Peptide-Based Drug-Delivery Systems in Biotechnological Applications: Recent Advances and Perspectives
Abstract
:1. Introduction
2. Peptide Self-Assembled Nanostructures
2.1. α-Helical and β-Sheet Peptides
2.2. Linear Peptides
2.3. Cyclic Peptides
2.4. Amphiphilic Peptides (PAs)
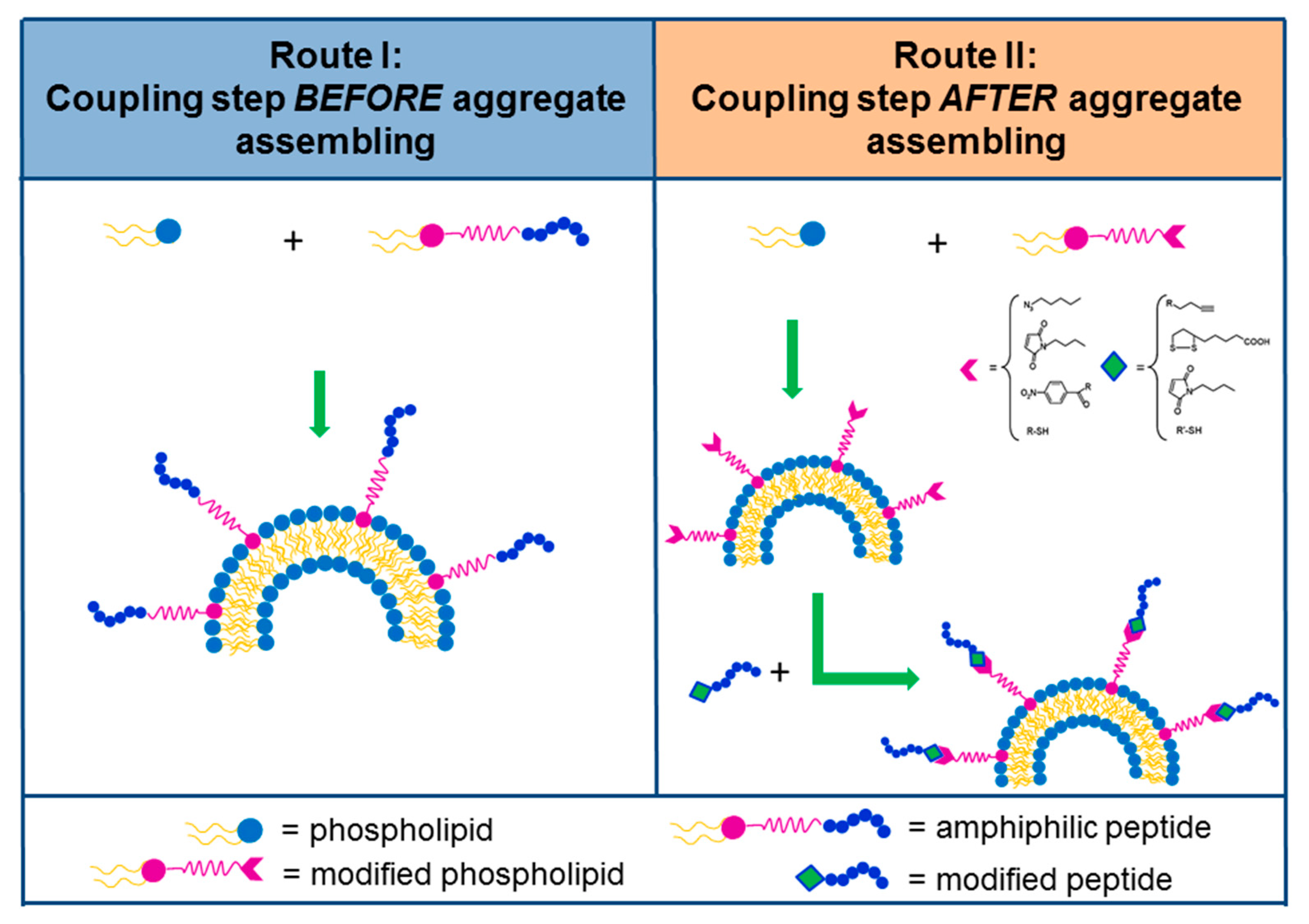
3. Self-Assembling PAs for Targeting in Nanostructures
3.1. Cell Penetrating Peptide (CPPs) and Smart Sequences
3.2. Peptide Able to Interact with Overexpressed Receptors
3.2.1. Peptide Target for Integrin Receptors
3.2.2. GPR Target Peptide
Somatostatin Receptors
Bombesin Receptors
CCK receptors
3.2.3. Supramolecular System Based on Disordered Linear Peptides
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruber Perez, C.M.; Stephanopoulos, N.; Sur, S.; Lee, S.S.; Newcomb, C.; Stupp, S.I. The Powerful Functions of Peptide-Based Bioactive Matrices for Regenerative Medicine. Ann. Biomed. Eng. 2015, 43, 501–514. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Labhasetwar, V. Nanotech approaches to drug delivery and imaging. Drug Discov. Today 2003, 8, 1112–1120. [Google Scholar] [CrossRef]
- Panda, J.J.; Chauhan, V.S. Short peptide based self-assembled nanostructures: Implications in drug delivery and tissue engineering. Polym. Chem. 2014, 5, 4418–4436. [Google Scholar] [CrossRef]
- Brack, A.; Orgel, L.E. ß structures of alternating polypeptides and their possible prebiotic significance. Nature 1975, 256, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Potekhin, S.A.; Melnik, T.N.; Popov, V.; Lanina, N.F.; Vazina, A.A.; Rigler, P.; Verdini, A.S.; Corradin, G.; Kajava, A.V. De novo design of fibrils made of short α-helical coiled coil peptides. Chem. Biol. 2001, 8, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Wagner, D.E.; Philips, C.L.; Ali, W.M.; Nybakken, G.E.; Crawford, E.D.; Schwab, A.D.; Smith, W.F.; Fairman, R. Toward the development of peptide nanofilaments and nanopores as smart materials. Proc. Nat. Acad. Sci. USA 2005, 102, 12656–12661. [Google Scholar] [CrossRef] [PubMed]
- Moutevelis, E.; Woolfson, D.N. A Periodic Table of Coiled-Coil Protein Structures. J. Mol. Biol. 2009, 385, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Aggeli, A.; Nyrkova, I.A.; Bell, M.; Harding, R.; Carrick, L.; McLeish, T.C.B.; Semenov, A.N.; Boden, N. Hierarchical self-assembly of chiral rod-like molecules as a model for peptide β-sheet tapes, ribbons, fibrils, and fibers. Proc. Nat. Acad. Sci. USA 2001, 98, 11857–11862. [Google Scholar] [CrossRef] [PubMed]
- Fishwick, C.W.G.; Beevers, A.J.L.; Carrick, M.; Whitehouse, C.D.; Aggeli, A.; Boden, N. Structures of helical β-tapes and twisted ribbons: The role of side-chain interactions on twist and bend behavior. Nano Lett. 2003, 3, 1475–1479. [Google Scholar] [CrossRef]
- Aggeli, A.; Bell, M.; Carrick, L.M.; Fishwick, C.W.G.; Harding, R.; Mawer, P.J.; Radford, S.E.; Strong, A.E.; Boden, N. pH as a trigger of peptide β-sheet self-assembly and reversible switching between nematic and isotropic phases. J. Am. Chem. Soc. 2003, 125, 9619–9628. [Google Scholar] [CrossRef]
- Schneider, J.P.; Pochan, D.J.; Ozbas, B.; Rajagopal, K.; Pakstis, L.; Kretsinger, J. Responsive hydrogels from the intramolecular folding and self-assembly of a designed peptide. J. Am. Chem. Soc. 2002, 124, 15030–15037. [Google Scholar] [CrossRef] [PubMed]
- Veiga, A.S.; Sinthuvanich, C.; Gaspar, D.; Franquelim, H.G.; Castanho, M.A.R.B.; Schneider, J.P. Arginine-rich self-assembling peptides as potent antibacterial gels. Biomaterials 2012, 33, 8907–8916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Görbitz, C.H. The structure of nanotubes formed by diphenylalanine, the core recognition motif of Alzheimer’s β-amyloid polypeptide. Chem. Comm. 2006, 22, 2332–2334. [Google Scholar] [CrossRef] [PubMed]
- Tamamis, P.; Adler-Abramovich, L.; Reches, M.; Marshall, K.; Sikorski, P.; Serpell, L.; Gazit, E.; Archontis, G. Self-Assembly of Phenylalanine Oligopeptides: Insights from Experiments and Simulations. Biophys. J. 2009, 96, 5020–5029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.; Zhu, P.; Li, J. Self-assembly and application of diphenylalanine-based nanostructures. Chem. Soc. Rev. 2010, 39, 1877–1890. [Google Scholar] [CrossRef] [PubMed]
- Adler-Abramovich, L.; Kol, N.; Yanai, I.; Barlam, D.; Shneck, R.Z.; Gazit, E.; Rousso, I. Self-assembled organic nanostructures with metallic-like stiffness. Angew. Chem. Int. Ed. 2010, 49, 9939–9942. [Google Scholar] [CrossRef]
- Wang, M.; Du, L.; Wu, X.; Xiong, S.; Chu, P.K. Charged Diphenylalanine Nanotubes and Controlled Hierarchical Self-Assembly. ACS Nano 2011, 5, 4448–4454. [Google Scholar] [CrossRef]
- Vasilev, S.; Zelenovskiy, P.; Vasileva, D.; Nuraeva, A.; ShurYa, V.; Kholkin, A.L. Piezoelectric properties of diphenylalaninemicrotubes prepared from the solution. J. Phys. Chem. Solids 2016, 93, 68–72. [Google Scholar] [CrossRef]
- Nikitin, T.; Kopyl, S.; Shur, V.Y.; Kopelevich, Y.V.; Kholkin, A.L. Low-temperature photoluminescence in self-assembled diphenylalanine microtubes. Phys. Lett. A 2016, 380, 1658–1662. [Google Scholar] [CrossRef]
- Handelman, A.; Kuritz, N.; Natan, A.; Rosenman, G. Reconstructive Phase Transition in Ultrashort Peptide Nanostructures and Induced Visible Photoluminescence. Langmuir 2016, 32, 2847–2862. [Google Scholar] [CrossRef] [PubMed]
- Handelman, A.; Apter, B.; Turko, N.; Rosenman, G. Linear and nonlinear optical waveguiding in bio-inspired peptide nanotubes. Acta Biomater. 2016, 30, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Adler-Abramovich, L.; Reches, M.; Sedman, V.L.; Allen, S.; Tendler, S.J.B.; Gazit, E. Thermal and chemical stability of diphenylalanine peptide nanotubes: Implications for nanotechnological applications. Langmuir 2006, 22, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Vargiu, A.V.; Styan, K.E. The Phe-Phe motif for peptide self-assembly in nanomedicine. Molecules 2015, 20, 19775–19788. [Google Scholar] [CrossRef] [PubMed]
- Adler-Abramovich, L.; Aronov, D.; Beker, P.; Yevnin, M.; Stempler, S.; Buzhansky, L.; Rosenman, G.; Gazit, E. Self-assembled arrays of peptide nanotubes by vapour deposition. Nat. Nanotechnol. 2009, 4, 849–854. [Google Scholar] [CrossRef]
- Scanlon, S.; Aggeli, A. Self-assembling peptide nanotubes. Nano Today 2008, 3, 22–30. [Google Scholar] [CrossRef]
- Hendler, N.; Sidelman, N.; Reches, M.; Gazit, E.; Rosenberg, Y.; Richter, S. Formation of well-organized self-assembled films from peptide nanotubes. Adv. Mater. 2007, 19, 1485–1488. [Google Scholar] [CrossRef]
- Silva, R.F.; Araujo, D.R.; Silva, E.R.; Ando, R.A.; Alves, W.A. l-Diphenylalanine Microtubes As a Potential Drug-Delivery System: Characterization, Release Kinetics, and Cytotoxicity. Langmuir 2013, 29, 10205–10212. [Google Scholar] [CrossRef]
- Bonetti, A.; Pellegrino, S.; Das, P.; Yuran, S.; Bucci, R.; Ferri, N.; Meneghetti, F.; Castellano, C.; Reches, M.; Gelmi, M.L. Dipeptide Nanotubes Containing Unnatural Fluorine-Substituted-Diarylamino Acid and L-Alanine as Candidates for Biomedical Applications. Org. Lett. 2015, 17, 4468–4471. [Google Scholar] [CrossRef]
- Emtiazi, G.; Zohrabi, T.; Lee, L.Y.; Habibi, N.; Zarrabi, A. Covalent diphenylalanine peptide nanotube conjugated to folic acid/magnetic nanoparticles for anti-cancer drug delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 90–98. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Chu, L.; Zhang, Y.; Xu, H.; Kong, D.; Yang, Z.; Yang, C.; Ding, D. Self-Assembling Peptide of D-Amino Acids Boosts Selectivity and Antitumor Efficacy of 10-Hydroxycamptothecin. ACS Appl. Mater. Interfaces 2014, 6, 5558–5565. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Kuang, Y.; Shi, J.; Du, X.; Zhou, J.; Wang, H.; Yang, Z.; Xu, B. Dephosphorylation of D-Peptide Derivatives to Form Biofunctional, Supramolecular Nanofibers/Hydrogels and Their Potential Applications for Intracellular Imaging and Intratumoral Chemotherapy. J. Am. Chem. Soc. 2013, 135, 9907–9914. [Google Scholar] [CrossRef]
- Zhang, H.; Fei, J.; Yan, X.; Wang, A.; Li, J. Enzyme-responsive release of doxorubicin from monodisperse dipeptide-based nanocarriers for highly efficient cancer treatment in vitro. Adv. Funct. Mater. 2015, 25, 1193–1204. [Google Scholar] [CrossRef]
- Das, P.; Yuran, S.; Yan, J.; Lee, P.S.; Reches, M. Sticky tubes and magnetic hydrogels co-assembled by a short peptide and melanin-like nanoparticles. Chem. Commun. 2015, 51, 5432–5435. [Google Scholar] [CrossRef] [PubMed]
- Liberato, M.S.; Kogikoski, S.; da Silva, E.R.; de Araujo, D.R.; Guha, S.; Alves, W.A. Polycaprolactone fibers with self-assembled peptide micro/nanotubes: A practical route towards enhanced mechanical strength and drug delivery applications. J. Mater. Chem. B 2016, 4, 1405–1413. [Google Scholar] [CrossRef]
- Li, Q.; Chen, M.; Chen, D.; Wu, L. One-Pot Synthesis of Diphenylalanine-Based Hybrid Nanospheres for Controllable pH- and GSH-Responsive Delivery of Drugs. Chem. Mater. 2016, 28, 6584–6590. [Google Scholar] [CrossRef]
- Jayawarna, V.; Ali, M.; Jowitt, T.A.; Miller, A.E.; Saiani, A.; Gough, J.E.; Ulijn, R.V. Nanostructured Hydrogels for Three-Dimensional Cell Culture Through Self-Assembly of Fluorenylmethoxycarbonyl-Dipeptides. Adv. Mater. 2006, 18, 611–614. [Google Scholar] [CrossRef]
- Mao, L.N.; Wang, H.M.; Tan, M.; Ou, L.L.; Kong, D.L.; Yang, Z.M. Conjugation of two complementary anti-cancer drugs confers molecular hydrogels as a co-delivery system. Chem. Commun. 2012, 48, 395–397. [Google Scholar] [CrossRef]
- Li, J.; Kuang, Y.; Gao, Y.; Du, X.; Shi, J.; Xu, B. D-amino acids boost the selectivity and confer supramolecular hydrogels of a nonsteroidal anti-inflammatory drug (NSAID). J. Am. Chem. Soc. 2013, 135, 542–545. [Google Scholar] [CrossRef]
- Liang, G.; Yang, Z.; Zhang, R.; Li, L.; Fan, Y.; Kuang, Y.; Gao, Y.; Wang, T.; Lu, W.W.; Xu, B. Supramolecular Hydrogel of a D-Amino Acid Dipeptide for Controlled Drug Release in Vivo. Langmuir 2009, 25, 8419–8422. [Google Scholar] [CrossRef]
- Erdogan, H.; Yilmaz, M.; Babur, E.; Duman, M.; Aydin, H.M.; Demirel, G. Fabrication of Plasmonic Nanorod-Embedded Dipeptide Microspheres via the Freeze-Quenching Method for Near-Infrared Laser-Triggered Drug-Delivery Applications. Biomacromolecules 2016, 17, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhao, J.; Huang, R.; Qi, W.; Wang, Y.; Su, R.; He, Z. Calcium-Ion-Triggered Co-assembly of Peptide and Polysaccharide into a Hybrid Hydrogel for Drug Delivery. Nanoscale Res. Lett. 2016, 11, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviv, M.; Halperin-Sternfeld, M.; Grigoriants, I.; Buzhansky, L.; Mironi-Harpaz, I.; Seliktar, D.; Einav, S.; Nevo, Z.; Adler-Abramovich, L. Improving the Mechanical Rigidity of Hyaluronic Acid by Integration of a Supramolecular Peptide Matrix. ACS Appl. Mater. Interfaces 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Xing, R.; Zhang, N.; Zou, Q.; Yan, X. Antitumor Photodynamic Therapy Based on Dipeptide Fibrous Hydrogels with Incorporation of Photosensitive Drugs. ACS Biomater. Sci. Eng. 2018, 4, 2046–2052. [Google Scholar] [CrossRef]
- Roth-Konforti, M.E.; Comune, M.; Halperin-Sternfeld, M.; Grigoriants, I.; Shabat, D.; Adler-Abramovich, L. UV Light-Responsive Peptide-Based Supramolecular Hydrogel for Controlled Drug Delivery. Macromol. Rapid Commun. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Diaferia, C.; Balasco, N.; Sibillano, T.; Ghosh, M.; Adler-Abramovich, L.; Giannini, C.; Vitagliano, L.; Morelli, G.; Accardo, A. Amyloid-Like Fibrillary Morphology Originated by Tyrosine-Containing Aromatic Hexapeptides. Chem. Eur. J. 2018, 24, 6804–6817. [Google Scholar] [CrossRef]
- Diaferia, C.; Balasco, N.; Sibillano, T.; Giannini, C.; Vitagliano, L.; Morelli, G.; Accardo, A. Structural Characterization of Self-Assembled Tetra-Tryptophan Based Nanostructures: Variations on a Common Theme. Chem. Phys. Chem. 2018, 19, 1635–1642. [Google Scholar] [CrossRef]
- Diaferia, C.; Gianolio, E.; Palladino, P.; Arena, F.; Boffa, C.; Morelli, G.; Accardo, A. Peptide Materials Obtained by Aggregation of Polyphenylalanine Conjugates as Gadolinium-Based Magnetic Resonance Imaging Contrast Agents. Adv. Funct. Mater. 2015, 25, 7003–7016. [Google Scholar] [CrossRef]
- Accardo, A.; Tesauro, D.; Aloj, L.; Pedone, C.; Morelli, G. Supramolecular aggregates containing lipophilic Gd(III) complexes as contrast agents in MRI. Coord. Chem. Rev. 2009, 253, 2193–2213. [Google Scholar] [CrossRef]
- Diaferia, C.; Gianolio, E.; Accardo, A.; Morelli, G. Gadolinium containing telechelic PEG-polymers end-capped by di-phenylalanine motives as potential supramolecular MRI contrast agents. J. Pept. Sci. 2017, 23, 122–130. [Google Scholar] [CrossRef]
- Diaferia, C.; Gianolio, E.; Sibillano, T.; Mercurio, F.A.; Leone, M.; Giannini, C.; Balasco, N.; Vitagliano, L.; Morelli, G.; Accardo, A. Cross-beta nanostructures based on dinaphthylalanine Gd-conjugates loaded with doxorubicin. Sci. Rep. 2017, 7, 307. [Google Scholar] [CrossRef] [PubMed]
- Handelman, A.; Natan, A.; Rosenman, G. Structural and optical properties of short peptides: Nanotubes-to-nanofibers phase transformation. J. Pept. Sci. 2014, 20, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Pinotsi, D.; Buell, A.K.; Dobson, C.M.; Kaminski, G.S.; Kaminski, C.F. A label-free, quantitative assay of amyloid fibril growth based on intrinsic fluorescence. ChemBioChem 2013, 14, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Diaferia, C.; Sibillano, T.; Balasco, N.; Giannini, C.; Roviello, V.; Vitagliano, L.; Morelli, G.; Accardo, A. Hierarchical analysis of self-assembled PEGylated hexaphenylalanine photoluminescent nanostructures. Chem. Eur. J. 2016, 22, 16586–16597. [Google Scholar] [CrossRef] [PubMed]
- Diaferia, C.; Sibillano, T.; Altamura, D.; Roviello, V.; Vitagliano, L.; Giannini, C.; Morelli, G.; Accardo, A. Structural Characterization of PEGylated Hexaphenylalanine Nanostructures Exhibiting Green Photoluminescence Emission. Chem. Eur. J. 2017, 23, 14039–14048. [Google Scholar] [CrossRef] [PubMed]
- Diaferia, C.; Sibillano, T.; Giannini, C.; Roviello, V.; Vitagliano, L.; Morelli, G.; Accardo, A. Photoluminescent Peptide-Based Nanostructures as FRET Donor for Fluorophore Dye. Chem. Eur. J. 2017, 23, 8741–8748. [Google Scholar] [CrossRef] [PubMed]
- De Santis, P.; Morosetti, S.; Rizzo, R. Conformational Analysis of Regular Enantiomeric Sequences. Macromolecules 1974, 7, 52–58. [Google Scholar] [CrossRef]
- Ghadiri, M.R.; Granja, J.R.; Milligan, R.A.; McRee, D.E.; Khazanovich, N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature 1993, 366, 324–327. [Google Scholar] [CrossRef]
- Chapman, R.; Danial, M.; Koh, M.L.; Jolliffe, K.A.; Perrier, S. Design and properties of functional nanotubes from the self-assembly of cyclic peptide templates. Chem. Soc. Rev. 2012, 41, 6023–6041. [Google Scholar] [CrossRef]
- Fernandez-Lopez, S.; Kim, H.S.; Choi, E.C.; Delgado, M.; Granja, J.R.; Khasanov, A.; Kraehenbuehl, K.; Long, G.; Weinberger, D.A.; Wilcoxen, K.M.; et al. Antibacterial agents based on the cyclic d,l-α-peptide architecture. Nature 2001, 412, 452–455. [Google Scholar] [CrossRef]
- Ishihara, Y.; Kimura, S. Nanofiber formation of amphiphilic cyclic tri-β-peptide. J. Pept. Sci. 2010, 16, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Hartgerink, J.D.; Granja, J.R.; Milligan, R.A.; Ghadiri, M.R. Peptide-amphiphile nanofibers: A versatile scaffold for the preparation of self-assembling materials. J. Am. Chem Soc. 1996, 118, 43–50. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, S.; Sun, L.; Huang, Y.; Lenaghan, S.C.; Zhang, M. Doxorubicin-loaded cyclic peptide nanotube bundles overcome chemoresistance in breast cancer cells. J. Biomed. Nanotechnol. 2014, 10, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. Self-assembly of amphiphilic peptides. Soft Matter. 2011, 7, 4122–4138. [Google Scholar] [CrossRef]
- Versluis, F.; Marsden, H.R.; Kros, A. Power struggles in peptide-amphiphile nanostructures. Chem. Soc. Rev. 2010, 39, 3434–3444. [Google Scholar] [CrossRef] [PubMed]
- Vauthey, S.; Santoso, S.; Gong, H.; Watson, N.; Zhang, S. Molecular self-assembly of surfactant-like peptides to form nanotubes and nanovesicles. Proc. Natl. Acad. Sci. USA 2002, 99, 5355–5360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoso, S.; Hwang, W.; Hartman, H.; Zhang, S. Self-assembly of surfactant-like peptides with variable glycine tails to form nanotubes and nanovesicles. Nano Lett. 2002, 2, 687–691. [Google Scholar] [CrossRef]
- Von Maltzahn, G.; Vauthey, S.; Santoso, S.; Zhang, S. Positively charged surfactant like peptides self-assemble into nanostructures. Langmuir 2003, 19, 4332–4337. [Google Scholar] [CrossRef]
- Dehsorkhi, A.; Castelletto, V.; Hamley, I.W.; Seitsonen, J.; Ruokolainen, J. Interaction between a cationic surfactant-like peptide and lipid vesicles and its relationship to antimicrobial activity. Langmuir 2013, 29, 14246–14253. [Google Scholar] [CrossRef]
- Zhabìng, S.; Holmes, T.; Lockshin, C.; Rich, A. Spontaneous assembly of a self complementaryoligopeptide to form stable microscopic membrane. Proc. Natl. Acad. Sci. USA 1993, 90, 3334–3338. [Google Scholar] [CrossRef]
- Liu, E.; Wang, H.; Shang, Y.; Liu, M.; Chen, P. Molecular binding self assembling peptide EAK16-II with anticancer agent EPT and its implication in cancer cell inhibition. J. Control. Release 2012, 160, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, C.; Schade, B.; Fuhrhop, J.H. Comparative cryo-electron microscopy of noncovalent N-dodecanoyl-(d- and l-) serine assemblies in vitreous toluene and water. Langmuir 2001, 17, 873–877. [Google Scholar] [CrossRef]
- Fuhrhop, J.H.; Spiroski, D.; Boettcher, C. Molecular monolayer rods and tubules made of .alpha.-(L-lysine),.omega.-(amino) bolaamphiphiles. J. Am. Chem. Soc. 1993, 115, 1600. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Peptide-amphiphilenanofibers: A versatile scaffold for the preparation of self- assembling materials. Proc. Natl. Acad. Sci. USA 2002, 99, 5133–5138. [Google Scholar] [CrossRef] [PubMed]
- Matson, J.B.; Newcomb, C.J.; Bitton, R.; Stupp, S.I. Nanostructure-templated control of drug release from peptide amphiphile nanofiber gels. Soft Matter 2012, 8, 3586–3595. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Muraoka, T.; Cheetham, A.G.; Stupp, S.I. Self-Assembly of Giant Peptide. Nanobelts 2018, 16, 9. [Google Scholar] [CrossRef]
- Yao, C.; Liu, J.Y.; Wu, X.; Tao, Z.G.; Gao, Y.; Zhu, Q.G.; Li, J.F.; Zhang, L.J.; Hu, C.L.; Gu, F.F.; et al. Reducible self-assembling cationic polypeptide-based micelles mediate co-delivery of doxorubicin and microRNA-34a for androgen- independent prostate cancer therapy. J. Control. Release 2016, 232, 203–214. [Google Scholar] [CrossRef]
- Tang, Q.; Cao, B.; Wu, H.; Cheng, G. Cholesterol-Peptide Hybrids to Form Liposome-Like Vesicles for Gene Delivery. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Missirlis, D.; Krogstad, D.V.; Tirrell, M. Subsequent Endosomal Disruption Results in SJSA-1. Mol Pharm. 2010, 7, 2173–2184. [Google Scholar] [CrossRef]
- Pujals, S.; Fernandez-Carneado, J.; Lopez-Iglesias, C.; Kogan, M.J.; Giralt, E. Mechanistic aspects of cell-penetrating peptide-mediated intracellular drug delivery: Relevance of CPP self-assembly. Biochim. Biophys. Acta Biomembr. 2006, 1758, 264–279. [Google Scholar] [CrossRef]
- Agrawal, P.; Bhalla, S.; Usmani, S.S.; Singh, S.; Chaudhary, K.; Raghava, G.P.; Gautam, A. CPPsite 2.0: Arepository of experimentally validated cell-penetrating peptides. Nucleic Acids Res. 2016, 44, D1098–D1103. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, A.; Tornesello, A.L.; Tornesello, M.L.; Buonaguro, F.M. Cell Penetrating Peptides as Molecular Carriers for Anti-Cancer Agents. Molecules 2018, 23, 295. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Dong, Y.; Feijen, J.; Zhong, Z. Peptide-decorated polymeric nanomedicines for precision cancer therapy. J. Control. Release 2018, 290, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Defaus, S.; Andreu, D. 1988–2018: Thirty years of drug smuggling at the nano scale. Challenges and opportunities of cell-penetrating peptides in biomedical research. Arch. Biochem. Biophys. 2019, 661, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tang, J.; Fu, L.; Ran, R.; Liu, Y.; Yuan, M.; He, Q. A pH-responsive α-helical cell penetrating peptide-mediated liposomal delivery system. Biomaterials 2013, 34, 7980–7993. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xiong, M.; Gong, J.; Zhang, Y.; Bai, N.; Luo, Y.; Li, L.; Wei, Y.; Liu, Y.; Tan, X. Legumain protease-activated TAT-liposome cargo for targeting tumours and their microenvironment. Nat. Commun. 2014, 5, 4280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Yang, Y.; Xie, X.; Cai, X.; Mei, X. Preparation and characterization of photo-responsive cell-penetrating peptide-mediated nanostructured lipid carrier. J. Drug Target. 2014, 22, 891–900. [Google Scholar] [CrossRef]
- Reubi, J.C. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef]
- Accardo, A.; Ringhieri, P.; Palumbo, R.; Morelli, G. Influence of PEG length on conformational and binding properties of CCK peptides exposed by supramolecular aggregates. Pept. Sci. 2014, 102, 304–312. [Google Scholar] [CrossRef]
- Ginj, M.; Zhang, H.; Waser, B.; Cescato, R.; Wild, D.; Wang, X.; Erchegyi, J.; Rivier, J.; Macke, H.R.; Reubi, J.C. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc. Natl. Acad. Sci. USA 2006, 103, 16436–16441. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.Y.; Vermeersch, S.; de Hoon, J.; Villalón, C.M.; Maassenvandenbrink, A. Potential mechanisms of prospective antimigraine drugs: A focus on vascular (side) effects. Pharmacol. Ther. 2011, 129, 332–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, F.H.; Pitchford, N.A. Conformational analysis from crystallographic data. In Structure Based Drug Design; Codding, P.W., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 1998; pp. 15–26. [Google Scholar]
- Pande, J.; Szewczyk, M.M.; Grover, A.K. Phage display: Concept, innovations, applications and future. Biotechnol. Adv. 2010, 28, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Ringhieri, P.; Mannucci, S.; Conti, G.; Nicolato, E.; Fracasso, G.; Marzola, P.; Morelli, G.; Accardo, A. Liposomes derivatized with multimeric copies of KCCYSL peptide as targeting agents for HER-2-overexpressing tumor cells. Int. J. Nanomed. 2017, 12, 501–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringhieri, P.; Diaferia, C.; Galdiero, S.; Palumbo, R.; Morelli, G.; Accardo, A. Liposomal doxorubicin doubly functionalized with CCK8 and R8 peptide sequences for selective intracellular drug delivery. J. Pept. Sci. 2015, 21, 415–425. [Google Scholar] [CrossRef]
- Accardo, A.; Ringhieri, P.; Tesauro, D.; Morelli, G. Liposomes derivatized with tetrabranchedneurotensin peptides via click chemistry reactions. New J. Chem. 2013, 37, 3528–3534. [Google Scholar] [CrossRef]
- Accardo, A.; Morelli, G. Review peptide-targeted liposomes for selective drug delivery: Advantages and problematic issues. Pept. Sci. 2015, 104, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Feldborg, L.N.; Jølck, R.I.; Andresen, T.L. Quantitative evaluation of bioorthogonal chemistries for surface functionalization of nanoparticles. Bioconjug. Chem. 2012, 23, 2444–2450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, T.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Targeted Polymeric Micelle System for Delivery of Combretastatin A4 to Tumor Vasculature In Vitro. Pharm. Res. 2010, 27, 1861–1868. [Google Scholar] [CrossRef]
- Miura, Y.; Takenaka, T.; Toh, K.; Wu, S.; Nishihara, H.; Kano, M.R.; Ino, Y.; Nomoto, T.; Matsumoto, Y.; Koyama, H.; et al. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood-brain tumor barrier. ACS Nano 2013, 7, 8583–8592. [Google Scholar] [CrossRef]
- Chen, Z.; Deng, J.; Zhao, Y.; Tao, T. Cyclic RGD peptide-modified liposomal drug delivery system: Enhanced cellular uptake in vitro and improved pharmacokinetics in rats. Int. J. Nanomed. 2012, 7, 3803–3811. [Google Scholar] [CrossRef]
- Amin, M.; Badiee, A.; Jaafari, M.R. Improvement of pharmacokinetic and antitumor activity of PEGylated liposomal doxorubicin by targeting with N-methylated cyclic RGD peptide in mice bearing C-26 colon carcinomas. Int. J. Pharm. 2013, 458, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Zhang, R.; Chen, Z.; Zhu, L. RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. J. Control. Release 2014, 196, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Battistini, L.; Burreddu, P.; Sartori, A.; Arosio, D.; Manzoni, L.; Paduano, L.; D’Errico, G.; Sala, R.; Reia, L.; Bonomini, S.; et al. Enhancement of the uptake and cytotoxicactivity of doxorubicin in cancercells by novel cRGD-semipeptide-anchoring liposomes. Mol. Pharm. 2014, 11, 2280–2293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hon, J.H.; Chan, F.; Skibba, M.; Liang, G.; Chen, M. RGD decorated lipid-polymer hybrid nanoparticles for targeted co-delivery of doxorubicin and sorafenib to enhance anti-hepatocellular carcinoma efficacy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Morisco, A.; Accardo, A.; Gianolio, E.; Tesauro, D.; Benedetti, E.; Morelli, G. Micelles derivatized with octreotide as potential target-selective contrast agents in MRI. J. Pept. Sci. 2009, 15, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Morisco, A.; Tesauro, D.; Pedone, C.; Morelli, G. Naposomes: A new class of peptide-derivatized, target-selective multimodal nanoparticles for imaging and therapeutic applications. Ther. Deliv. 2011, 2, 235–257. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Mangiapia, G.; Paduano, L.; Morelli, G.; Tesauro, D. Octreotide labeled aggregates containing platinum complexes as nanovectors for drug delivery. J. Peptsci. 2013, 19, 190–197. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. The eradication of breast cancer and cancer stem cells using octreotide modified paclitaxel active targeting micelles and salinomycin passive targeting micelles. Biomaterials 2012, 33, 679–691. [Google Scholar] [CrossRef]
- Zou, A.; Chen, Y.; Huo, M.; Wang, J.; Zhang, Y.; Zhou, J.; Zhang, Q. In vivo studies of octreotidemodified N-octyl-O, N-carboxymethyl chitosan micelles loaded with doxorubicin for tumor-targeted delivery. J. Pharm. Sci. 2013, 102, 126–135. [Google Scholar] [CrossRef]
- Jaskula-Sztul, R.; Xu, W.; Chen, G.; Harrison, A.; Dammalapati, A.; Nair, R.; Cheng, Y.; Gong, S.; Chen, H. Thailandepsin A-loaded and octreotide-functionalized unimolecular micelles for targeted neuroendocrine cancer therapy. Biomaterials 2016, 91, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Helbok, A.; Rangger, C.; von Guggenberg, E.; Saba-Lepek, M.; Radolf, T.; Thurner, G.; Andreae, F.; Prassl, R.; Decristoforo, C. Targeting properties of peptide-modified radiolabeled liposomal nanoparticles. Nanomedicine 2012, 8, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, D.; Minjie, S.; Ping, Q. Effect of ligand density and PEG modification on octreotide-targetedliposome via somatostatin receptor in vitro and in vivo. Drug Deliv. 2016, 23, 3562–3572. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.L.; Binderup, T.; Jølck, R.I.; Rasmussen, P.; Henriksen, J.R.; Pfeifer, A.K.; Kjær, A.; Andresen, T.L. Positron emission tomography evaluation of somatostatin receptor targeted 64Cu-TATE-liposomes in a human neuroendocrine carcinoma mouse model. J. Control. Release 2012, 160, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jaskula-Sztul, R.; Harrison, A.; Dammalapati, A.; Chen, H.; Gong, S.; Xube, W.; Cheng, Y. KE108-conjugated unimolecular micelles loaded with a novel HDAC inhibitor thailandepsin-A for targeted neuroendocrine cancer therapy. Biomaterials 2016, 97, 22–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaskula-Sztul, R.; Chen, G.; Dammalapati, A.; Harrison, A.; Tang, W.; Gong, S.; Chen, H. AB3-loaded and tumor-targeted unimolecular micelles for medullary thyroid cancer treatment. J. Mater. Chem. B 2017, 5, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Accardo, A.; Mansi, R.; Morisco, A.; Mangiapia, G.; Paduano, L.; Tesauro, D.; Radulescu, A.; Aurilio, M.; Aloj, L.; Arra, C.; et al. Peptide modified nanocarriers for selective targeting of bombesin receptors. Mol. Biosyst. 2010, 6, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Salzano, G.; Morisco, A.; Aurilio, M.; Parisi, A.; Maione, F.; Cicala, C.; Tesauro, D.; Aloj, L.; De Rosa, G.; et al. Peptide-modified liposomes for selective targeting of bombesin receptors overexpressed by cancer cells: A potential theranostic agent. Int. J. Nanomed. 2012, 7, 2007–2017. [Google Scholar] [CrossRef]
- Ringhieri, P.; Iannitti, R.; Nardon, C.; Palumbo, R.; Fregona, D.; Morelli, G.; Accardo, A. Target selective micelles for bombesin receptors incorporating Au(III)-dithiocarbamato complexes. Int. J. Pharmaceut. 2014, 473, 194–202. [Google Scholar] [CrossRef]
- Accardo, A.; Mansi, R.; Salzano, G.; Morisco, A.; Aurilio, M.; Parisi, A.; Maione, F.; Cicala, C.; Ziaco, B.; Tesauro, D.; et al. Bombesin peptide antagonist for target-selective delivery of liposomal doxorubicin on cancer cells. J. Drug Target. 2013, 21, 240–249. [Google Scholar] [CrossRef]
- Accardo, A.; Tesauro, D.; Morelli, G.; Gianolio, E.; Aime, S.; Vaccaro, M.; Mangiapia, G.; Paduano, L.; Schillen, K. High-relaxivity supramolecular aggregates containing peptide and Gd complexes agents in MRI. J. Biol. Inorg. Chem. 2007, 12, 267–276. [Google Scholar] [CrossRef]
- Gasparini, G.; Brooks, P.C.; Biganzoli, E.; Vermeulen, P.B.; Bonoldi, E.; Dirix, L.Y.; Ranieri, G.; Miceli, R.; Cheresh, D.A. Vascular integrin αvβ3: A new prognostic indicator in breast cancer. Clin. Cancer Res. 1998, 4, 2625–2634. [Google Scholar] [PubMed]
- Liu, S. Radiolabeled multimeric cyclic RGD peptides as integrin alphavbeta3 targeted radiotracers for tumor imaging. Mol. Pharm. 2006, 3, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.B.; Huang, Y.; Lu, W.L.; Zhang, X.; Zhang, H.; Nagai, T.; Zhang, Q. Enhanced intracellular delivery and improved antitumor efficacy of doxorubicin by sterically stabilized liposomes modified with a synthetic RGD mimetic. J. Control. Release 2005, 107, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Le Breton, A.; Préat, V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Schiffelers, R.M.; Koning, G.A.; ten Hagen, T.L.M.; Fens, M.H.A.M.; Schraa, A.J.; Janssen, A.P.C.A.; Kok, R.J.; Molema, G.; Storm, G. Anti-tumor efficacy of tumor vasculature-targeted liposomal doxorubicin. J. Control. Release 2003, 115–122. [Google Scholar] [CrossRef]
- Murphy, E.A.; Majeti, B.K.; Barnes, L.A.; Makale, M.; Weis, S.M.; Lutu-Fuga, K.; Wrasidlo, W.; Cheresh, D.A. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl. Acad. Sci. USA 2008, 105, 9343–9348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, X.; Hu, X.; Liu, S.; Sun, X.; Gai, X. Cyclic RGD targeting cisplatin micelles for near-infrared imaging-guided chemotherapy. RSC Advances 2016, 6, 1151–1157. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.-C.; Sun, Q.-S.; Luo, C.-L.; Zhang, Q. RGD-based strategies for improving antitumor activity of paclitaxel-loaded liposomes in nude mice xenografted with human ovarian cancer. J. Drug Target. 2009, 17, 10–18. [Google Scholar] [CrossRef]
- Meng, S.; Su, B.; Li, W.; Ding, Y.; Tang, L.; Zhou, W.; Song, Y.; Li, H.; Zhou, C. Enhanced antitumor effect of novel dual-targeted paclitaxel liposomes. Nanotechnology 2010, 21, 415103. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Sun, Y.; Ren, Z.; Li, X.; Cui, G. RGD-fatty alcohol-modified docetaxel liposomes improve tumor selectivity in vivo. Int. J. Pharm. 2014, 468, 133–141. [Google Scholar] [CrossRef]
- Pattillo, C.B.; Sari-Sarraf, F.; Nallamothu, R.; Moore, B.M.; Wood, G.C.; Kiani, M.F. Targeting of the antivascular drug combretastatin to irradiated tumors results in tumor growth delay. Pharm. Res. 2005, 22, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.K.; Mishra, V.; Jain, S.; Mahor, S.; Vyas, S.P. Liposomes modified with cyclic RGD peptide for tumor targeting. J. Drug Target. 2004, 12, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Tisdale, A.W.; Haidari, E.; Kokkoli, E. Targeting colon cancer cells using PEGylated liposomes modified with a fibronectin-mimetic peptide. Int. J. Pharm. 2009, 366, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kessler, H.; Kutscher, B.; Klein, A. Peptidkonformationen, 39. NMR-studien zur konformation von cyclopentapeptidanalogen des thymopoietins. Liebigs Ann. Chem. 1986, 1986, 893–913. [Google Scholar] [CrossRef]
- Kessler, H.; Diefenbach, B.; Finsinger, D.; Geyer, A.; Gurrath, M.; Goodman, S.L.; Hölzemann, G.; Haubner, R.; Jonczyk, A.; Müller, G.; et al. Design of superactive and selective integrin receptor antagonists containing the RGD sequence. Lett. Pep. Sci. 1995, 2, 155–166. [Google Scholar] [CrossRef]
- Haubner, R.; Gratias, R.; Diefenbach, B.; Goodman, S.; Jonczyk, A.; Kessler, H. Structural and functional aspects of RGD-containing cyclic pentapeptides as highly potent and selective integrin v 3 antagonists. J. Am. Chem. Soc. 1996, 118, 7461–7472. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhang, Y.; Yang, S.; Wang, J.; Zhang, X.; Zhang, Q. RGD-modified polymeric micelles as potential carriers for targeted delivery to integrin-overexpressing tumor vasculature and tumor cells. J. Drug Target. 2009, 17, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Kazuki, N.; TambetTeesalu, S.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors. Cancer Cell 2009, 6, 510–520. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, J.; Fu, C.; Xie, X.; Peng, F.; You, J.; Tang, H.; Wang, Z.; Li, P.; Chen, J. iRGD-modified lipid–polymer hybrid nanoparticles loaded with isoliquiritigenin to enhance anti-breast cancer effect and tumor-targeting ability. Int. J. Nanomed. 2017, 12, 4147–4162. [Google Scholar] [CrossRef]
- Jiang, Y.; Pang, X.; Liu, R.; Xiao, Q.; Wang, P.; Leung, A.W.; Luan, Y.; Xu, C. Design of an AmphiphilicRGD Peptide and Self-Assembling Nanovesicles for Improving Tumor Accumulation and Penetration and the Photodynamic Efficacy of the Photosensitizer. ACS Appl. Mater. Interfaces 2018, 10, 31674–31685. [Google Scholar] [CrossRef]
- Hu, H.; Wan, J.; Huang, X.; Tang, Y.; Xiao, C.; Xu, H.; Yang, X.; Li, Z. iRGD-decorated reduction-responsive nanoclusters for targeted drug delivery. Nanoscale 2018, 10, 10514–10527. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, S.W. Octreotide: The Next Decade; BioScientifica: Bristol, UK, 1999. [Google Scholar]
- Melacini, G.; Zhu, Q.; Goodman, M. Multiconformational NMR Analysis of Sandostatin (Octreotide): Equilibrium between beta-Sheet and Partially Helical Structures. Biochemistry 1997, 36, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Cuttitta, F.; Carney, D.N.; Mulshine, J.; Moody, T.W.; Fedorko, J.; Fischler, A.; Minna, J.D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature 1985, 16, 823–826. [Google Scholar] [CrossRef]
- Patel, O.; Shulkes, A.; Baldwin, G.S. Gastrin-releasing peptide and cancer. Biochim. Biophys. Acta 2006, 1766, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Volkert, W.A.; Hoffman, T.J. Radiolabeled peptide conjugates for targeting of the bombesin receptor superfamily subtypes. Nucl. Med. Biol. 2005, 32, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Parry, J.J.; Kelly, T.S.; Andrews, R.; Rogers, B.E. In vitro and in vivo evaluation of 64Cu-labeled DOTA-linker-bombesin(7–14) analogues containing different amino acid linker moieties. Bioconjug. Chem. 2007, 18, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Jamous, M.; Tamma, M.L.; Gourni, E.; Waser, B.; Reubi, J.C.; Maecke, H.R.; Mansi, R. RPEG spacers of different length influence the biological profile of bombesin-based radiolabeled antagonists. Nucl. Med. Biol. 2014, 41, 464–470. [Google Scholar] [CrossRef]
- Bernhard, Y.; Gigot, E.; Goncalves, V.; Moreau, M.; Sok, N.; Richard, P.; Decréau, R.A. Direct subphthalocyanine conjugation to bombesin vs. indirect conjugation to its lipidic nanocarrier. Org. Biomol. Chem. 2016, 14, 4511–4518. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Mierke, D.F. Molecular complex of cholecystokinin-8 and N-terminus of the cholecystokinin A receptor by NMR spectroscopy. Biochemistry 1999, 38, 14775–14783. [Google Scholar] [CrossRef] [PubMed]
- Morelli, G.; De Luca, S.; Tesauro, D.; Saviano, M.; Pedone, C.; Dolmella, A.; Visentin, R.; Mazzi, U. CCK8 peptide derivatized with diphenylphosphine for rhenium labelling: Synthesis and molecular mechanics calculations. Pept. Sci. 2002, 8, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Morisco, A.; Palladino, P.; Palumbo, R.; Tesauro, D.; Morelli, G. Amphiphilic CCK peptides assembled in supramolecular aggregates: Structural investigations and in vitro studies. Mol. Biosyst. 2011, 7, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Tesauro, D.; Accardo, A.; Gianolio, E.; Paduano, L.; Teixeira, J.; Schillen, K.; Aime, S.; Morelli, G. Peptide derivatizedlamellar aggregates as target-specific MRI contrast agents. Chembiochem 2007, 8, 950–995. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Tesauro, D.; Aloj, L.; Tarallo, L.; Arra, C.; Mangiapia, G.; Vaccaro, M.; Pedone, C.; Paduano, L.; Morelli, G. Peptide-containing aggregates as selective nanocarriers for therapeutics. Chem. Med. Chem. 2008, 3, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, M.; Accardo, A.; Costantini, S.; Scala, S.; Portella, L.; Trotta, A.; Ronga, L.; Guillon, J.; Leone, M.; Colonna, G.; et al. Intrinsically disordered amphiphilic peptides as potential targets in drug delivery vehicles. Mol. Biosyst. 2015, 11, 2925–2932. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Leone, M.; Tesauro, D.; Aufiero, R.; Bénarouche, A.; Cavalier, J.F.; Longhi, S.; Carriere, F.; Rossi, F. Solution conformational features and interfacial properties of an intrinsically disordered peptide coupled to alkyl chains: A new class of peptide amphiphiles. Mol. Biosyst. 2013, 9, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, M.; Costantini, S.; Scala, S.; Tesauro, D.; Accardo, A.; Leone, M.; Colonna, G.; Guillon, J.; Portella, L.; Trotta, A.; et al. Conformational ensembles explored dynamically from disordered peptides targeting chemokine receptor CXCR4. Int. J. Mol. Sci. 2015, 16, 12159–12173. [Google Scholar] [CrossRef] [PubMed]
- Banta, S.; Megeed, Z.; Casali, M.; Rege, K.; Yarmush, M.L. Engineering protein and peptide building blocks for nanotechnology. J. Nanosci. Nanotechnol. 2007, 7, 387–401. [Google Scholar] [CrossRef]



| Receptor | Peptide Sequence | Peptide Derivative | Drug | Ref. |
|---|---|---|---|---|
| Integrin receptor Avβ3 | c(RGDfK) | c(RGDfK)-NHS-PEG-PLA | CA4 | [99] |
| c(RGDfK) | c(RGDfK)-SH post liposome modification | CDDP | [100] | |
| c(RGDfC) | MBPE-c(RGDfC) post-insertion | DOX | [101] | |
| c(RGDf[N-Met]K) | c(RGDf[N-Met]K(Ac-SCH2CO)) | DOX | [102] | |
| c(RGDyK) | DSPE-PEG- c(RGDyK) | CDDP | [103] | |
| cAbaRGD cAmpRGD | DSPE-PEG-cAbaRGD DSPE-PEG- cAmpRGD | DOX DOX | [104] | |
| iRGD | iRGD-HES-SS-C18 NCs | DOX/sorafenib | [105] | |
| G-Protein coupled receptor | Octreotide | OCA-DOTA/ OCA-DTPAGlu | Gd-complex | [106] |
| Octreotide | (C18)2(AdOO)5OCT | Gd-complex | [107] | |
| Octreotide | (C18)2(AdOO)5OCT | CDDP/DOX | [108] | |
| Octreotide | OCT-(PTX)-PEG-b-PCL | PTX | [109] | |
| Octreotide | Oct-Phe-PEG-SA | DOX | [110] | |
| Octreotide | H40-PLA-PEG-OCT | TDP-A | [111] | |
| Octreotide | SAMA-TOC post liposome modification | 111In-DTPA | [112] | |
| Octreotide | HSPE-PEG4000-OCT | DOX | [113] | |
| [Tyr3]-Octreotate | Maleimido-TATE | 64Cu-DOTA | [114] | |
| KE108 | KE108 post micelle modification via NHS | TDP-A AB3 | [115] [116] | |
| [7–14]BN wild-type | (C18)2-L5-[7–14]BN (C18)2-PEG3000-[7–14]BN | 111In-DOTA | [117] | |
| [7–14]BN-AA1 analogue | MonY-BN-AA1 | DOX AUL12 DOX | [118] [119] [120] | |
| CCK8 | (C18)2-L5CCK8 | Gd-DOTA/Gd-DTPA | [121] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesauro, D.; Accardo, A.; Diaferia, C.; Milano, V.; Guillon, J.; Ronga, L.; Rossi, F. Peptide-Based Drug-Delivery Systems in Biotechnological Applications: Recent Advances and Perspectives. Molecules 2019, 24, 351. https://doi.org/10.3390/molecules24020351
Tesauro D, Accardo A, Diaferia C, Milano V, Guillon J, Ronga L, Rossi F. Peptide-Based Drug-Delivery Systems in Biotechnological Applications: Recent Advances and Perspectives. Molecules. 2019; 24(2):351. https://doi.org/10.3390/molecules24020351
Chicago/Turabian StyleTesauro, Diego, Antonella Accardo, Carlo Diaferia, Vittoria Milano, Jean Guillon, Luisa Ronga, and Filomena Rossi. 2019. "Peptide-Based Drug-Delivery Systems in Biotechnological Applications: Recent Advances and Perspectives" Molecules 24, no. 2: 351. https://doi.org/10.3390/molecules24020351
APA StyleTesauro, D., Accardo, A., Diaferia, C., Milano, V., Guillon, J., Ronga, L., & Rossi, F. (2019). Peptide-Based Drug-Delivery Systems in Biotechnological Applications: Recent Advances and Perspectives. Molecules, 24(2), 351. https://doi.org/10.3390/molecules24020351