Antitumor Efficacy of Liposome-Encapsulated NVP-BEZ235 Combined with Irreversible Electroporation for Head and Neck Cancer
Abstract
1. Introduction
2. Results
2.1. Synthesis and Characterization of Liposomal NVP-BEZ235
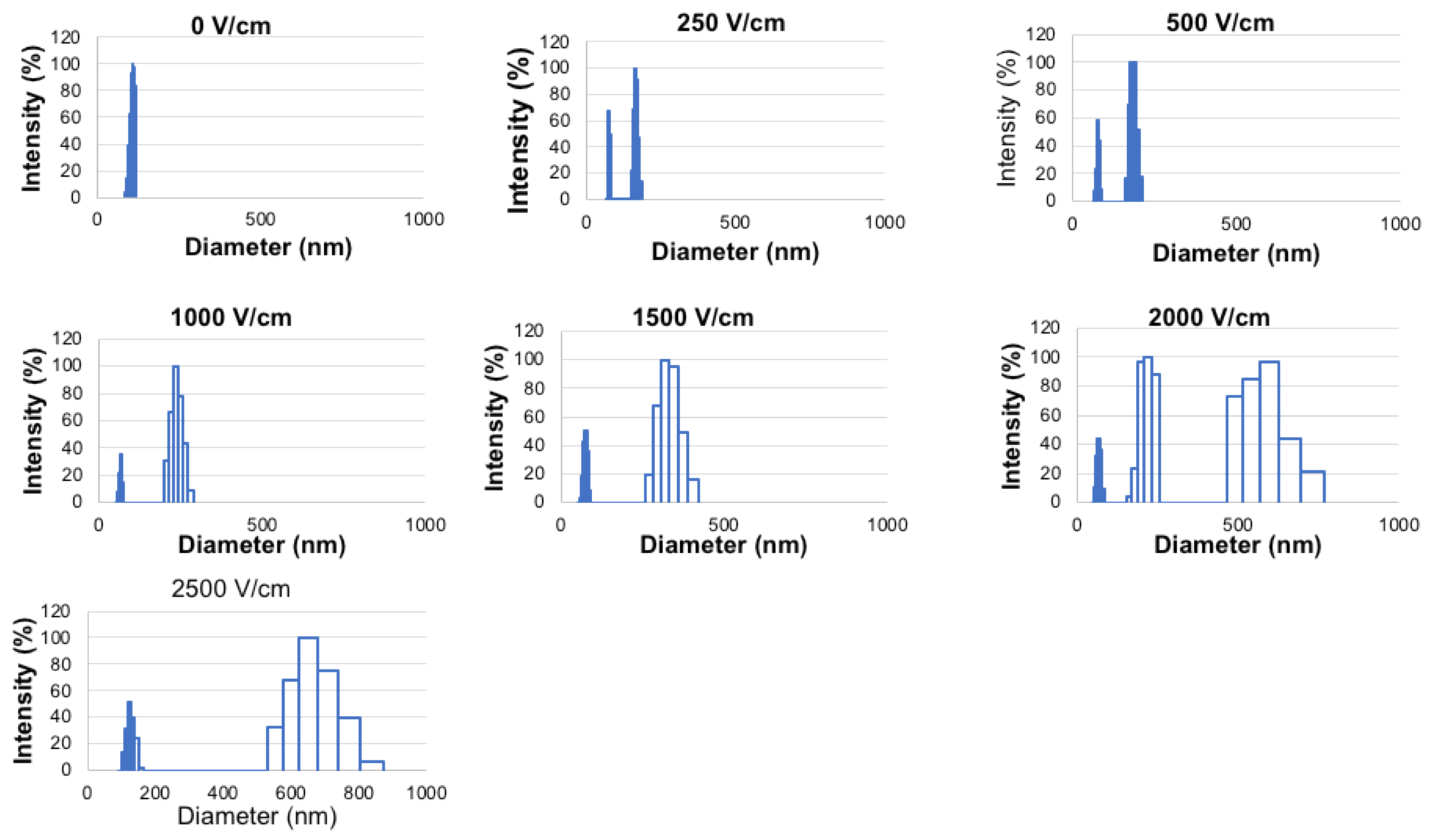
2.2. Effect of Electroporation Field Strength on Liposomal NVP-BEZ235 Stability
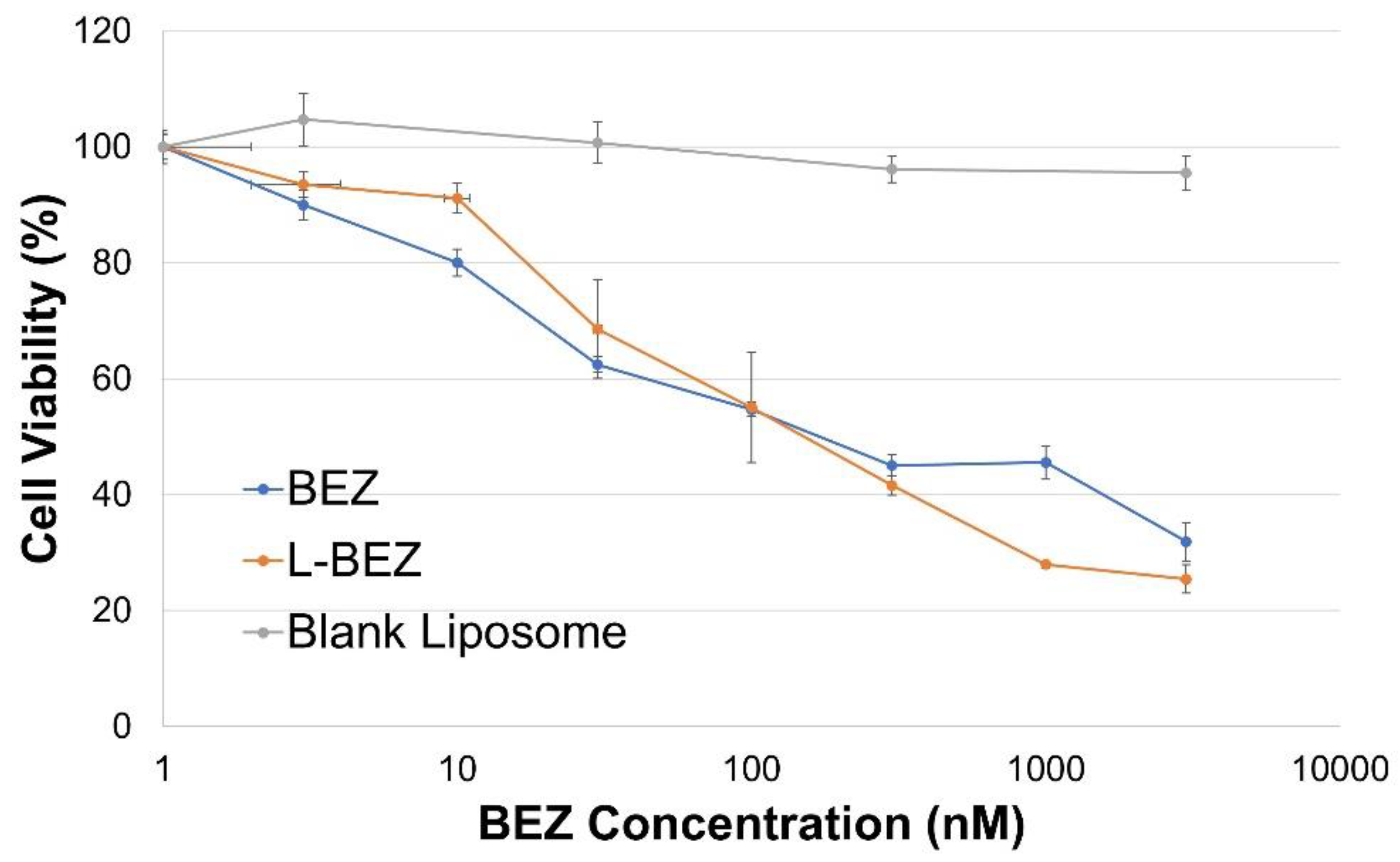
2.3. Liposomal NVP-BEZ235 Cytotoxicity against HN5 Cells
2.4. Effect of Electroporation at Different Field Strengths on HN5 Cells
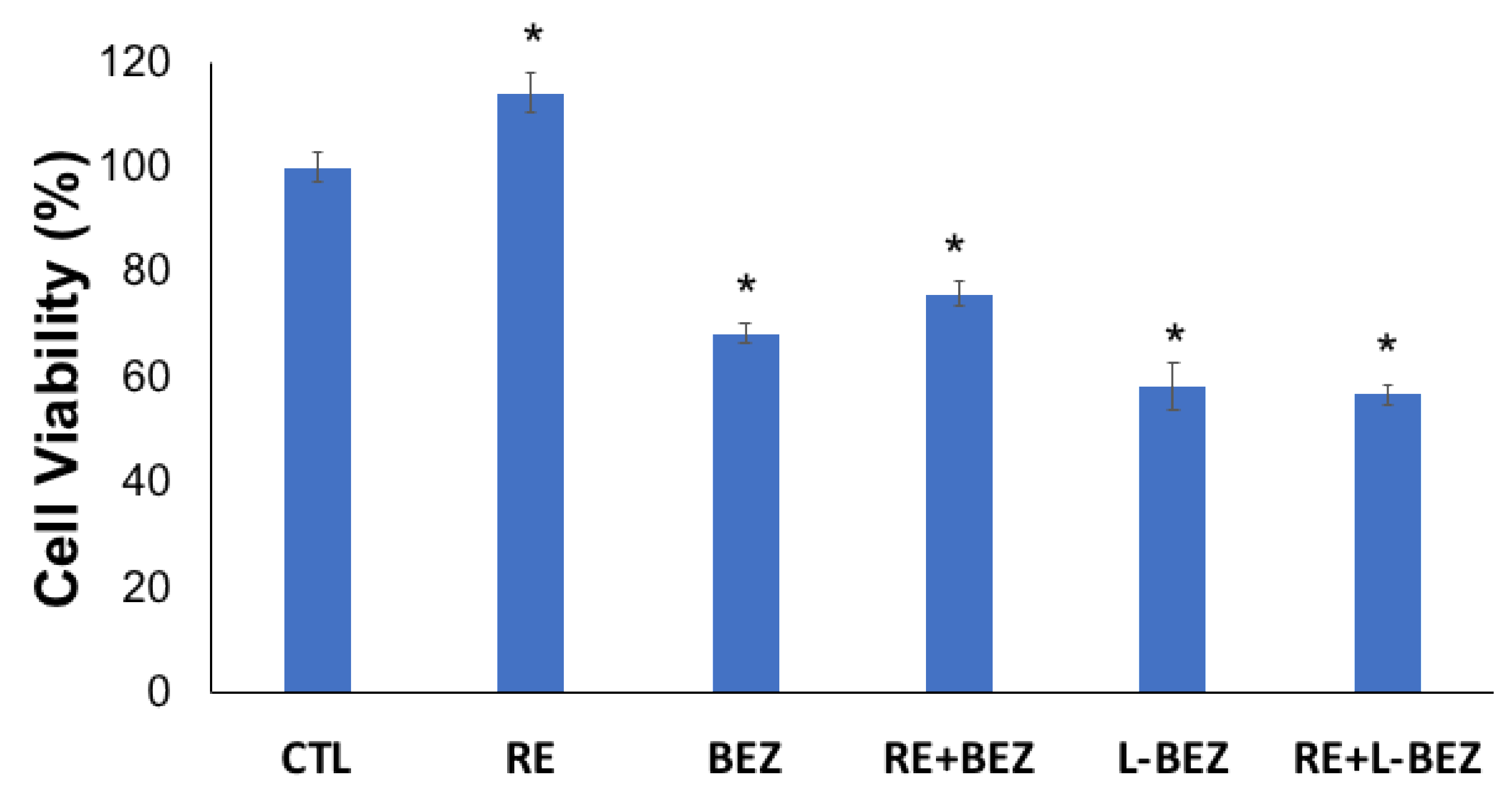
2.5. Effect of Reversible Electroporation, NVP-BEZ235, Liposomal NVP-BEZ235, and Their Combinations on HN5 Cells
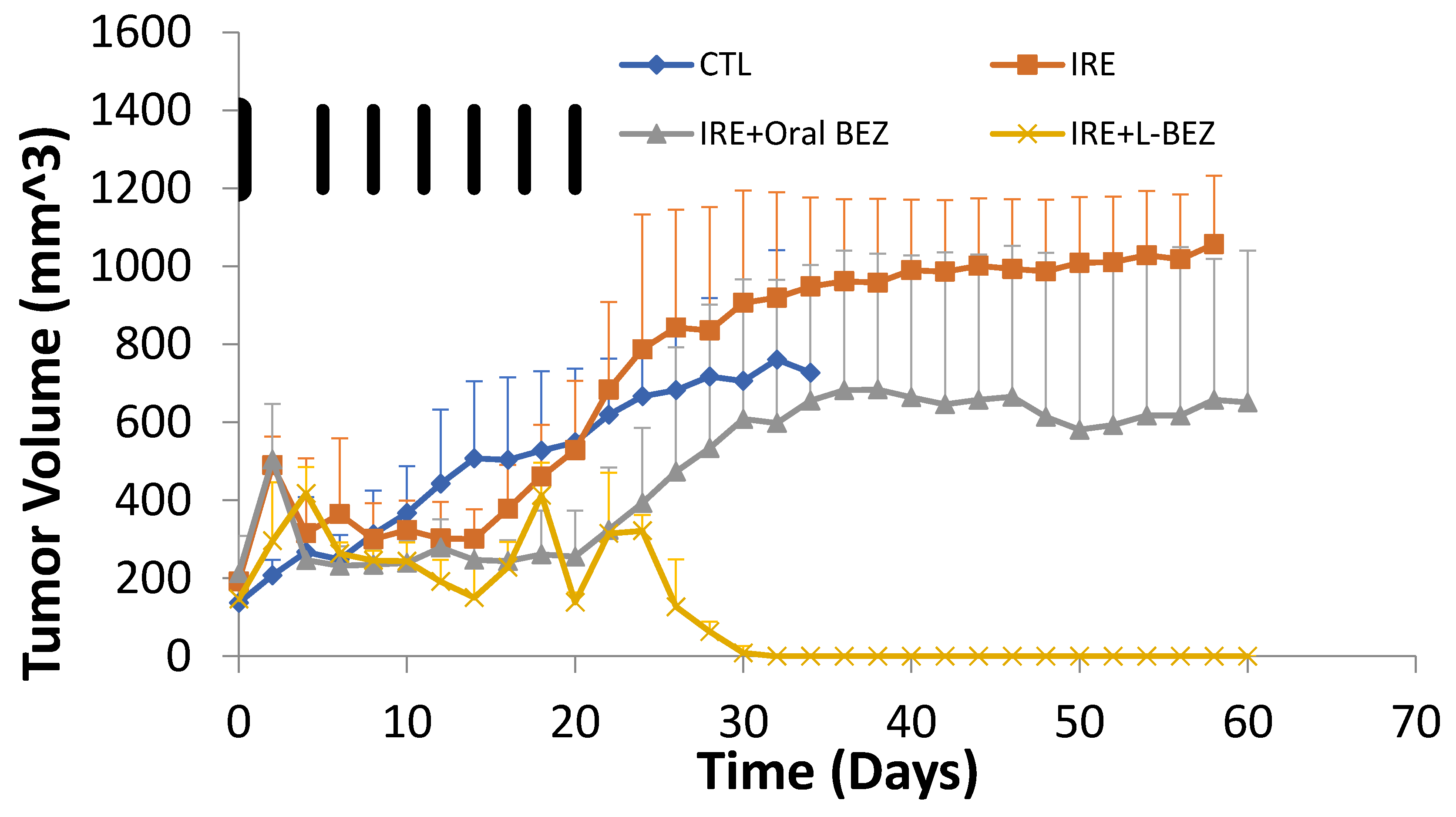
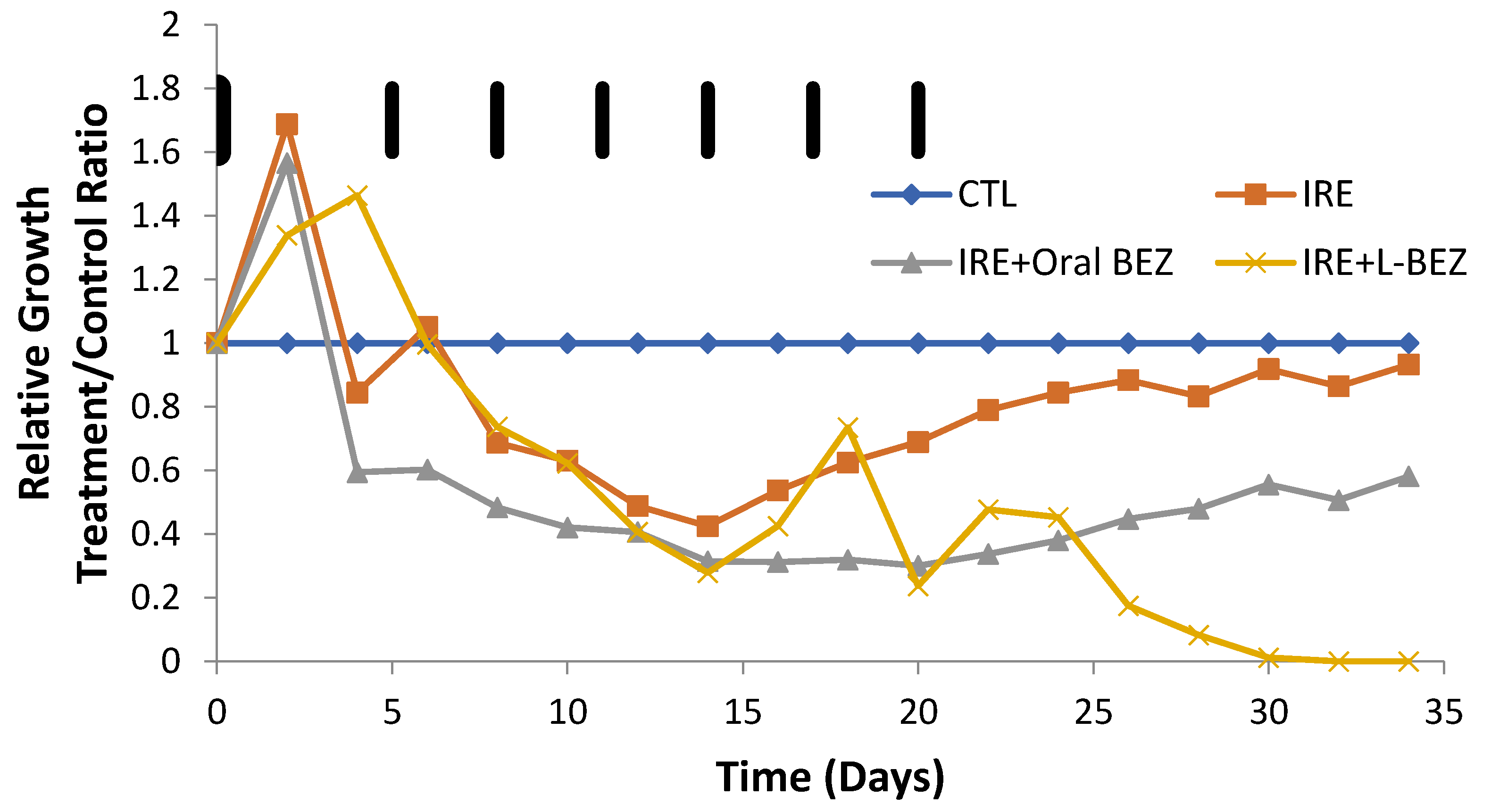
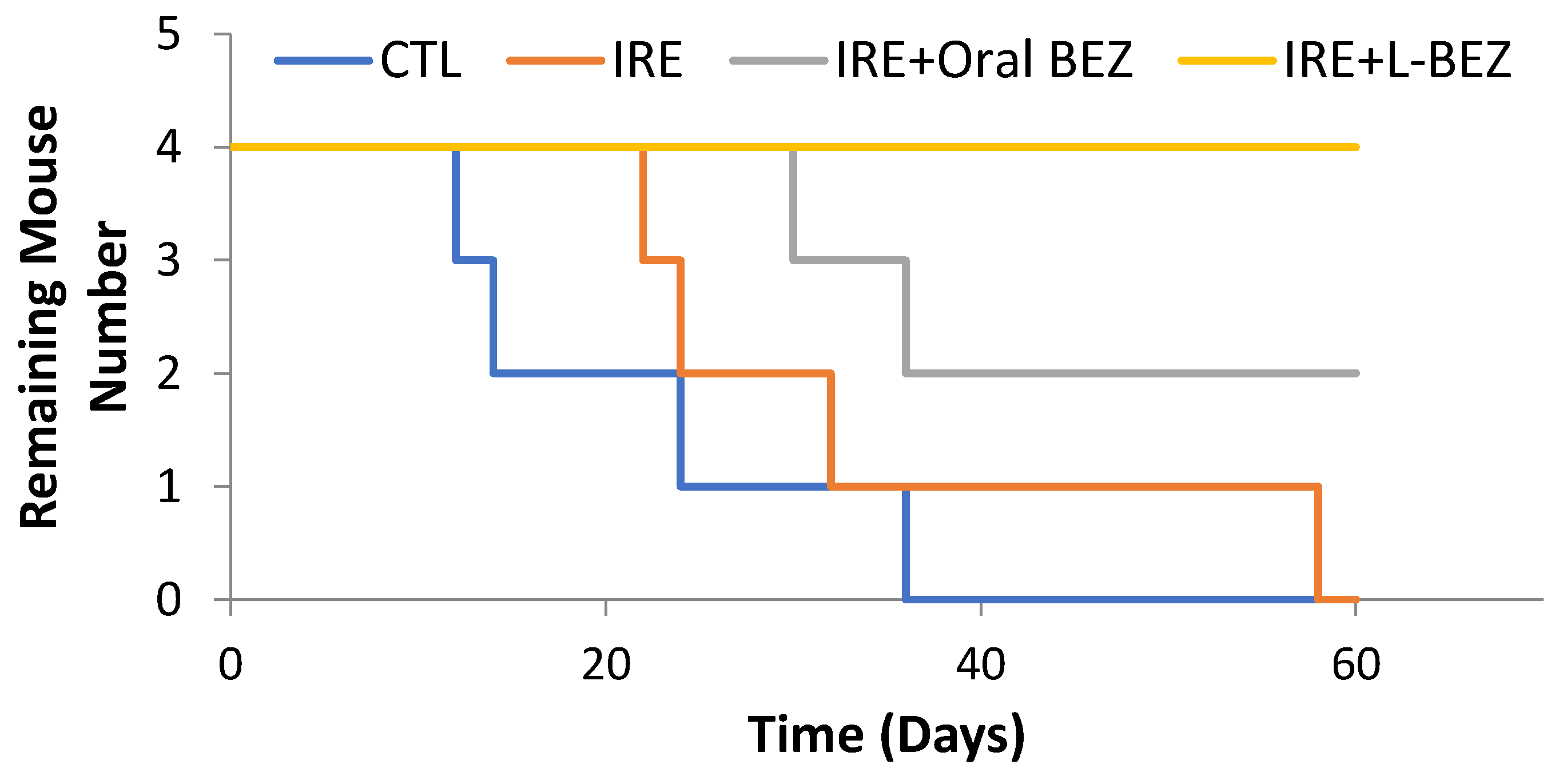
2.6. In Vivo Efficacy of Irreversible Electroporation Plus Liposomal NVP-BEZ235
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Liposome Preparation
4.3. NVP-BEZ235 Quantification
4.4. Cell Culture and Liposomal NVP-BEZ235 Cytotoxicity Assays
4.5. In Vitro Electroporation and Cell Viability Assays
4.6. Treatment of Mouse Xenograft Tumors with Irreversible Electroporation and NVP-BEZ235
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Society of Clinical Oncology. Head and Neck Cancer: Statistics. Available online: www.Cancer.Net/Cancer-Types/Head-and-Neck-Cancer/Statistics (accessed on 2 June 2019).
- Fu, K.K.; Pajak, T.F.; Trotti, A.; Jones, C.U.; Spencer, S.A.; Phillips, T.L.; Garden, A.S.; Ridge, J.R.; Cooper, J.S.; Ang, K.K. A Radiation Therapy Oncology Group (Rtog) Phase Iii Randomized Study to Compare Hyperfractionation and Two Variants of Accelerated Fractionation to Standard Fractionation Radiotherapy for Head and Neck Squamous Cell Carcinomas: First Report of Rtog 9003. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 7–16. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J. Radiotherapy Plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, M.R.; Scheffer, H.J.; de Bree, R.; Sedee, R.J. Percutaneous Irreversible Electroporation for Recurrent Thyroid Cancer—A Case Report. J. Vasc. Interv. Radiol. 2015, 26, 1180–1182. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, A.; Ralli, M.; Longo, L.; Mancini, P.; Attanasio, G.; Atturo, F.; Vincentiis, M.D.; Greco, A. Electrochemotherapy in Head and Neck Cancer: A Review of an Emerging Cancer Treatment. Oncol. Lett. 2018, 16, 3415–3423. [Google Scholar] [CrossRef]
- Golberg, A.; Bruinsma, B.G.; Uygun, B.E.; Yarmush, M.L. Tissue Heterogeneity in Structure and Conductivity Contribute to Cell Survival During Irreversible Electroporation Ablation by Electric Field Sinks. Sci. Rep. 2015, 5, 8485. [Google Scholar] [CrossRef]
- Miklavcic, D.; Davalos, R.V. Electrochemotherapy (Ect) and Irreversible Electroporation (Ire)-Advanced Techniques for Treating Deep-Seated Tumors Based on Electroporation. Biomed. Eng. Online 2015, 14, I1. [Google Scholar] [CrossRef]
- Zhao, J.; Qiao, Y.; Zhou, M.; Wallace, M.; Gupta, S.; Li, C.; Melancon, M.P. Antitumor Efficacy of Irreversible Electroporation and Doxorubicin-Loaded Polymeric Micelles. ACS Macro Lett. 2015, 4, 1081–1084. [Google Scholar] [CrossRef]
- Yair, G.; Rubinsky, B. Methods of Optimization of Electrical Impedance Tomography for Imaging Tissue Electroporation. Physiol. Meas. 2007, 28, 1135. [Google Scholar] [CrossRef]
- Jiang, C.; Davalos, R.V.; Bischof, J.C. A Review of Basic to Clinical Studies of Irreversible Electroporation Therapy. IEEE Trans. Biomed. Eng. 2015, 62, 4–20. [Google Scholar] [CrossRef]
- Tian, L.; Qiao, Y.; Lee, P.; Wang, L.; Chang, A.; Ravi, S.; Rogers, T.A.; Lu, L.; Singhana, B.; Zhao, J. Antitumor Efficacy of Liposome-Encapsulated Nvp-Bez 235 in Combination with Irreversible Electroporation. Drug Deliv. 2018, 25, 668–678. [Google Scholar] [CrossRef]
- Philips, P.; Hays, D.; Martin, R.C. Irreversible Electroporation Ablation (Ire) of Unresectable Soft Tissue Tumors: Learning Curve Evaluation in the First 150 Patients Treated. PLoS ONE 2013, 8, e76260. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle Therapeutics: An Emerging Treatment Modality for Cancer. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific Nature Publishing Group: London, UK, 2009; pp. 239–250. [Google Scholar] [CrossRef]
- Sriram, V.; Rhodes, C.T. Preparation and Characterization of Liposomes as Therapeutic Delivery Systems: A Review. Pharm. Acta Helv. 1995, 70, 95–111. [Google Scholar]
- Horn, D.; Hess, J.; Freier, K.; Hoffmann, J.; Freudlsperger, C. Targeting Egfr-Pi3k-Akt-Mtor Signaling Enhances Radiosensitivity in Head and Neck Squamous Cell Carcinoma. Expert Opin. Ther. Targets 2015, 19, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Zumsteg, Z.S.; Morse, N.; Krigsfeld, G.; Gupta, G.; Higginson, D.S.; Lee, N.Y.; Morris, L.; Ganly, I.; Shiao, S.L.; Powell, S.N. Taselisib (Gdc-0032), a Potent Β-Sparing Small Molecule Inhibitor of Pi3k, Radiosensitizes Head and Neck Squamous Carcinomas Containing Activating Pik3ca Alterations. Clin. Cancer Res. 2016, 22, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Serra, V.; Markman, B.; Scaltriti, M.; Eichhorn, P.J.; Valero, V.; Guzman, M.; Botero, M.L.; Llonch, E.; Atzori, F.; Cosimo, S.D. Nvp-Bez235, a Dual Pi3k/Mtor Inhibitor, Prevents Pi3k Signaling and Inhibits the Growth of Cancer Cells with Activating Pi3k Mutations. Cancer Res. 2008, 68, 8022–8030. [Google Scholar] [CrossRef]
- Fokas, E.; Yoshimura, M.; Prevo, R.; Higgins, G.; Hackl, W.; Maira, S.M.; Bernhard, E.J.; McKenna, W.; Muschel, R.J. Nvp-Bez235 and Nvp-Bgt226, Dual Phosphatidylinositol 3-Kinase/Mammalian Target of Rapamycin Inhibitors, Enhance Tumor and Endothelial Cell Radiosensitivity. Radiat. Oncol. 2012, 7, 48. [Google Scholar] [CrossRef]
- Maira, S.M.; Stauffer, F.; Brueggen, J.; Furet, P.; Schnell, C.; Fritsch, C.; Brachmann, S.; Chène, P.; Pover, A.D.; Schoemaker, K. Identification and Characterization of Nvp-Bez235, a New Orally Available Dual Phosphatidylinositol 3-Kinase/Mammalian Target of Rapamycin Inhibitor with Potent in Vivo Antitumor Activity. Mol. Cancer Ther. 2008, 7, 1851–1863. [Google Scholar] [CrossRef]
- Elad, M.; Ivorra, A.; Leor, J.; Rubinsky, B. Irreversible Electroporation Attenuates Neointimal Formation after Angioplasty. IEEE Trans. Biomed. Eng. 2008, 55, 2268–2274. [Google Scholar] [CrossRef]
- Gary, O.; Mikus, P.; Rubinsky, B. Irreversible Electroporation: Implications for Prostate Ablation. Technol. Cancer Res. Treat. 2007, 6, 295–300. [Google Scholar] [CrossRef]
- Melancon, M.P.; Appleton Figueira, T.; Fuentes, D.T.; Tian, L.; Qiao, Y.; Gu, J.; Gagea, M.; Ensor, J.E.; Muñoz, N.M.; Maldonado, K.M. Development of an Electroporation and Nanoparticle-Based Therapeutic Platform for Bone Metastases. Radiology 2017, 286, 149–157. [Google Scholar] [CrossRef]
- Neal, B.; Agle, S.; Li, Y.; Li, S.; Martin, R.C.G. Irreversible Electroporation Enhances Delivery of Gemcitabine to Pancreatic Adenocarcinoma. J. Surg. Oncol. 2016, 114, 181–186. [Google Scholar] [CrossRef]
- Abdus, S.; Sultana, Y.; Aqil, M. Liposomal Drug Delivery Systems: An Update Review. Curr. Drug Deliv. 2007, 4, 297–305. [Google Scholar]
- Allen, T.M.; Pieter, R.C. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Ickenstein, L.M.; Sandström, M.C.; Mayer, L.D.; Edwards, K. Effects of Phospholipid Hydrolysis on the Aggregate Structure in Dppc/Dspe-Peg2000 Liposome Preparations after Gel to Liquid Crystalline Phase Transition. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.; Johnsson, M.; Karlsson, G.; Silvander, M. Effect of Polyethyleneglycol-Phospholipids on Aggregate Structure in Preparations of Small Unilamellar Liposomes. Biophys. J. 1997, 73, 258–266. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Lipid Composition (DPPC:DSPE-PEG Molar Ratio) | Total Lipid Weight (mg) | Added BEZ (mg) | Extrusion | Particle Size (nm) | Polydispersity | L-BEZ Concentration (mg/mL) | Loading Efficiency (%) |
|---|---|---|---|---|---|---|---|
| 95:5 | 40 | 0.4 | Y | 71.6 ± 0.8 | 0.18 | 0.017 | 11% |
| 90:10 | 40 | 0.4 | Y | 82.3 ± 0.8 | 0.18 | 0.017 | 11% |
| 85:15 | 40 | 0.4 | Y | 90.5 ± 1.2 | 0.19 | 0.017 | 11% |
| 95:5 | 80 | 0.8 | Y | 84.3 ± 1.8 | 0.17 | 0.029 | 9% |
| 95:5 | 80 | 4 | N | 276.1 ± 40.2 | 0.28 | 1.16 | 73% |
| 95:5 | 80 | 8 | N | 411.4 ± 75.4 | 0.36 | 2.42 | 75% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Wang, L.; Qiao, Y.; Lu, L.; Lee, P.; Chang, A.; Ravi, S.; Rogers, T.A.; Melancon, M.P. Antitumor Efficacy of Liposome-Encapsulated NVP-BEZ235 Combined with Irreversible Electroporation for Head and Neck Cancer. Molecules 2019, 24, 3560. https://doi.org/10.3390/molecules24193560
Tian L, Wang L, Qiao Y, Lu L, Lee P, Chang A, Ravi S, Rogers TA, Melancon MP. Antitumor Efficacy of Liposome-Encapsulated NVP-BEZ235 Combined with Irreversible Electroporation for Head and Neck Cancer. Molecules. 2019; 24(19):3560. https://doi.org/10.3390/molecules24193560
Chicago/Turabian StyleTian, Li, Lucas Wang, Yang Qiao, Linfeng Lu, Patrick Lee, Ashley Chang, Saisree Ravi, Thomas A. Rogers, and Marites P. Melancon. 2019. "Antitumor Efficacy of Liposome-Encapsulated NVP-BEZ235 Combined with Irreversible Electroporation for Head and Neck Cancer" Molecules 24, no. 19: 3560. https://doi.org/10.3390/molecules24193560
APA StyleTian, L., Wang, L., Qiao, Y., Lu, L., Lee, P., Chang, A., Ravi, S., Rogers, T. A., & Melancon, M. P. (2019). Antitumor Efficacy of Liposome-Encapsulated NVP-BEZ235 Combined with Irreversible Electroporation for Head and Neck Cancer. Molecules, 24(19), 3560. https://doi.org/10.3390/molecules24193560







