Considerations when Measuring Biocatalyst Performance
Abstract
1. Introduction
2. Motivation for the Assessment of Biocatalyst Performance
3. Existing Methods for Measuring Biocatalyst Performance
- When converting non-natural substrates, it might be of great importance, because the KM might be particularly high (low affinity).
- When poorly water-soluble substrates are supplied from a second organic phase, it is important to know KM in order to ensure that the enzyme is used effectively.
- When enzymes are used for degrading specific compounds in waste streams, the final required concentration of the substrate in the effluent stream may be well below KM, resulting in an inefficient use of the enzyme.
- When high conversions of substrate to product are required, the final part of the reaction may be carried out with substrate concentrations below KM, affecting the amount of enzyme required.
- When using an enzyme in bimolecular reactions, the KM on both substrates needs to be checked to ensure that the concentration of one of the substrates is not beneath its KM.
4. Use of Biocatalyst Yield
5. Complementary Metrics
5.1. Product Concentration
5.2. Productivity
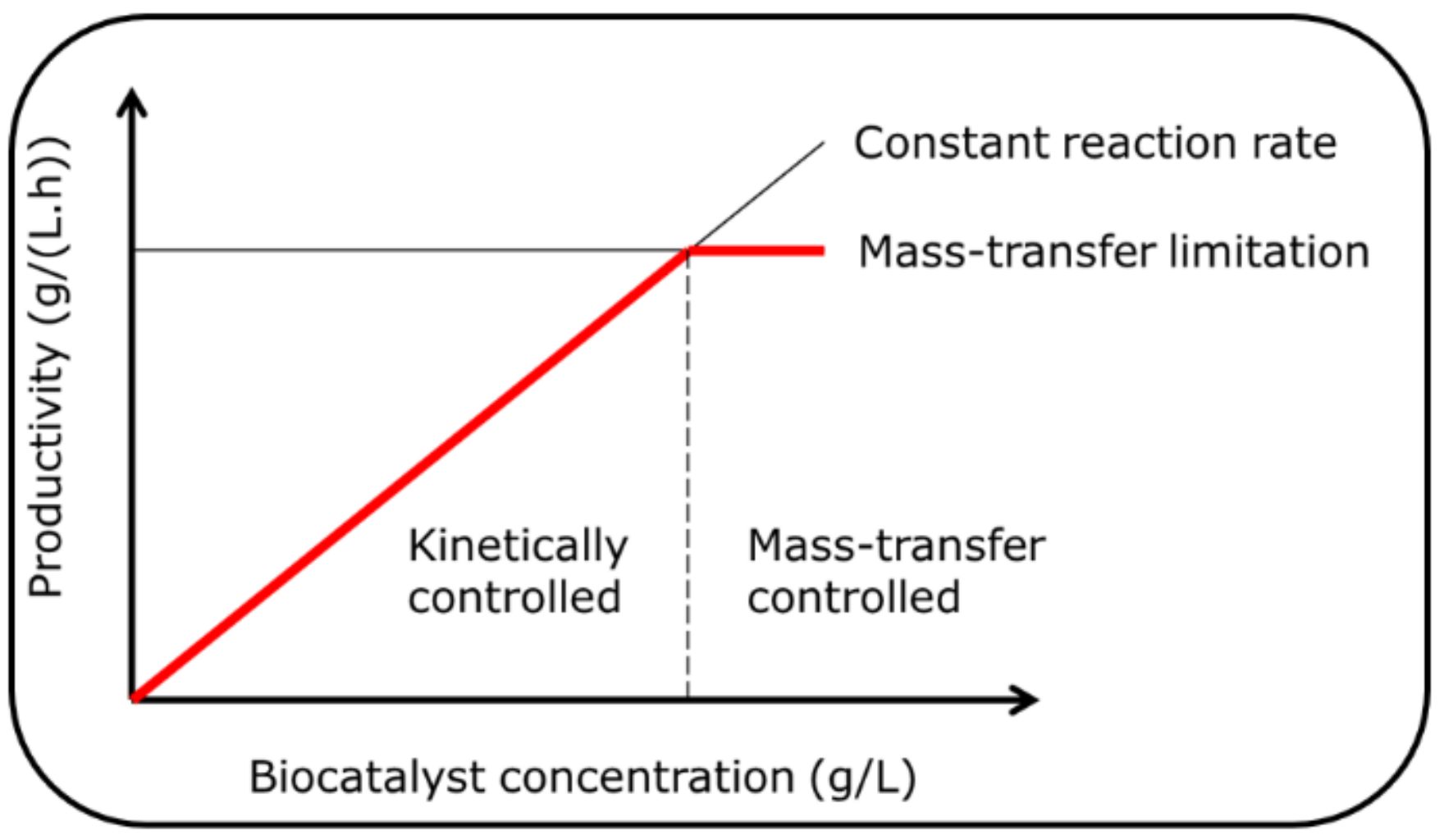
- The first is when the reaction rate is sufficient to become limited not by the enzyme reaction rate, but by the mass transfer of the substrate to the enzyme. This is schematically shown in Figure 1. This is the case with many immobilized enzymes where diffusional limitations mean that the enzyme may operate at less than the maximum rate of reaction. Hence, in such cases, it is always good to evaluate the enzyme activity both with a soluble and an immobilized enzyme. Interestingly, improvements in enzyme activity due to protein engineering make this problem more complicated, since for the same load of protein, the maximum possible activity will be higher. Likewise, with poorly-water soluble substrates, problems can arise. The well-known case of oxygen supply to oxidases (or oxygenases) highlights this point well. Here, the maximum transfer rate of oxygen is limited by the low water solubility of oxygen, meaning that the maximum rates will always be low (even in cases with a high mass transfer coefficient of oxygen from a gas to aqueous phase). A more detailed explanation is given in several recent publications on the topic [38,39].
- The second case is when the amount of protein loaded in the reactor exceeds what is practical from an operational perspective. For example, an immobilized enzyme will reach a maximum in a stirred tank at around 10% by volume. Above this value, particle-particle collisions will result in attrition (making downstream filtration problematic). Likewise, in a packed bed, to allow for the void space between particles and to achieve adequate flow through the bed, a maximum of around 60% by volume is to be expected. A soluble enzyme can also have an upper limit, dependent upon downstream recovery (ultrafiltration) or removal strategies. In pharmaceutical processes, regulatory demands mean that all residual protein must be removed from solution prior to the final processing steps and product formulation.
6. Towards a Systematic Approach
6.1. Initial Tests of Intrinsic Metrics
6.2. Conditions for Establishing Metrics Closer to Industrial Operation
6.3. Modelling
7. Concluding Remarks
Funding
Conflicts of Interest
References
- Woodley, J.M. New opportunities for biocatalysis: making pharmaceutical processes greener. Trends Biotechnol. 2008, 26, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. The Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–834. [Google Scholar] [CrossRef] [PubMed]
- Arnold, F.H. Directed evolution: Bringing new chemistry to life. Angew. Chem. Int. Ed. 2018, 57, 4143–4148. [Google Scholar] [CrossRef] [PubMed]
- Currin, A.; Swainston, N.; Day, P.J.; Kell, D.B. Synthetic biology for the directed evolution of protein biocatalysts: navigating sequence space intelligently. Chem. Soc. Rev. 2015, 44, 1172–1239. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.P.; Brown, M.J.B.; Diaz-Rodriguez, A.; Lloyd, R.C.; Roiban, G.-D. Biocatalysis: A pharma perspective. Adv. Synth. Catal. 2019, 361, 2421–2432. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Brady, D. The limits to biocatalysis: pushing the envelope. Chem. Commun. 2018, 54, 6088–6104. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T. What are the limitations of enzymes in synthetic organic chemistry? Chem. Rec. 2016, 16, 2449–2459. [Google Scholar] [CrossRef]
- Bommarius, A.S. Biocatalysis: A status report. Annu. Rev. Chem. Biomol. Engng. 2015, 6, 319–345. [Google Scholar] [CrossRef]
- Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J.M. Guidelines and cost analysis for catalyst production in biocatalytic processes. Org. Proc. Res. Dev. 2011, 15, 266–274. [Google Scholar] [CrossRef]
- Polizzi, K.M.; Bommarius, A.S.; Broering, J.M.; Chaparro-Riggers, J.F. Stability of biocatalysts. Curr. Opin. Chem. Biol. 2007, 11, 220–225. [Google Scholar] [CrossRef]
- Klein-Marcuschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, H.W. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 2012, 109, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; Luley-Goedl, C.; Leitner, E.; Sawangwan, T.; Nidetzky, B. Production of glucosyl glycerol by immobilized sucrose phosphorylase: Options for enzyme fixation on a solid support and application in microscle format. J. Biotechnol. 2017, 257, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.P.; Penafiel, I.; Cosgrove, S.C.; Turner, N.J. Biocatalysis using immobilized enzymes in continuous flow for the synthesis of fine chemicals. Org. Proc. Res. Dev. 2018, 23, 9–18. [Google Scholar] [CrossRef]
- Tamborini, L.; Fernandes, P.; Paradisi, F.; Molinari, F. Flow bioreactors as complementary tools for biocatalytic process intensification. Trends Biotechnol. 2017, 36, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, J.; Khinast, J. The future of pharmaceutical manufacturing sciences. J. Pharm. Sci. 2015, 104, 3612–3638. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.; Jamison, T.F. The assembly and use of continuous flow systems for chemical synthesis. Nature Protocol. 2017, 12, 2423–2446. [Google Scholar] [CrossRef] [PubMed]
- Przybysz, A.; Volmer, A.A.; Westphal, A.H.; van Berkel, W.J.H. Bifunctional immobilization of a hyperthermostable endo-β-1,3-glucanase. Appl. Microbiol. Biotechnol. 2014, 98, 1155–1163. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Gorines, B.C.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Effect of protein load on stability of immobilized enzymes. Enzyme. Microb. Technol. 2017, 98, 18–25. [Google Scholar] [CrossRef]
- Vennestrøm, P.N.R.; Christensen, C.H.; Pedersen, S.; Grunwaldt, J.-D.; Woodley, J.M. Next generation catalysis for renewables: Combining enzymatic with inorganic heterogeneous catalysis for bulk chemical production. ChemCatChem. 2010, 2, 249–258. [Google Scholar] [CrossRef]
- Corey, E.J. The logic of chemical synthesis: Multistep synthesis of complex carbohenic molecules. Angew. Chem. Int. Ed. Engl. 1991, 30, 455–465. [Google Scholar] [CrossRef]
- Turner, N.J.; O’Reilly, E. Biocatalytic retrosynthesis. Nature Chem. Biol. 2013, 9, 285–288. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.O.M.A.; Miranda, L.S.M.; Bornscheuer, U.T. A retrosynthetic approach for biocatalysis in organic synthesis. Chem. Eur. J. 2017, 23, 12040–12063. [Google Scholar] [CrossRef] [PubMed]
- Hönig, M.; Sondermann, O.; Turner, N.J.; Carreira, E.M. Enantioselective chemo- and biocatalysis: Partners in retrosynthesis. Angew. Chem. Int. Ed. 2017, 56, 8942–8973. [Google Scholar] [CrossRef] [PubMed]
- Kohls, H.; Steffen-Munsbery, F.; Höhne, M. Recent achievements in developing the biocatalytic toolbox for chiral amine synthesis. Curr. Opin. Chem. Biol. 2014, 19, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Ghislieri, D.; Turner, N.J. Biocatalytic approaches to the synthesis of enantiomerically pure chiral amines. Top. Catal. 2014, 57, 284–300. [Google Scholar] [CrossRef]
- Batista, V.F.; Galman, J.L.; Pinto, D.C.G.A.; Silva, A.M.S.; Turner, N.J. Monoamine oxidase: Tunable activity for amine resolution and functionalization. ACS Catal. 2018, 8, 11889–11907. [Google Scholar] [CrossRef]
- Woodley, J.M. Integrating Protein Engineering with Process Design for Biocatalysis. Phil. Trans. R. Soc. (Lond.) A 2017, 376, 20170062. [Google Scholar] [CrossRef]
- Bommarius, A.S.; Blum, J.K.; Abrahamson, M.J. Status of protein engineering for biocatalysts: How to design an industrially useful biocatalyst. Curr. Opin. Chem. Biol. 2011, 15, 194–200. [Google Scholar] [CrossRef]
- Rogers, T.A.; Bommarius, A.S. Utilizing simple biochemical measurements to predict lifetime output of biocatalysts in continuous isothermal processes. Chem. Eng. Sci. 2010, 65, 2118–2124. [Google Scholar] [CrossRef]
- Carro, J.; Fernandez-Fueyo, E.; Fernandez-Alonso, C.; Cañada, J.; Ullrich, R.; Hofrichter, M.; Alcalde, M.; Ferreira, P.; Martínez, A.T. Self-sustained enzymatic cascade for the production of 2,5-furandicarboxylic acid from 5-methoxymethylfurfural. Biotechnol. Biofuels 2018, 11, 86. [Google Scholar] [CrossRef]
- Kozuch, S.; Martin, J.M.L. ‘Turning over’ definitions in catalytic cycles. ACS Catal. 2012, 2, 2787–2794. [Google Scholar] [CrossRef]
- Bommarius, A.S.; Paye, M.F. Stabilizing biocatalysts. Chem. Soc. Rev. 2013, 42, 6534–6565. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Holtmann, D.; Hollmann, F. How green is biocatalysis? To calculate is to know. ChemCatChem. 2014, 6, 930–943. [Google Scholar] [CrossRef]
- Hinzmann, A.; Glinsky, S.; Worm, M.; Gröger, H. Enzymatic synthesis of aliphatic nitriles at a substrate loading of 1.4 kg/L: A biocatalytic record achieved with a heme protein. J. Org. Chem. 2019, 84, 4867–4872. [Google Scholar] [CrossRef] [PubMed]
- Bornadel, A.; Bisagni, S.; Pushpanath, A.; Montgomery, S.L.; Turner, N.J.; Dominguez, B. Technical considerations for scale-up of imine-reductase –catalyzed reductive amination: A case study. Org. Proc. Res. Dev. 2019, 23, 1262–1268. [Google Scholar] [CrossRef]
- Lye, G.J.; Woodley, J.M. Application of in situ product-removal techniques to biocatalytic processes. Trends Biotechnol. 1999, 17, 395–402. [Google Scholar] [CrossRef]
- Hülsewede, D.; Meyer, L.-E.; von Langermann, J. Application of in.situ product crystallization and related techniques in biocatalytic processes. Chem. Eur. J. 2019, 25, 4871–4884. [Google Scholar] [CrossRef] [PubMed]
- Toftgaard Pedersen, A.; Birmingham, W.R.; Rehn, G.; Charnock, S.J.; Turner, N.J.; Woodley, J.M. Process requirements of galactose oxidase based oxidation of alcohols. Org. Proc. Res. Dev. 2015, 19, 1580–1589. [Google Scholar] [CrossRef]
- Woodley, J.M. Reaction Engineering for the Industrial Implementation of Biocatalysis. Topics Catal. 2019. [Google Scholar] [CrossRef]
- Duggleby, R.G. Analysis of progress curves for enzyme-catalyzed reactions: Application to unstable enzymes, coupled reactions and transient-state kinetics. Biochim. Biophys. Acta. 1994, 1205, 268–274. [Google Scholar] [CrossRef]
- Duggleby, R.G. Quantitative analysis of the time course of enzyme catalyzed reactions. Methods 2001, 24, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Blackmond, D.G. Reaction progress kinetic analysis: A powerful methodology for mechanistic studies of complex catalytic reactions. Angew. Chem. Int. Ed. 2005, 44, 4302–4320. [Google Scholar] [CrossRef] [PubMed]
- Selwyn, M.J. A simple test for inactivation of an enzyme during assay. Biochim. Biophys. Acta. 1965, 105, 193–195. [Google Scholar] [CrossRef]
- Nordblad, M.; Dias Gomes, M.; Meissner, M.P.; Ramesh, H.; Woodley, J.M. Scoping Biocatalyst Performance using Reaction Trajectory Analysis. Org. Proc. Res. Dev. 2018, 22, 1101–1114. [Google Scholar] [CrossRef]
- Thomas, C.R.; Nienow, A.W.; Dunnill, P. Action of shear on enzymes: Studies with alcohol dehydrogenase. Biotechnol. Bioeng. 1979, 21, 2263–2278. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.R.; Geer, D. Effects of shear on proteins in solution. Biotechnol. Lett. 2011, 33, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Bommarius, A.S.; Karau, A. Dectivation of formate dehydrogenase (FDH) in solution and at gas-liquid interfaces. Biotechnol. Prog. 2005, 21, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.S.; Ghadge, R.S.; Sawant, S.B.; Joshi, J.B. Lipase deactivation at gas-liquid interface and its subsequent reactivation. AIChE. J. 2000, 46, 1280–1283. [Google Scholar] [CrossRef]
- Bhagia, S.; Dhir, R.; Kumar, R.; Wyman, C.E. Deactivation of cellulose at the air-liquid interface is the main cause of incomplete cellulose conversion at low enzyme loadings. Nature Sci. Rep. 2018, 8, 1350. [Google Scholar] [CrossRef]
- Dias Gomes, M.; Bommarius, B.R.; Anderson, S.R.; Feske, B.; Woodley, J.M.; Bommarius, A.S. Bubble Column Enables Higher Reaction Rate for Deracemization of (R,S)-1-phenylethanol with Coupled Alcohol Dehydrogenase/NADH Oxidase System. Adv. Synth. Catal. 2019, 361, 2574–2581. [Google Scholar] [CrossRef]
- Egger, T.; Egger, L.S.; Fieg, G. Scale and causes of catalyst activity loss in enzymatic catalyzed reactive distillation. Chem. Eng. Sci. 2018, 178, 324–334. [Google Scholar] [CrossRef]
- Henley, J.P.; Sadana, A. Categorization of enzyme deactivations using a series-type mechanism. Enzyme Microb. Technol. 1985, 7, 50–60. [Google Scholar] [CrossRef]
- Sadana, A.; Henley, J.P. Single-step unimolecular non-first-order enzyme deactivation kinetics. Biotechnol. Bioeng. 1987, 30, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, P.R.; Uehara, C.S.; Neunert, U.; Bommarius, A.S. Accelerated biocatalyst stability testing for process optimization. Biotechnol. Prog. 2005, 21, 762–774. [Google Scholar] [CrossRef]
- Bommarius, A.S.; Broering, J.M.; Polizzi, K.M. High-throughput screening for enhanced protein stability. Curr. Opin. Biotechnol. 2006, 17, 606–610. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Metric | Definition | Advantages | Main Limitations |
|---|---|---|---|
| kcat/KM | mol P/(mol Biocat.t.KM) | Biochemists standard measurement | Disregards stability |
| TON | mol P/mol Biocat | Often used to compare with other enzymes | Disregards rate |
| TOF | mol P/mol Biocat.t | Good for stable enzymes | Disregards stability |
| TTN * | mol P/mol Biocat | Good for laboratory use with pure enzymes | Disregards enzyme purity |
| BY * | mass P/mass B | Good for economic assessment | Disregards other metrics ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias Gomes, M.; Woodley, J.M. Considerations when Measuring Biocatalyst Performance. Molecules 2019, 24, 3573. https://doi.org/10.3390/molecules24193573
Dias Gomes M, Woodley JM. Considerations when Measuring Biocatalyst Performance. Molecules. 2019; 24(19):3573. https://doi.org/10.3390/molecules24193573
Chicago/Turabian StyleDias Gomes, Mafalda, and John M. Woodley. 2019. "Considerations when Measuring Biocatalyst Performance" Molecules 24, no. 19: 3573. https://doi.org/10.3390/molecules24193573
APA StyleDias Gomes, M., & Woodley, J. M. (2019). Considerations when Measuring Biocatalyst Performance. Molecules, 24(19), 3573. https://doi.org/10.3390/molecules24193573






