Crystal Structure of Human EOLA1 Implies Its Possibility of RNA Binding
Abstract
:1. Introduction
2. Results
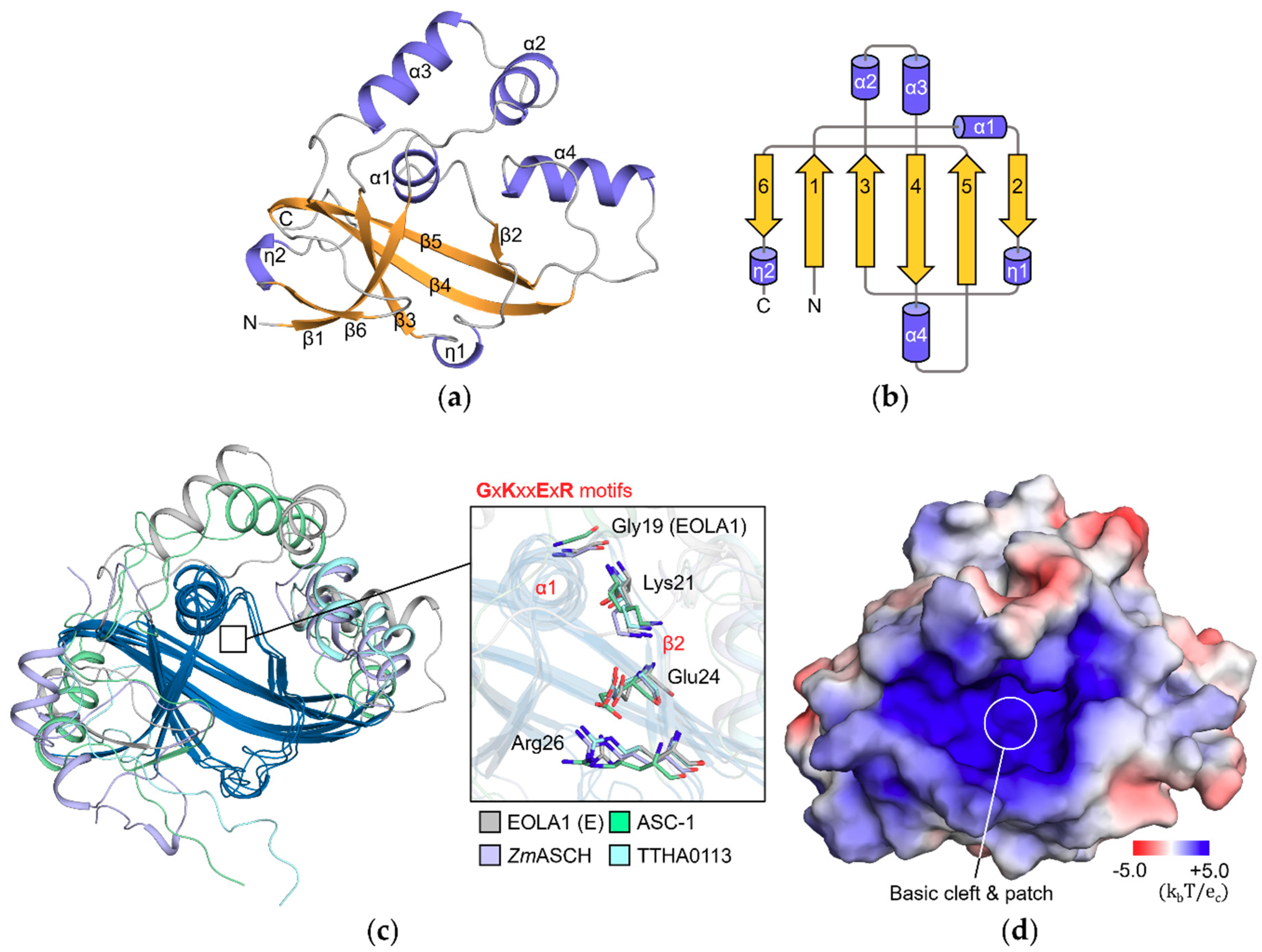
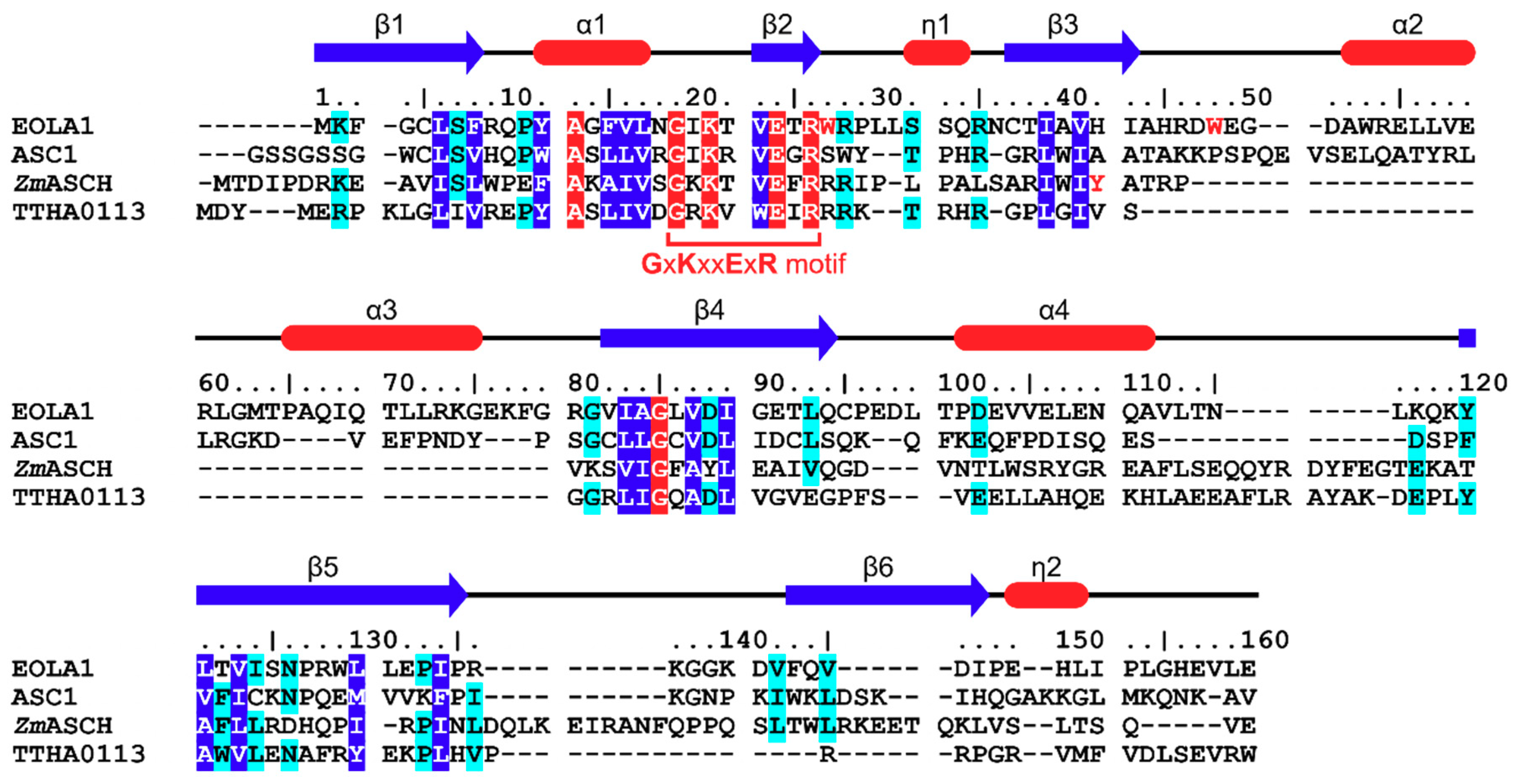
2.1. Endothelial-Overexpressed Lipopolysaccharide-Associated Factor 1 (EOLA1) Contains Typical Activating Signal Cointegrator-1 Homology (ASCH) Structure
2.2. Structural Analyses Imply That RNA-Binding Mode of EOLA1 is Different from That of Pseudouridine Synthase and Archaeosine Transglycosylase (PUA) Domains
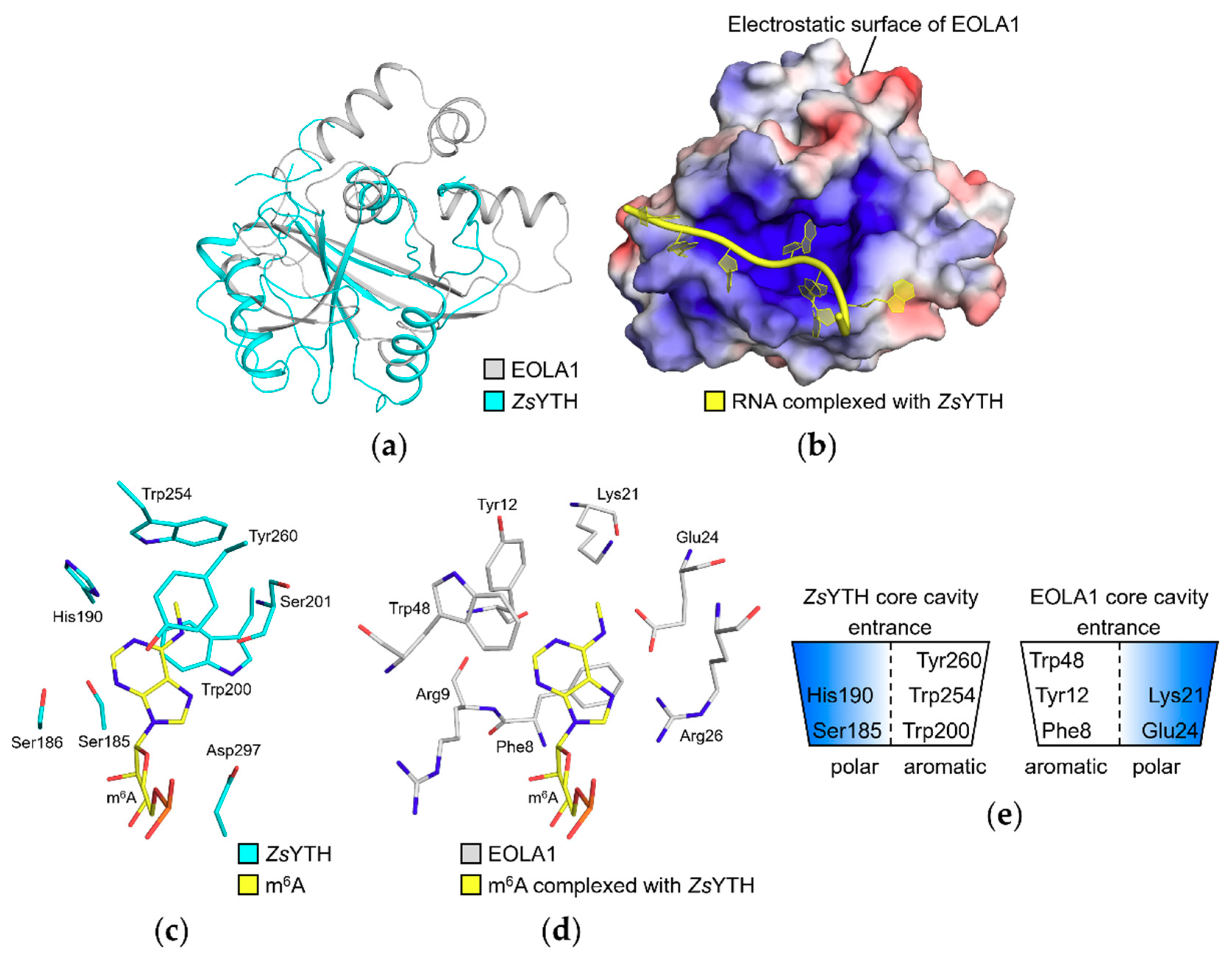
2.3. Structural Comparison with Zygosaccharomyces Rouxii YTH Domain (ZsYTH) Revealed a Cavity in EOLA1 for A Base Substrate
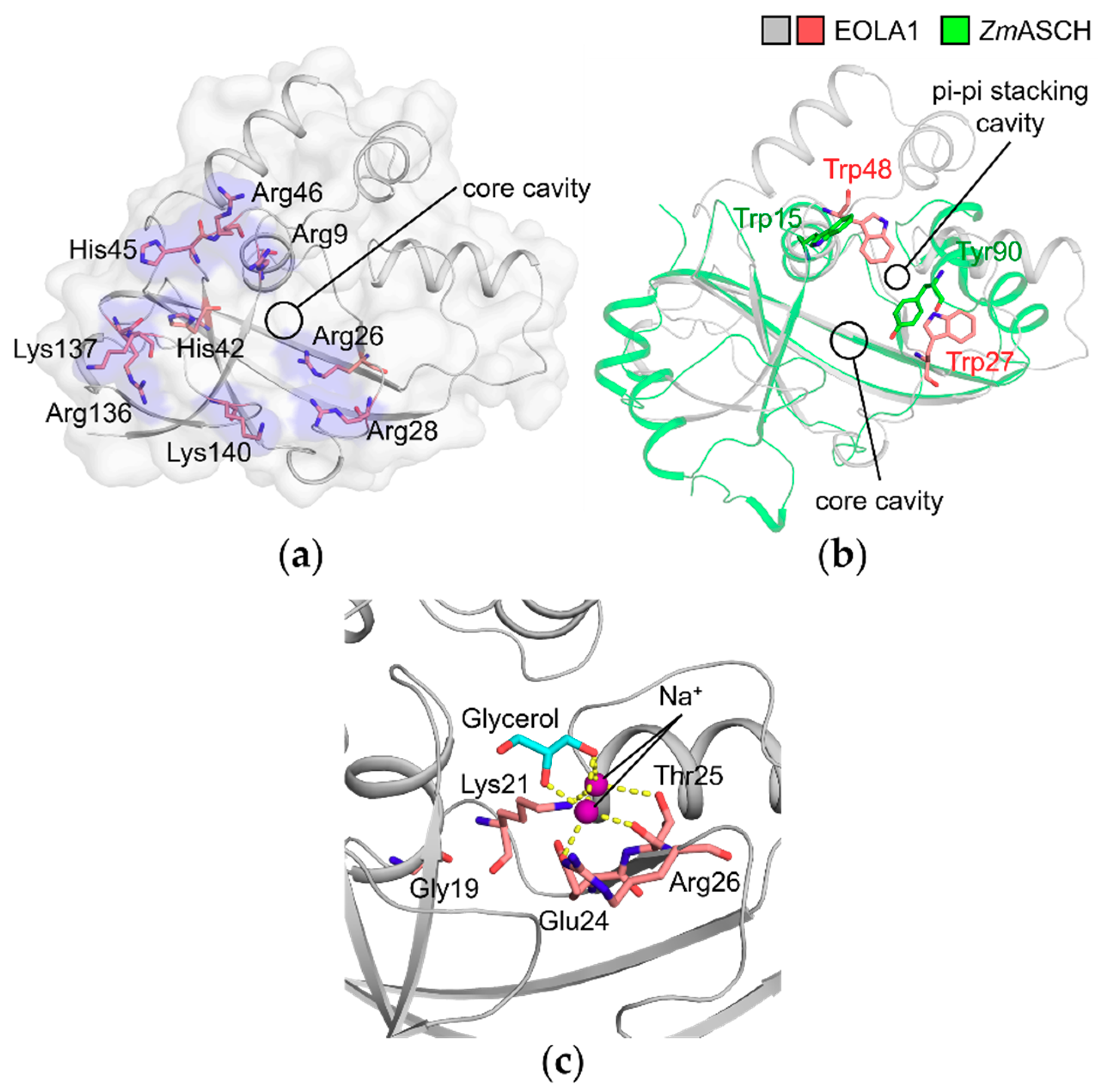
2.4. Basic Patch of EOLA1 Could Provide a Binding Interface for Nucleic Acid Backbones
2.5. Molecules in the Core Cavity of EOLA1 Could Be Reminiscent of Nucleotide Binding
3. Discussion
4. Materials and Methods
4.1. Cloning, Protein Expression, and Purification of EOLA1
4.2. Crystallization, Data Collection, and Structure Determination
4.3. Data Availability
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liang, Z.; Yang, Z. Identification and characterization of a novel gene EOLA1 stimulating ECV304 cell proliferation. Biochem. Biophys. Res. Commun. 2004, 325, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-B.; Wong, F.; Harlan, J.M.; Chaudhary, P.M.; Hood, L.; Karsan, A. Lipopolysaccharide Mediates Endothelial Apoptosis by a FADD-dependent Pathway. J. Boil. Chem. 1998, 273, 20185–20188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, M.; Kuwano, K.; Kunitake, R.; Hagimoto, N.; Miyazaki, H.; Kaneko, Y.; Kawasaki, M.; Maeyama, T.; Hara, N. Endothelial Cell Apoptosis in Lipopolysaccharide–Induced Lung Injury in Mice. Int. Arch. Allergy Immunol. 1998, 117, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Swanson, P.E.; Freeman, B.D.; Tinsley, K.W.; Cobb, J.P.; Matuschak, G.M.; Buchman, T.G.; Karl, I.E. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 1999, 27, 1230–1251. [Google Scholar] [CrossRef] [PubMed]
- Fries, J.W.; Williams, A.J.; Atkins, R.C.; Newman, W.; Lipscomb, M.F.; Collins, T. Expression of VCAM-1 and E-selectin in an in vivo model of endothelial activation. Am. J. Pathol. 1993, 143, 725–737. [Google Scholar] [PubMed]
- Erridge, C.; Spickett, C.M.; Webb, D.J. Non-enterobacterial endotoxins stimulate human coronary artery but not venous endothelial cell activation via Toll-like receptor 2. Cardiovasc. Res. 2007, 73, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, N.; Yoshida, M.; Umeda, M.; Huang, Y.; Kitajima, S.; Inoue, Y.; Ishikawa, I.; Iwai, T. Extended exposure of lipopolysaccharide fraction from Porphyromonas gingivalis facilitates mononuclear cell adhesion to vascular endothelium via Toll-like receptor-2 dependent mechanism. Atherosclerosis 2008, 196, 59–67. [Google Scholar] [CrossRef]
- Anand, A.R.; Bradley, R.; Ganju, R.K. LPS-induced MCP-1 expression in human microvascular endothelial cells is mediated by the tyrosine kinase, Pyk2 via the p38 MAPK/NF-kappaB-dependent pathway. Mol. Immunol. 2009, 46, 962–968. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, L.; Liu, D.; Wang, J.; Zhang, X.; Shu, R.; Liang, J. Role of p38 Mitogen-Activated Protein Kinase Pathway inPorphyromonas gingivalisLipopolysaccharide–Induced VCAM-1 Expression in Human Aortic Endothelial Cells. J. Periodontol. 2012, 83, 955–962. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Chen, W.; Yang, T.; Zhang, W. EOLA1 protects lipopolysaccharide induced IL-6 production and apoptosis by regulation of MT2A in human umbilical vein endothelial cells. Mol. Cell. Biochem. 2014, 395, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Iyer, L.M.; Burroughs, A.M.; Aravind, L. The ASCH superfamily: Novel domains with a fold related to the PUA domain and a potential role in RNA metabolism. Bioinformatics 2006, 22, 257–263. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yi, J.-Y.; Sung, H.-S.; Moore, D.D.; Jhun, B.H.; Lee, Y.C.; Lee, J.W. Activating Signal Cointegrator 1, a Novel Transcription Coactivator of Nuclear Receptors, and Its Cytosolic Localization under Conditions of Serum Deprivation. Mol. Cell. Boil. 1999, 19, 6323–6332. [Google Scholar] [CrossRef] [Green Version]
- Jung, D.-J.; Sung, H.-S.; Goo, Y.-W.; Lee, H.M.; Park, O.K.; Jung, S.-Y.; Lim, J.; Kim, H.-J.; Lee, S.-K.; Kim, T.S.; et al. Novel Transcription Coactivator Complex Containing Activating Signal Cointegrator 1. Mol. Cell. Boil. 2002, 22, 5203–5211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.-N.; Shin, M.; Ha, S.C.; Park, S.-Y.; Seo, P.-W.; Hofmann, A.; Kim, J.-S. Crystal structure of an ASCH protein from Zymomonas mobilis and its ribonuclease activity specific for single-stranded RNA. Sci. Rep. 2017, 7, 12303. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Tong, L. Molecular basis for the recognition of methylated adenines in RNA by the eukaryotic YTH domain. Proc. Natl. Acad. Sci. USA 2014, 111, 13834–13839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Wang, X.; Liu, K.; A Roundtree, I.; Tempel, W.; Li, Y.; Lu, Z.; He, C.; Min, J. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat. Methods 2014, 10, 927–929. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, Y.; Bao, H.; Jiang, Y.; Xu, C.; Wu, J.; Shi, Y. A novel RNA-binding mode of the YTH domain reveals the mechanism for recognition of determinant of selective removal by Mmi1. Nucleic Acids Res. 2016, 44, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Stowell, J.A.W.; Wagstaff, J.L.; Hill, C.H.; Yu, M.; McLaughlin, S.H.; Freund, S.M.V.; Passmore, L.A. A low-complexity region in the YTH domain protein Mmi1 enhances RNA binding. J. Boil. Chem. 2018, 293, 9210–9222. [Google Scholar] [CrossRef] [Green Version]
- Holm, L.; Laakso, L.M. Dali server update. Nucleic Acids Res. 2016, 44, W351–W355. [Google Scholar] [CrossRef]
- Bertonati, C.; Punta, M.; Fischer, M.; Yachdav, G.; Forouhar, F.; Zhou, W.; Kuzin, A.P.; Seetharaman, J.; Abashidze, M.; Ramelot, T.A.; et al. Structural genomics reveals EVE as a new ASCH/PUA-related domain. Proteins: Struct. Funct. Bioinform. 2009, 75, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Arellano, I.; Gallego, J.; Cervera, J.; Pérez-Arellano, I. The PUA domain − a structural and functional overview. FEBS J. 2007, 274, 4972–4984. [Google Scholar] [CrossRef] [PubMed]
- Ishitani, R.; Nureki, O.; Nameki, N.; Okada, N.; Nishimura, S.; Yokoyama, S. Alternative Tertiary Structure of tRNA for Recognition by a Posttranscriptional Modification Enzyme. Cell 2003, 113, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Theobald, D.L.; Mitton-Fry, R.M.; Wuttke, D.S. Nucleic acid recognition by OB-fold proteins. Annu. Rev. Biophys. Biomol. Struct. 2003, 32, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ye, K. Crystal structure of an H/ACA box ribonucleoprotein particle. Nature 2006, 443, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Leng, W.; Lei, X.; Meng, H.; Ouyang, X.; Liang, Z. EOLA1 Inhibits Lipopolysaccharide-Induced Vascular Cell Adhesion Molecule-1 Expression by Association with MT2A in ECV304 Cells. Int. J. Inflamm. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, W.; Adhikari, S.; Dahal, U.; Chen, Y.-S.; Hao, Y.-J.; Sun, B.-F.; Sun, H.-Y.; Li, A.; Ping, X.-L.; Lai, W.-Y.; et al. Nuclear m 6 A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell 2016, 61, 507–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otwinowski, Z.; Minor, W. [20] Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [PubMed]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.-W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D Boil. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Terwilliger, T.C. SOLVE and RESOLVE: Automated Structure Solution and Density Modification. Methods Enzym. 2003, 374, 22–37. [Google Scholar]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K.D. Features and development of Coot. Acta Crystallogr. Sect. D Boil. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Afonine, P.V.; Grosse-Kunstleve, R.W.; Echols, N.; Headd, J.J.; Moriarty, N.W.; Mustyakimov, M.; Terwilliger, T.C.; Urzhumtsev, A.; Zwart, P.H.; Adams, P.D. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. Sect. D Boil. Crystallogr. 2012, 68, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Skubák, P.; Murshudov, G.N.; Pannu, N.S. Direct incorporation of experimental phase information in model refinement. Acta Crystallogr. Sect. D Boil. Crystallogr. 2004, 60, 2196–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, V.B.; Arendall, W.B., 3rd; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| Endothelial-Overexpressed Lipopolysaccharide-Associated Factor 1 (EOLA1, PDB ID: 5Y7D) | SeMet-EOLA1 (SAD, Peak) | |
|---|---|---|
| Data collection | ||
| Beamline | PLS-7A | PLS-5C |
| Space group | P41212 | P41212 |
| Cell dimensions | ||
| a, b, c (Å) | 49.77, 49.77, 175.71 | 49.65, 49.65, 176.08 |
| α, β, γ (°) | 90, 90, 90 | 90, 90, 90 |
| Wavelength (Å) | 1.0000 | 0.9795 |
| Resolution (Å) | 50.00–1.71 (1.74–1.71) 1 | 50.00–1.93 (1.96–1.93) 1 |
| No. of reflections | 24953 | 17540 |
| Rmerge2 | 0.062 (0.579) 1 | 0.081 (0.577) 1 |
| <I>/<σ(I)> | 53.11 (6.13) 1 | 30.28 (5.46) 1 |
| Completeness (%) | 99.8 (99.8) 1 | 99.9 (100.0) 1 |
| Redundancy | 29.4 (24.1) 1 | 15.1 (15.5) 1 |
| Refinement | ||
| Resolution (Å) | 32.93–1.71 | |
| Rwork/Rfree3 (%) | 19.9/24.7 | |
| No. of atoms | 1530 | |
| Macromolecule | 1283 | |
| Ligand/ion 4 | 26 | |
| Water | 221 | |
| RMSD | ||
| Bond lengths (Å) | 0.005 | |
| Bond angles (°) | 1.08 | |
| Overall B factor | 31.4 | |
| Macromolecule | 29.7 | |
| Ligand/ion 4 | 39.2 | |
| Water | 40.2 | |
| Ramachandran favored/outliers (%) | 98.1/0.0 | |
| Poor rotamers (%) | 0.0 | |
| PDB ID | Z-score (DALI) | Cα RMSD | Description | Source |
|---|---|---|---|---|
| 2E50 | 12.4 | 3.4 | Activating signal cointegrator-1 | Homo sapiens |
| 5GUQ | 10.4 | 2.3 | ASCH protein | Zymomonas mobilis |
| 2DP9 | 9.2 | 2.4 | TTHA0113 | Thermus thermophilus |
| 2KKU | 7.1 | 3.0 | Uncharacterized protein AF_2351 | Archaeoglobus fulgidus |
| 2EVE | 6.2 | 3.7 | EVE protein | Pseudomonas syringae |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Park, S.H.; Park, J.S.; Kim, H.-J.; Han, B.W. Crystal Structure of Human EOLA1 Implies Its Possibility of RNA Binding. Molecules 2019, 24, 3529. https://doi.org/10.3390/molecules24193529
Kim M, Park SH, Park JS, Kim H-J, Han BW. Crystal Structure of Human EOLA1 Implies Its Possibility of RNA Binding. Molecules. 2019; 24(19):3529. https://doi.org/10.3390/molecules24193529
Chicago/Turabian StyleKim, Minju, Sang Ho Park, Joon Sung Park, Hyun-Jung Kim, and Byung Woo Han. 2019. "Crystal Structure of Human EOLA1 Implies Its Possibility of RNA Binding" Molecules 24, no. 19: 3529. https://doi.org/10.3390/molecules24193529







