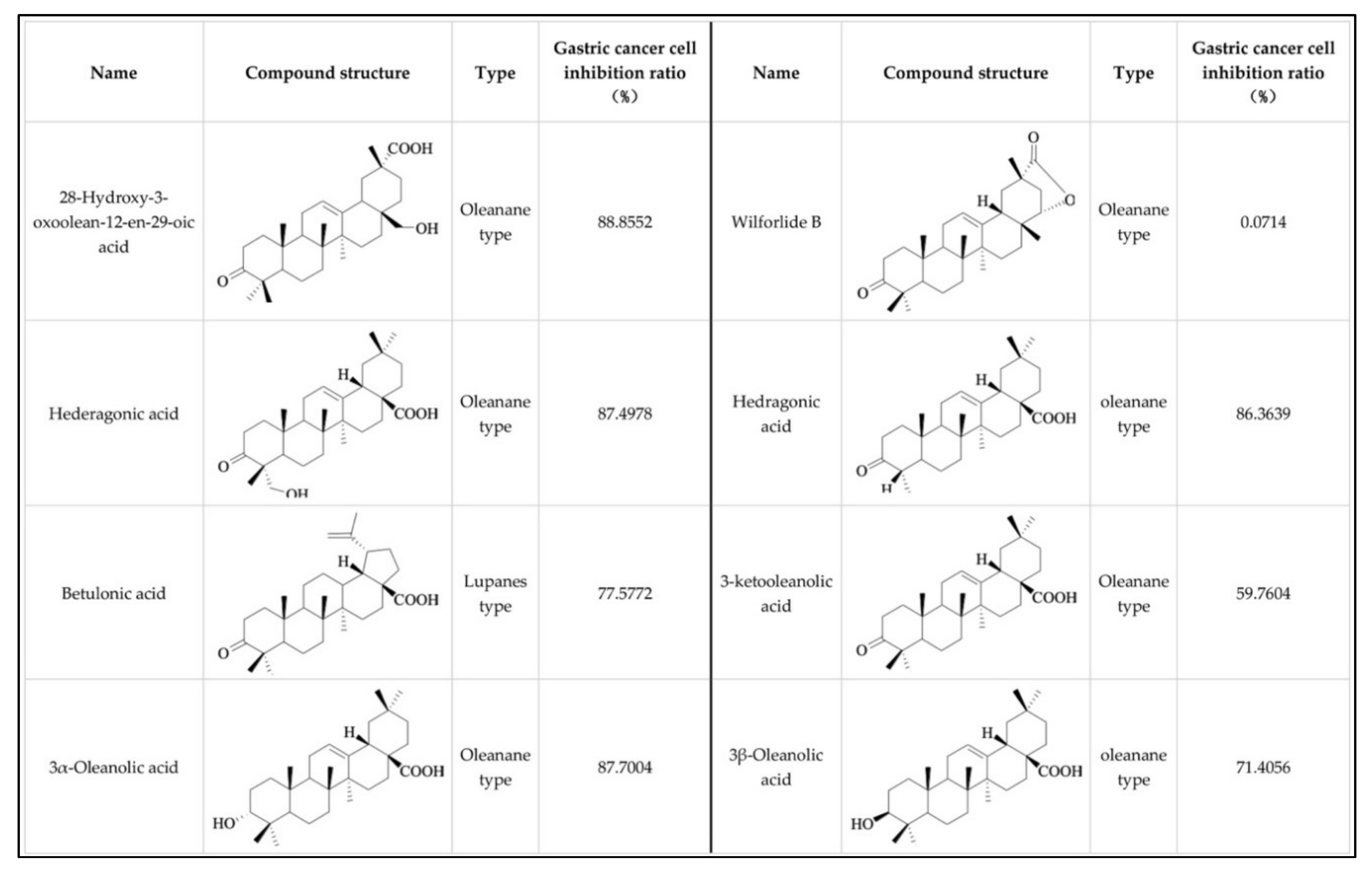
2.1. Effect of Oleanane-Type Triterpenoid Acid from Celastrus Orbiculatus Thunb. on the Viability of Human Gastric Cancer Cells
2.2. Effect of 28-Hydroxy-3-oxoolean-12-en-29-oic Acid on the Viability of Human Gastric Cancer Cells
2.3. 28-Hydroxy-3-oxoolean-12-en-29-oic Acid Prevents the Migration and Invasion of Gastric Cancer Cells
2.4. PerkinElmer Operetta CLS High-Content Imaging System Analysis
2.5. Effect of 28-Hydroxy-3-oxoolean-12-en-29-oic Acid on the Expression of Matrix Metalloproteinases (MMPs) in Human Gastric Cancer Cells
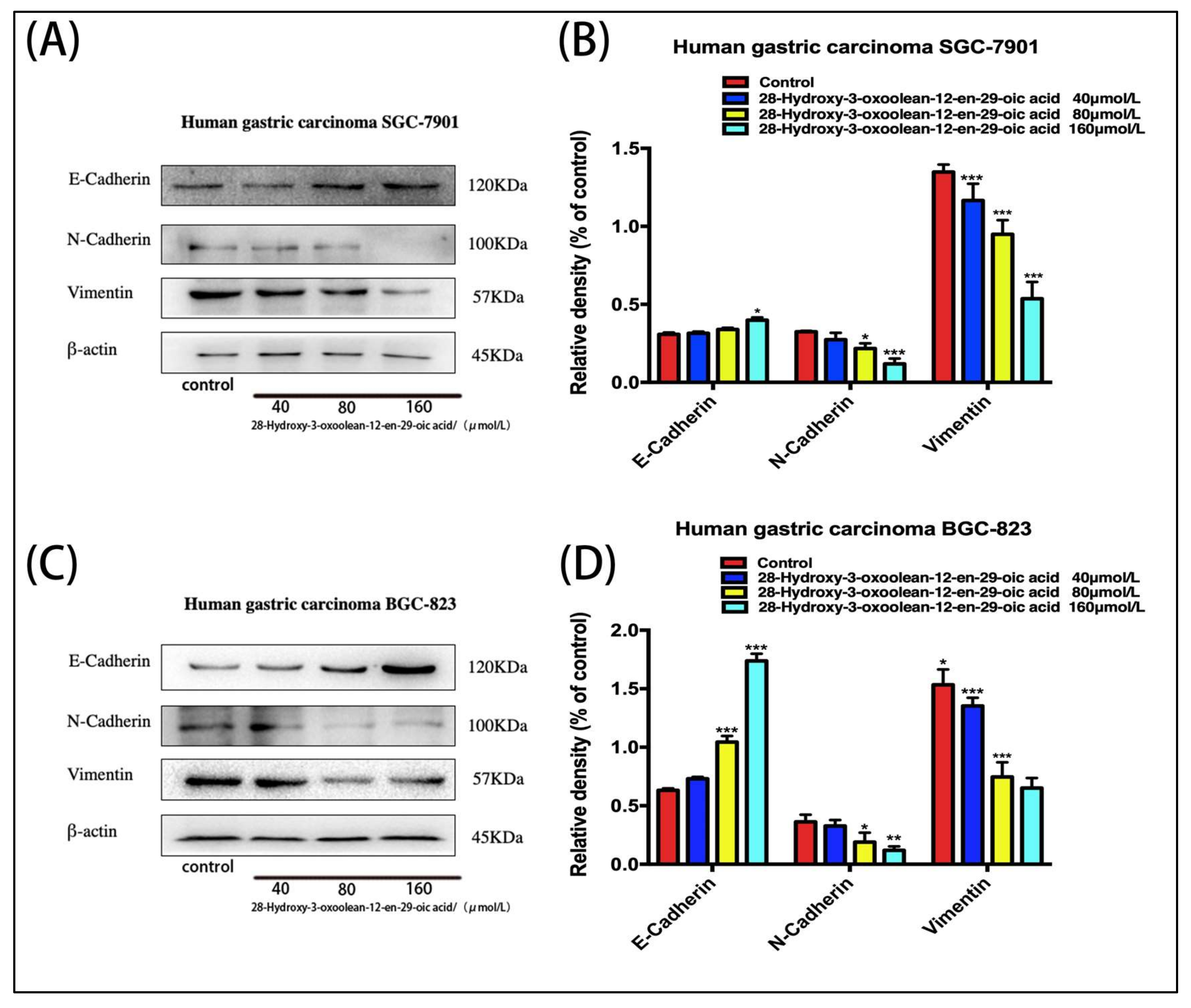
2.6. Effect of 28-Hydroxy-3-oxoolean-12-en-29-oic Acid on Expression of Epithelial–Mesenchymal Transition (EMT)-Related Proteins in Human Gastric Cells
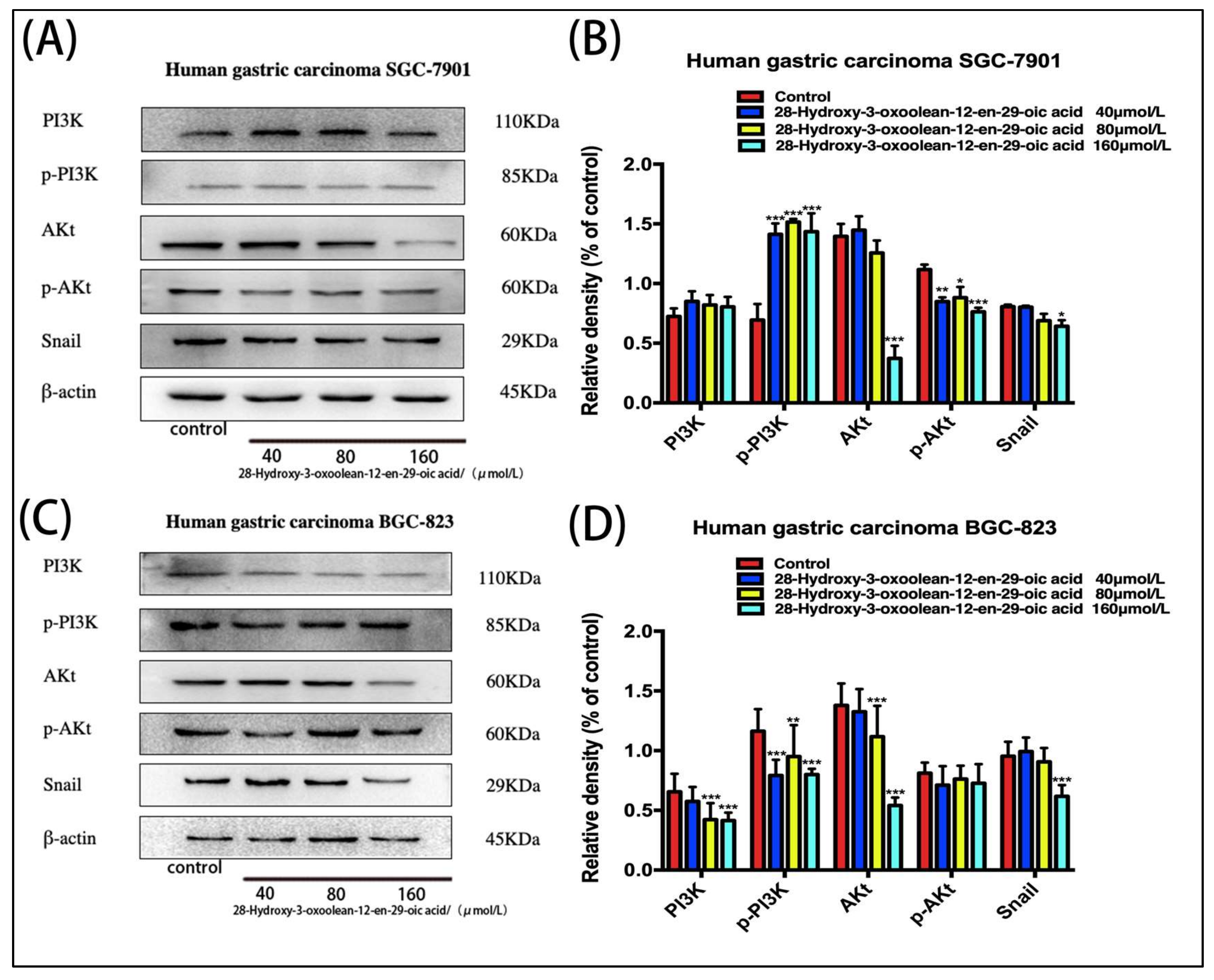
2.7. Effect of 28-Hydroxy-3-oxoolean-12-en-29-oic Acid on the Expression of PI3K/Akt/Snail Signaling Proteins in Human Gastric Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Drug
4.2. Reagents
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Cell Invasion and Migration Assay
4.6. PerkinElmer Operetta CLS High-Content Imaging System Analysis
4.7. Western Blot Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, J.J.; Hu, X.; Wang, P.; Huang, B.; Sun, W.; Xiong, C.; Hu, Z.; Chen, S. Investigation on Species Authenticity for Herbal Products of and from Markets Using ITS2 Barcoding. Molecules 2018, 23, 967. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Q.; Zheng, R.S.; Peter, D. Cancer Statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.W.; Wang, P.; Huang, H.W. A proposal of a personalized surveillance strategy for gastric cancer: A retrospective analysis of 9191 patients. Gastroenterol. Res. Pract. 2019, 2019, 3248727. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Q.; Wang, J.F. Transforming growth factor-β (TGF-β) induces the expression of chondrogenesis-related genes through TGF-β receptor II (TGFRII)–AKT–mTOR signaling in primary cultured mouse precartilaginous stem cells. Biochem. Biophys. Res. Commun. 2014, 450, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, S.; Deng, L. Decreased MT1-MMP in gastric cancer suppressed cell migration and invasion via regulating MMPs and EMT. Tumor Biol. 2015, 36, 6883–6889. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Tao, L.; Ni, T.Y.; Gu, H.; Jin, F.; Dai, X.J. Anticancer efficacy of the ethyl acetate extract from the traditional chinese medicine herb celastrus orbiculatus against human gastric cancer. J. Ethnopharmacol. 2017, 205, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, M.; Zhang, Z.S.; Wang, X.Y.; Jin, F.; Wang, H.B.; Shi., Y.Y.; Liu, Y.Q. COE inhibits vasculogenic mimicry in hepatocellular carcinoma via suppressing Notch1 signaling. J. Ethnopharmacol. 2017, 208, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, X.; Wang, J.; Xu, J.; Li, N. A new alkaloid from the fruits of Celastrus orbiculatus. Fitoterapia 2005, 76, 273–275. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Liu, Y.Q.; Qian, Y.Y.; Zhang, H.; Li, G.Q.; Yang, L. Extracts of Celastrus orbiculatus exhibit anti-proliferative and anti-invasive effects on human gastric adenocarcinoma cells. Chin. J. Integr. Med. 2014, 10, 1–9. [Google Scholar] [CrossRef]
- Wang, W.M.; Zhou, Y.; Yao, Q.; Xiang, L.L.; Ni, T.Y.; Dai, X.J.; Liu, Y.Q. Extract Potentiates the Sensitivity of Cisplatin Caspase-Depenent Apoptosis in Gastric Cancer. Anticancer Agents Med. Chem. 2018, 18, 2206–2211. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.Q.; Wang, M.; Qian, Y.Y.; Dai, X.J.; Chen, J.; Guo, S.Y. Celastrus orbiculatus extract triggers apoptosis and autophagy via PI3K/Akt/mTOR inhibition in human colorectal cancer cells. Oncol. Lett. 2016, 12, 3771–3778. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.Q.; Qian, Y.Y.; Dai, X.J.; Chen, J. Antimetastatic Effects of Celastrus orbiculatus on Human Gastric Adenocarcinoma by Inhibiting Epithelial-Mesenchymal Transition and NF-kB/Snail Signaling Pathway. Integr. Cancer Ther. 2015, 14, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Feng, J.; Wang, H.B.; Qian, Y.Y.; Chen, J.; Yang, L.; Lu, S.H.; Liu, Y.Q. Celastrus orbiculatus extract inhibits the migration and invasion of human glioblastoma cells in vitro. BMC Complement Altern. Med. 2016, 16, 387. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Su, P.; Meng, S.S.; Chen, Y.P. Role of matrix metalloproteinase-2/9 (MMP2/9) in lead-induced changes in an in vitro blood-brain barrier model. Int. J. Biol. Sci. 2017, 13, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.Y.; Liu, H.M.; Xie, D.Y.; Xiao, Q. Differentially expressed genes and are potential prognostic biomarkers for gastric cancer. Oncol. Lett. 2019, 17, 3191–3202. [Google Scholar]
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in translation: from molecule to brain physiology, pathology, and therapy. J. Neurochem. 2016, 139, 91–114. [Google Scholar] [CrossRef]
- Hong, Y.L.; Qin, H.F.; Li, Y.; Zhang, Y.H.; Zhuang, X.R.; Liu, L.; Lu, K.; Li, L.; Deng, X.L.; Liu, F.; et al. FNDC3B circular RNA promotes the migration and invasion of gastric cancer cells via the regulation of E-cadherin and CD44 expression. J. Cell Physiol. 2019, 234, 19895–19910. [Google Scholar] [CrossRef]
- Gloushankova, N.A. Changes in Regulation of Cell-Cell Adhesion during Tumor Transformation. Biochemistry 2008, 73, 742–750. [Google Scholar] [CrossRef]
- Camand, E.; Peglion, F.; Osmani, N.; Sanson, M.; Etienne-Manneville, S. N-cadherin expression level modulates integrin-mediated polarity and strongly impacts on the speed and directionality of glial cell migration. J. Cell. Sci. 2012, 125, 844–857. [Google Scholar] [CrossRef]
- Lin, H.; Huang, B.; Wang, H.; Liu, X.C.; Hong, Y.X.; Qiu, S.P.; Zheng, J.H. MTHFD2 Overexpression Predicts Poor Prognosis in Renal Cell Carcinoma and is Associated with Cell Proliferation and Vimentin-Modulated Migration and Invasion. Cell Physiol. Biochem. 2018, 51, 991–1000. [Google Scholar] [CrossRef]
- Tania, M.; Khan, M.A.; Fu, J. Epithelial to mesenchymal transition inducing transcription factors and metastatic cancer. Tumor Biol. 2014, 35, 7335–7342. [Google Scholar] [CrossRef] [PubMed]
- Pawan, K.; Leonard, A.; Lidija, K. Activating Mutations in β-Catenin in Colon Cancer Cells Alter Their Interaction with Macrophages; The Role of Snail. PLoS ONE 2012, 7, e45462. [Google Scholar]
- Nieto, M.A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell. Biol. 2003, 3, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Zhang, J.Z.; Dong, H.; Wang, W.J. Nimotuzuma restrains proliferation and induces apoptosis in human osteosarcoma cells by regulation of EGFR/PI3K/AKT signal pathway. J. Cell. Physiol. 2019, 234, 11. [Google Scholar] [CrossRef] [PubMed]
- Bachelder, R.E. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J. Cell Biol. 2004, 168, 29–33. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Z.; Wang, H.; Ni, T.; Tao, L.; Xiang, L.; Zhou, Z.; Qian, Y.; Sunagawa, M.; Liu, Y. 28-Hydroxy-3-oxoolean-12-en-29-oic Acid, a Triterpene Acid from Celastrus Orbiculatus Extract, Inhibits the Migration and Invasion of Human Gastric Cancer Cells In Vitro. Molecules 2019, 24, 3513. https://doi.org/10.3390/molecules24193513
Chu Z, Wang H, Ni T, Tao L, Xiang L, Zhou Z, Qian Y, Sunagawa M, Liu Y. 28-Hydroxy-3-oxoolean-12-en-29-oic Acid, a Triterpene Acid from Celastrus Orbiculatus Extract, Inhibits the Migration and Invasion of Human Gastric Cancer Cells In Vitro. Molecules. 2019; 24(19):3513. https://doi.org/10.3390/molecules24193513
Chicago/Turabian StyleChu, Zewen, Haibo Wang, Tengyang Ni, Li Tao, Liangliang Xiang, Zhen Zhou, Yayun Qian, Masataka Sunagawa, and Yanqing Liu. 2019. "28-Hydroxy-3-oxoolean-12-en-29-oic Acid, a Triterpene Acid from Celastrus Orbiculatus Extract, Inhibits the Migration and Invasion of Human Gastric Cancer Cells In Vitro" Molecules 24, no. 19: 3513. https://doi.org/10.3390/molecules24193513
APA StyleChu, Z., Wang, H., Ni, T., Tao, L., Xiang, L., Zhou, Z., Qian, Y., Sunagawa, M., & Liu, Y. (2019). 28-Hydroxy-3-oxoolean-12-en-29-oic Acid, a Triterpene Acid from Celastrus Orbiculatus Extract, Inhibits the Migration and Invasion of Human Gastric Cancer Cells In Vitro. Molecules, 24(19), 3513. https://doi.org/10.3390/molecules24193513





