Plant-Derived Oleanolic Acid (OA) Ameliorates Risk Factors of Cardiovascular Diseases in a Diet-Induced Pre-Diabetic Rat Model: Effects on Selected Cardiovascular Risk Factors
Abstract
:1. Introduction
2. Results
2.1. Fasting Blood Glucose
2.2. Mean Arterial Pressure
2.3. Heart and Body Weights; Cardiac C-Reactive Protein (CRP)
2.4. Plasma Lipid Profile
2.5. Inflammatory Markers (IL-6 and TNFα)
2.6. Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Extraction Method
4.3. Animal Studies
4.3.1. Experimental Design
4.3.2. Experimental Protocol
4.3.3. Experimental Procedures
Blood Collection and Tissue Harvesting
4.4. Biochemical Analysis
4.5. MDA Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van Dieren, S.; Beulens, J.W.J.; van der, S.Y.T.; Grobbee, D.E.; Nealb, B. The global burden of diabetes and its complications: An emerging pandemic. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, s3–s8. [Google Scholar]
- Isomaa, B.; Almgren, P.; Tuomi, T.; Forsén, B.; Lahti, K.; Nissén, M.; Taskinen, M.-R.; Groop, L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001, 24, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.-H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J. Am. Coll. Cardiol. 2012, 59, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B. Adipose tissue, inflammation and atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Giles, T.D.; Sander, G.E.; Nossaman, B.D.; Kadowitz, P.J. Impaired vasodilation in the pathogenesis of hypertension: Focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J. Clin. Hypertens. 2012, 14, 198–205. [Google Scholar] [CrossRef]
- Hsieh, H.-J.; Liu, C.-A.; Huang, B.; Tseng, A.H.; Wang, D. Shear-induced endothelial mechanotransduction: The interplay between reactive oxygen species (ROS) and nitric oxide (No) and the pathophysiological implications. J. Biomed. Sci. 2014, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Foran, J.P.; Jain, A.K.; Casserly, I.P.; Kandzari, D.E.; Rocha-Singh, K.J.; Witkowski, A.; Katzen, B.T.; Deaton, D.; Balmforth, P.; Sobotka, P.A. The ROX coupler: Creation of a fixed iliac arteriovenous anastomosis for the treatment of uncontrolled systemic arterial hypertension, exploiting the physical properties of the arterial vasculature. Catheter. Cardiovasc. Interv. 2015, 85, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Herouvi, D.; Karanasios, E.; Karayianni, C.; Karavanaki, K. Cardiovascular disease in childhood: The role of obesity. Eur. J. Pediatr. 2013, 172, 721–732. [Google Scholar] [CrossRef]
- Castro, A.V.B.; Kolka, C.M.; Kim, S.P.; Bergman, R.N. Obesity, insulin resistance and comorbidities? mechanisms of association. Arq. Bras. Endocrinol. Metabol. 2014, 58, 600–609. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.-P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; DeFronzo, R.A.; Einhorn, D.; Fonseca, V.A.; et al. Consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm—2016 executive summary. Endocrinol. Pract. 2016, 22, 84–113. [Google Scholar] [CrossRef] [PubMed]
- Devonish, J.A.; Singh, S.; Tomkinson, E.; Morse, G.D. Novel considerations about diabetes management strategies in chinese immigrants in america: Possible corollaries of the use of traditional chinese medicines. Innov. Pharm. 2017, 8, 4. [Google Scholar] [CrossRef]
- Polonsky, W.H.; Henry, R.R. Poor medication adherence in type 2 diabetes: Recognizing the scope of the problem and its key contributors. Patient Prefer. Adher. 2016, 10, 1299. [Google Scholar] [CrossRef]
- Luo, H.; Liu, J.; Ouyang, Q.; Xuan, C.; Wang, L.; Li, T.; Liu, J. The effects of oleanolic acid on atherosclerosis in different animal models. Acta Biochim. Biophys. Sin. 2017, 49, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. The effects of plant-derived oleanolic acid on selected parameters of glucose homeostasis in a diet-induced pre-diabetic rat model. Molecules 2018, 23, 794. [Google Scholar] [CrossRef] [PubMed]
- Vernochet, C.; Damilano, F.; Mourier, A.; Bezy, O.; Mori, M.A.; Smyth, G.; Rosenzweig, A.; Larsson, N.-G.; Kahn, C.R. Adipose tissue mitochondrial dysfunction triggers a lipodystrophic syndrome with insulin resistance, hepatosteatosis, and cardiovascular complications. FASEB J. 2014, 28, 4408–4419. [Google Scholar] [CrossRef] [Green Version]
- Patel, T.P.; Rawal, K.; Bagchi, A.K.; Akolkar, G.; Bernardes, N.; da Silva Dias, D.; Gupta, S.; Singal, P.K. Insulin resistance: An additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail. Rev. 2016, 21, 11–23. [Google Scholar] [CrossRef]
- Costantino, S.; Paneni, F.; Battista, R.; Castello, L.; Capretti, G.; Chiandotto, S.; Tanese, L.; Russo, G.; Pitocco, D.; Lanza, G.A. Impact of glycemic variability on chromatin remodeling, oxidative stress and endothelial dysfunction in type 2 diabetic patients with target hba1c levels. Diabetes 2017, 66, 2472–2482. [Google Scholar] [CrossRef]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Luvuno, M.; Mabandla, M.; Khathi, A. Voluntary ingestion of a high-fat high-carbohydrate diet: A model for prediabetes. Ponte Int. Sci. Res. J. 2018, 74. [Google Scholar]
- Mazzotti, A.; Caletti, M.T.; Marchignoli, F.; Forlani, G.; Marchesini, G. Which treatment for type 2 diabetes associated with non-alcoholic fatty liver disease? Digest. Liver Dis. 2017, 49, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Putta, S.; Sastry Yarla, N.; Kumar Kilari, E.; Surekha, C.; Aliev, G.; Basavaraju Divakara, M.; Sridhar Santosh, M.; Ramu, R.; Zameer, F.; Prasad, M. Therapeutic potentials of triterpenes in diabetes and its associated complications. Curr. Top. Med. Chem. 2016, 16, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, J.; Borzym-Kluczyk, M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem. Rev. 2015, 14, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Khathi, A.; Mbatha, B.; Musabayane, C.T. The hypoglycaemic and antioxidant properties of oleanolic acid ameliorate blood pressure and kidney function of experimental animals. Endocrinol. Abs 2015, 38, P224. [Google Scholar] [CrossRef]
- Committee, I.E. International expert committee report on the role of the a1c assay in the diagnosis of diabetes. Diabetes Care 2009, 32, 1327–1334. [Google Scholar] [CrossRef]
- Rosqvist, F.; Kullberg, J.; Orho-Melander, M.; Cederholm, T.; Ahlström, H.; Risérus, U. Effects of overfeeding polyunsaturated and saturated fat on lean tissue, liver fat and visceral fat accumulation in overweight and obese humans. Diabetes 2014, 63, 2356–2368. [Google Scholar] [CrossRef]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Chang, C.-I.; Chou, C.-H.; Liao, M.-H.; Chen, T.-M.; Cheng, C.-H.; Anggriani, R.; Tsai, C.-P.; Tseng, H.-I.; Cheng, H.-L. Bitter melon triterpenes work as insulin sensitizers and insulin substitutes in insulin-resistant cells. J. Funct. Foods 2015, 13, 214–224. [Google Scholar] [CrossRef]
- da Luz, G.; Frederico, M.J.S.; Castro, A.J.G.; Moraes, A.L.L.; de Carvalho, F.K.; Espíndola, L.; Schmidt, É.C.; Bouzon, Z.L.; Pizzolatti, M.G.; Silva, F.R.M.B. Triterpene derivative: A potential signaling pathway for the fern-9 (11)-ene-2α, 3β-diol on insulin secretion in pancreatic islet. Life Sci. 2016, 154, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, S.; Xu, J.; Wang, D.-B.; Chen, Y.; Yang, G.-Z. Triterpenoid saponins from stauntonia chinensis ameliorate insulin resistance via the amp-activated protein kinase and IR/IRS-1/PI3K/Akt pathways in insulin-resistant HepG2 cells. Int. J. Mol. Sci. 2014, 15, 10446–10458. [Google Scholar] [CrossRef] [PubMed]
- Kayama, Y.; Raaz, U.; Jagger, A.; Adam, M.; Schellinger, I.; Sakamoto, M.; Suzuki, H.; Toyama, K.; Spin, J.; Tsao, P. Diabetic cardiovascular disease induced by oxidative stress. Int. J. Mol. Sci. 2015, 16, 25234–25263. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco, J.; Holgado, F.; Márquez-Ruiz, G.; Ruiz-Méndez, M.V. Concentrates of triterpenic acids obtained from crude olive pomace oils: Characterization and evaluation of their potential antioxidant activity. J. Sci. Food Agric. 2018, 98, 4837–4844. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, B.; Hedjazifar, S.; Gogg, S.; Hammarstedt, A.; Smith, U. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Met. 2015, 26, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Caprio, S.; Perry, R.; Kursawe, R. Adolescent obesity and insulin resistance: Roles of ectopic fat accumulation and adipose inflammation. Gastroenterology 2017, 152, 1638–1646. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Chapman, M.J.; Ginsberg, H.N.; Amarenco, P.; Andreotti, F.; Borén, J.; Catapano, A.L.; Descamps, O.S.; Fisher, E.; Kovanen, P.T.; Kuivenhoven, J.A. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur. Heart J. 2011, 32, 1345–1361. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ. Res. 2016, 118, 547. [Google Scholar] [CrossRef]
- Xia, B.; Cai, G.H.; Yang, H.; Wang, S.P.; Mitchell, G.A.; Wu, J.W. Adipose tissue deficiency of hormone-sensitive lipase causes fatty liver in mice. PLoS Genet. 2017, 13, e1007110. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egert, S.; Boesch-Saadatmandi, C.; Wolffram, S.; Rimbach, G.; Müller, M.J. Serum lipid and blood pressure responses to quercetin vary in overweight patients by apolipoprotein e genotype. J. Nutr. 2010, 140, 278–284. [Google Scholar] [CrossRef] [PubMed]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (JNC 8). JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Li, H. Therapeutic effect of enhancing endothelial nitric oxide synthase (enos) expression and preventing enos uncoupling. Br. J. Pharmacol. 2011, 164, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R. Oleanolic acid and related triterpenoids from olives on vascular function: Molecular mechanisms and therapeutic perspectives. Curr. Med. Chem. 2015, 22, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef]
- Hu, D.; Xu, T.; Li, J.; Wang, W.; Lu, X. Advances in the relationship between leptin and hypertensive-left ventricular hypertrophy. Zhong Nan Da Xue Xue Bao. Yi Xue Ban= J. Cent. South Univ. Medi. Sci. 2015, 40, 811–815. [Google Scholar]
- Mapanga, R.F.; Essop, M.F. Benfotiamine: A novel cardioprotective agent that blunts hyperglycemia-induced cardiac dysfunction. FASEB J. 2012, 26. [Google Scholar]
- Mapanga, R.; Joseph, D.; Symington, B.; Garson, K.L.; Kimar, C.; Kelly-Laubscher, R.; Essop, M.F. Detrimental effects of acute hyperglycaemia on the rat heart. Acta Physiol. 2014, 210, 546–564. [Google Scholar] [CrossRef]
- Ferreira, L.; Teixeira-de-Lemos, E.; Pinto, F.; Parada, B.; Mega, C.; Vala, H.; Pinto, R.; Garrido, P.; Sereno, J.; Fernandes, R. Effects of sitagliptin treatment on dysmetabolism, inflammation, and oxidative stress in an animal model of type 2 diabetes (zdf rat). Mediat. Inflamm. 2010, 2010, 592760. [Google Scholar] [CrossRef] [PubMed]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. 2017, 57, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, M.; Kuda, O.; Rossmeisl, M.; Flachs, P.; Kopecky, J. Lipid signaling in adipose tissue: Connecting inflammation & metabolism. BBA-Mol. Cell Biol. Lipids 2015, 1851, 503–518. [Google Scholar]
- Barbui, T.; Carobbio, A.; Finazzi, G.; Vannucchi, A.M.; Barosi, G.; Antonioli, E.; Guglielmelli, P.; Pancrazzi, A.; Salmoiraghi, S.; Zilio, P. Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: Different role of c-reactive protein and pentraxin 3. Haematologica 2011, 96, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Khathi, A.; Masola, B.; Musabayane, C.T. Effects of syzygium aromaticum-derived oleanolic acid on glucose transport and glycogen synthesis in the rat small intestine. J. Diabetes 2013, 5, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
 = p <0.05 denotes comparison with NC; α = p < 0.05 denotes comparison with PC.
= p <0.05 denotes comparison with NC; α = p < 0.05 denotes comparison with PC.
 = p <0.05 denotes comparison with NC; α = p < 0.05 denotes comparison with PC.
= p <0.05 denotes comparison with NC; α = p < 0.05 denotes comparison with PC.
 = p < 0.05 denotes comparison with NC; α = p < 0.05 denotes comparison with PC.
= p < 0.05 denotes comparison with NC; α = p < 0.05 denotes comparison with PC.
 = p < 0.05 denotes comparison with NC; α = p < 0.05 denotes comparison with PC.
= p < 0.05 denotes comparison with NC; α = p < 0.05 denotes comparison with PC.
 p < 0.05 denotes comparison with NC; α p < 0.05 denotes comparison with PC.
p < 0.05 denotes comparison with NC; α p < 0.05 denotes comparison with PC.
 p < 0.05 denotes comparison with NC; α p < 0.05 denotes comparison with PC.
p < 0.05 denotes comparison with NC; α p < 0.05 denotes comparison with PC.
 p < 0.05 denotes comparison with NC; α p < 0.05 denotes comparison with PC.
p < 0.05 denotes comparison with NC; α p < 0.05 denotes comparison with PC.
 p < 0.05 denotes comparison with NC; α p < 0.05 denotes comparison with PC.
p < 0.05 denotes comparison with NC; α p < 0.05 denotes comparison with PC.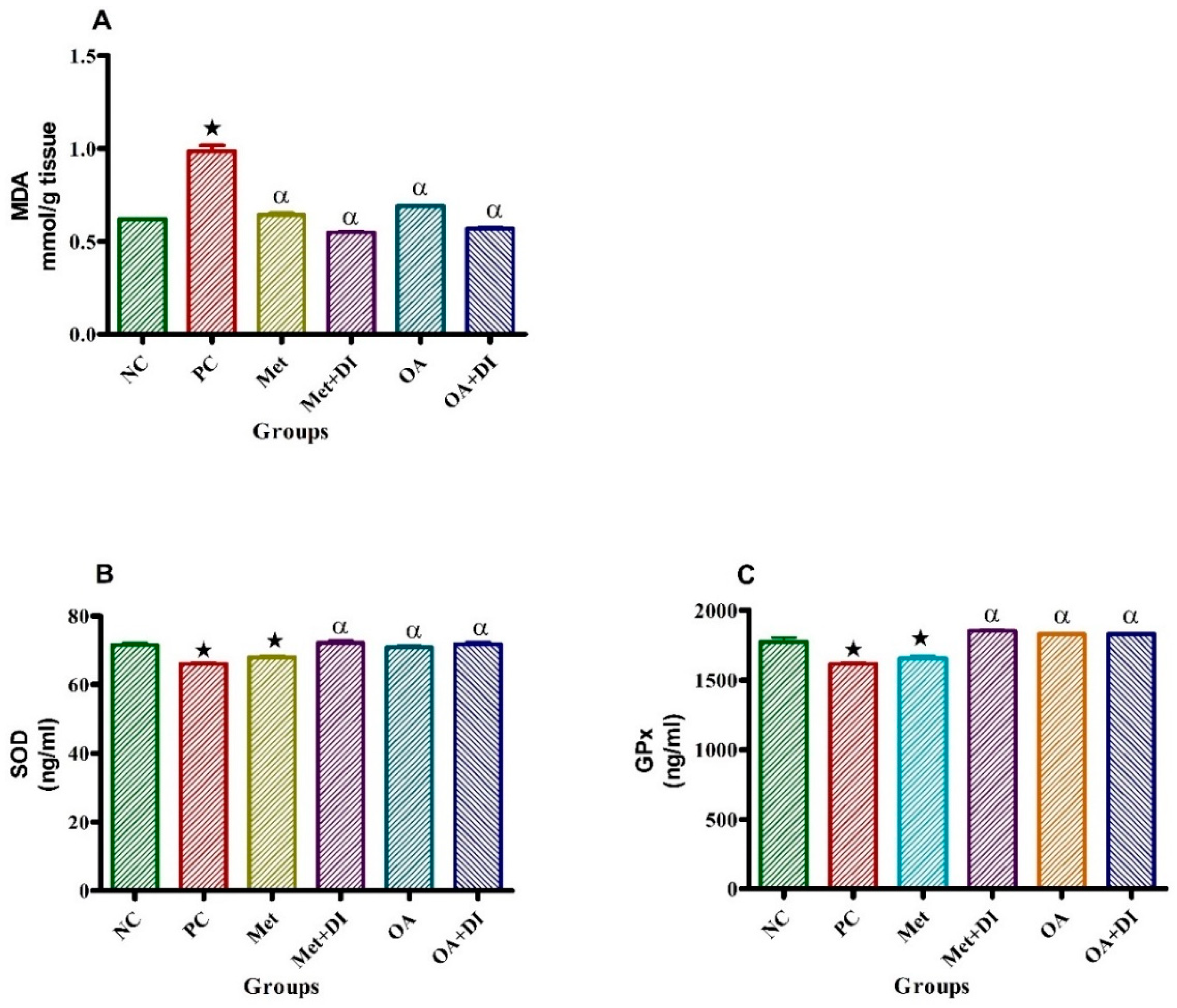
| Experimental Group | Heart Weights (g) | Body Weights (g) | Heart/Body Ratio (H/B) | Cardiac CRP (ng/mL) |
|---|---|---|---|---|
| NC | 1.56 ± 0.08 | 387.50 ± 11.18 | 0.0040 ± 0.00029 | 9.63 ± 1.08 |
| PC | 1.72 ± 0.05 * | 679.75 ± 78.52 * | 0.0027 ± 0.00029 * | 17.24 ± 0.35 * |
| Met | 1.62 ± 0.03 α | 500.50 ± 2.59 *α | 0.0032 ± 0.00028 α | 16.61 ± 0.17 * |
| Met + DI | 1.73 ± 0.01 * | 443.00 ± 13.86 *α | 0.0039 ± 0.00012 α | 15.03 ± 0.27 * |
| OA | 1.61 ± 0.12 α | 516.75 ± 8.28 *α | 0.0031 ± 0.00019 α | 12.10 ± 0.45 α |
| OA+DI | 1.49 ± 0.04 α | 434.25 ± 12.19 α | 0.0034 ± 0.00011 α | 9.56 ± 0.58 α |
| Experimental Groups (n = 6) | TGs (mmol/L) | Total-C (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) |
|---|---|---|---|---|
| NC | 1.23 ± 0.03 | 4.15 ± 0.05 | 1.71 ± 0.12 | 2.39 ± 0.03 |
| PC | 5.62 ± 0.32 * | 4.12 ± 0.08 | 0.85 ± 0.04 * | 8.88 ± 0.19 * |
| Met | 3.29 ± 0.19 *α | 4.12 ± 0.03 | 0.97 ± 0.04 | 5.93 ± 0.04 *α |
| Met + DI | 0.91 ± 0.04 *α | 4.10 ± 0.01 | 1.23 ± 0.04 α | 1.89 ± 0.05α |
| OA | 1.94 ± 0.15 α | 4.05 ± 0.06 | 1.88 ± 0.02 α | 5.69 ± 0.07 * |
| OA+DI | 1.08 ± 0.05 α | 4.10 ± 0.04 | 1.76 ± 0.02 α | 2.04 ± 0.05 α |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. Plant-Derived Oleanolic Acid (OA) Ameliorates Risk Factors of Cardiovascular Diseases in a Diet-Induced Pre-Diabetic Rat Model: Effects on Selected Cardiovascular Risk Factors. Molecules 2019, 24, 340. https://doi.org/10.3390/molecules24020340
Gamede M, Mabuza L, Ngubane P, Khathi A. Plant-Derived Oleanolic Acid (OA) Ameliorates Risk Factors of Cardiovascular Diseases in a Diet-Induced Pre-Diabetic Rat Model: Effects on Selected Cardiovascular Risk Factors. Molecules. 2019; 24(2):340. https://doi.org/10.3390/molecules24020340
Chicago/Turabian StyleGamede, Mlindeli, Lindokuhle Mabuza, Phikelelani Ngubane, and Andile Khathi. 2019. "Plant-Derived Oleanolic Acid (OA) Ameliorates Risk Factors of Cardiovascular Diseases in a Diet-Induced Pre-Diabetic Rat Model: Effects on Selected Cardiovascular Risk Factors" Molecules 24, no. 2: 340. https://doi.org/10.3390/molecules24020340
APA StyleGamede, M., Mabuza, L., Ngubane, P., & Khathi, A. (2019). Plant-Derived Oleanolic Acid (OA) Ameliorates Risk Factors of Cardiovascular Diseases in a Diet-Induced Pre-Diabetic Rat Model: Effects on Selected Cardiovascular Risk Factors. Molecules, 24(2), 340. https://doi.org/10.3390/molecules24020340





