M860, a Monoclonal Antibody against Human Lactoferrin, Enhances Tumoricidal Activity of Low Dosage Lactoferrin via Granzyme B Induction
Abstract
:1. Introduction
2. Results
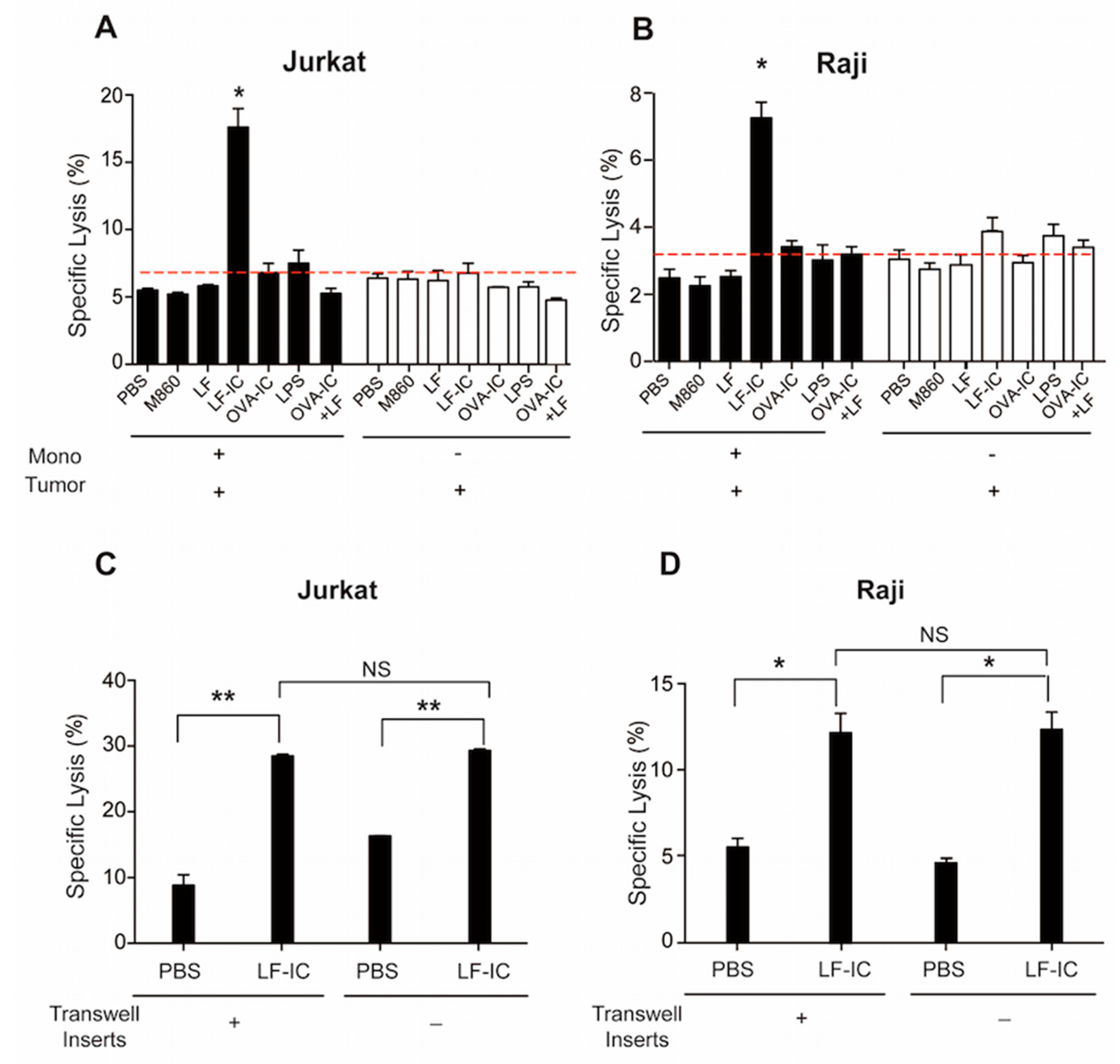
2.1. Human Monocytes Acquire Potent Tumoricidal Activity Following LF-IC Stimulation
2.2. The Tumoricidal Function of LF-IC-Primed Human Monocytes Is Independent of TNFα Production
2.3. Granzyme B Is a Key Mediator of the Tumoricidal Function of LF-IC-Primed Monocytes
2.4. Role of CD32a (FcγRIIa) and Membrane-Bound CD14 in LF-IC Priming of Human Monocytes
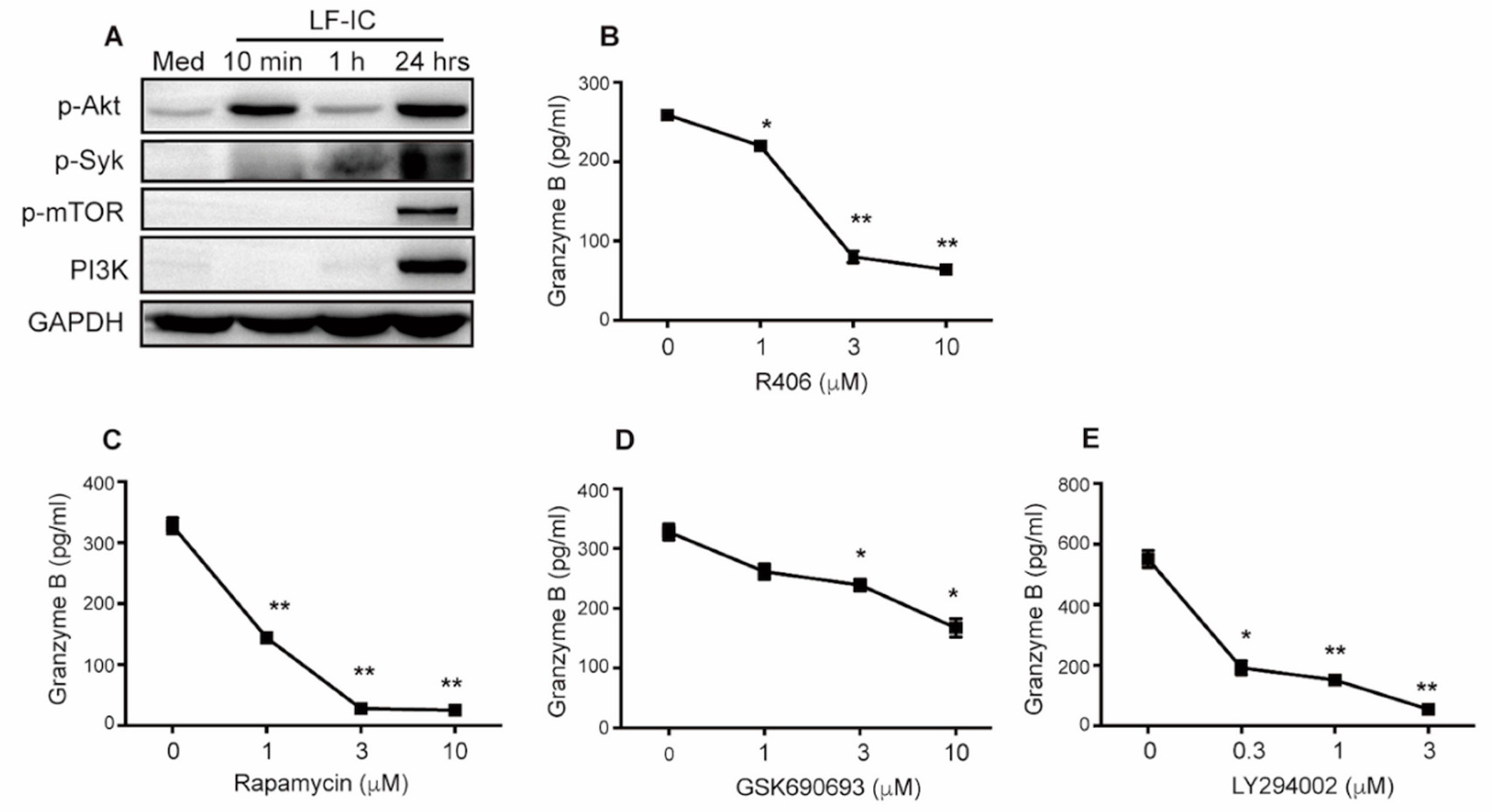
2.5. LF-IC Induces GzB Expression in Human Monocytes via the syk-PI3K-AKT Pathway
2.6. Unresponsiveness of Murine Monocytes to LF-IC Stimulation
3. Discussion
4. Materials and Methods
4.1. Reagents and Abs
4.2. Peripheral Blood Monocyte Isolation and Cell Culture
4.3. Mouse Monocyte Isolation
4.4. Anti-Tumor Effects
4.5. ELISA
4.6. Real-Time RT-PCR
4.7. Tumor Models
4.8. Western Blotting Analysis
4.9. Study Approval
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vogel, H.J. Lactoferrin, a bird’s eye view. Biochem. Cell Biol. 2012, 90, 233. [Google Scholar] [CrossRef] [PubMed]
- Damiens, E.; El Yazidi, I.; Mazurier, J.; Duthille, I.; Spik, G.; Boilly-Marer, Y. Lactoferrin inhibits G1 cyclin-dependent kinases during growth arrest of human breast carcinoma cells. J. Cell. Biochem. 2015, 74, 486–498. [Google Scholar] [CrossRef]
- Mader, J.S.; Salsman, J.; Conrad, D.M.; Hoskin, D.W. Bovine lactoferricin selectively induces apoptosis in human leukemia and carcinoma cell lines. Mol. Cancer 2005, 4, 612. [Google Scholar] [CrossRef] [PubMed]
- Hoedt, E.; Hardivillé, S.; Mariller, C.; Elass, E.; Perraudin, J.P.; Pierce, A. Discrimination and evaluation of lactoferrin and delta-lactoferrin gene expression levels in cancer cells and under inflammatory stimuli using TaqMan real-time PCR. Biometals 2010, 23, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Benaïssa, M.; Peyrat, J.P.; Hornez, L.; Mariller, C.; Mazurier, J.; Pierce, A. Expression and prognostic value of lactoferrin mRNA isoforms in human breast cancer. Int. J. Cancer 2005, 114, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Sang, W.P.; Pyo, C.W.; Yoo, N.K.; Kim, J.; Choi, S.Y. Requirement of the JNK-associated Bcl-2 pathway for human lactoferrin-induced apoptosis in the Jurkat leukemia T cell line. Biochimie 2009, 91, 0–108. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.X.; Jiang, H.R.; Li, H.B.; Zhang, T.N.; Zhou, Q.; Liu, N. Apoptosis of stomach cancer cell SGC-7901 and regulation of Akt signaling way induced by bovine lactoferrin. J. Dairy Sci. 2010, 93, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lönnerdal, B. Bovine lactoferrin and lactoferricin exert antitumor activities on human colorectal cancer cells (HT-29) by activating various signaling pathways. Biochem. Cell Biol. 2016, 95, 1. [Google Scholar] [CrossRef] [PubMed]
- Birgens, H.S.; Karle, H.; Hansen, N.E.; Ostergaard, K.L. Lactoferrin receptors in normal and leukaemic human blood cells. Scand. J. Haematol. 2010, 33, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Damiens, E.; Mazurier, J.; Yazidi, I.E.; Masson, M.; Duthille, I.; Spik, G.; Boilly-Marer, Y. Effects of human lactoferrin on NK cell cytotoxicity against haematopoietic and epithelial tumour cells. Biochim. Biophys. Acta 1998, 1402, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Ben, A.; Qian, G.; Mark, W. Bovine lactoferrin structures promoting corneal epithelial wound healing in vitro. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2719–2726. [Google Scholar]
- Mario, F.D.; Aragona, G.; Bò, N.D.; Cavestro, G.M.; Cavallaro, L.; Iori, V.; Comparato, G.; Leandro, G.; Pilotto, A.; Franzè, A. Use of bovine lactoferrin for Helicobacter pylori eradication. Dig. Liver Dis. 2003, 35, 706–710. [Google Scholar]
- Valenti, P.; Antonini, G. Lactoferrin: An important host defence against microbial and viral attack. Cell. Mol. Life Sci. Cmls 2005, 62, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, L.; Calvo, M.; Brock, J.H. Biological role of lactoferrin. Haematologia 1985, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.N.; Rulan, J.; Xiaou, D. Bovine lactoferrin can be taken up by the human intestinal lactoferrin receptor and exert bioactivities. J. Pediatric Gastroenterol. Nutr. 2011, 53, 606–614. [Google Scholar]
- Debanne, M.T.; Regoeczi, E.; Sweeney, G.D.; Krestynski, F. Interaction of human lactoferrin with the rat liver. Am. J. Physiol. 1985, 248, G463. [Google Scholar] [CrossRef]
- Bennett, R.M.; Davis, J. Lactoferrin binding to human peripheral blood cells: An interaction with a B-enriched population of lymphocytes and a subpopulation of adherent mononuclear cells. J. Immunol. 1981, 127, 1211. [Google Scholar]
- Hu, X.M.; Xu, Y.R.; Yan, R.; Sun, S.L.; Dong, H.L.; Wang, J.; Gao, X.M. A Novel Murine Anti-Lactoferrin Monoclonal Antibody Activates Human Polymorphonuclear Leukocytes through Membrane-Bound Lactoferrin and TLR4. Biomed Res. Int. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Hu, L.; Hu, X.; Long, K.; Gao, C.; Dong, H.L.; Zhong, Q.; Gao, X.M.; Gong, F.Y. Extraordinarily potent proinflammatory properties of lactoferrin-containing immunocomplexes against human monocytes and macrophages. Sci Rep 2017, 1, 1–4230. [Google Scholar] [CrossRef]
- Alberto, M.; Silvano, S.; Massimo, L.; Paola, A.; Antonio, S. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar]
- Gao, C.H.; Dong, H.L.; Tai, L.; Gao, X.M. Lactoferrin-Containing Immunocomplexes Drive the Conversion of Human Macrophages from M2- into M1-like Phenotype. Front. Immunol. 2018, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, T.; Saddawi-Konefka, R.; Vermi, W.; Koebel, C.M.; Arthur, C.; White, J.M.; Uppaluri, R.; Andrews, D.M.; Ngiow, S.F.; Teng, M.W.; et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J. Exp. Med. 2012, 209, 1869–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonio, S.; Vincenzo, B. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig. 2007, 117, 1155–1166. [Google Scholar]
- Qian, B.Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, T.S.; Wiley, S.R.; Kubin, M.Z.; Sedger, L.M.; Maliszewski, C.R.; Fanger, N.A. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J. Exp. Med. 2015, 189, 1343–1354. [Google Scholar] [CrossRef]
- Lindholm, P.F.; Sivapurapu, N.; Jovanovic, B.; Kajdacsy, B.A. Monocyte-Induced Prostate Cancer Cell Invasion is Mediated by Chemokine ligand 2 and Nuclear Factor-κB Activity. J. Clin. Cell. Immunol. 2015, 6, 308. [Google Scholar] [CrossRef] [PubMed]
- Lauren, B.E.; David, S.; Fang, T.; Hyung-Gu, K.; Takushi, N.; César, M.O.F.; Anna, M.; Aaronson, S.A.; Lee, S.W. Expression of the p53 target CDIP correlates with sensitivity to TNFα-induced apoptosis in cancer cells. Cancer Res. 2012, 72, 2373. [Google Scholar]
- Wang, C.Y.; Mayo, M.W.; Korneluk, R.G.; Goeddel, D.V.; Baldwin, A.S., Jr. NF-kappaB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998, 281, 1680–1683. [Google Scholar] [CrossRef]
- Zhong, Q.; Gong, F.Y.; Gong, Z.; Hua, S.H.; Gao, X.M. IgG Immunocomplexes Sensitize Human Monocytes for Inflammatory Hyperactivity via Transcriptomic and Epigenetic Reprogramming in Rheumatoid Arthritis. J. Immunol. 2018, 200, 3913–3925. [Google Scholar] [CrossRef] [Green Version]
- Russell, J.H.; Ley, T.J. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 2002, 20, 323–370. [Google Scholar] [CrossRef]
- Mahrus, S.; Craik, C.S. Selective Chemical Functional Probes of Granzymes A and B Reveal Granzyme B Is a Major Effector of Natural Killer Cell-Mediated Lysis of Target Cells. Chem. Biol. 2005, 12, 567–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parren, P.W.; Warmerdam, P.A.; Boeije, L.C.; Arts, J.; Westerdaal, N.A.; Vlug, A.; Capel, P.J.; Aarden, L.A.; Van de Winkel, J.G. On the interaction of IgG subclasses with the low affinity Fc gamma RIIa (CD32) on human monocytes, neutrophils, and platelets. J. Clin. Investig. 1992, 90, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.M.; Jönsson, F.; Mancardi, D.A.; Tu, N.; Beutier, H.; Van Rooijen, N.; Macdonald, L.E.; Murphy, A.J.; Bruhns, P. Mechanisms of anaphylaxis in human low-affinity IgG receptor locus knock-in mice. J. Allergy Clin. Immunol 2017, 139, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Veri, M.-C.; Gorlatov, S.; Li, H.; Burke, S.; Johnson, S.; Stavenhagen, J.; Stein, K.E.; Bonvini, E.; Koenig, S. Monoclonal antibodies capable of discriminating the human inhibitory Fcγ-receptor IIB (CD32B) from the activating Fcγ-receptor IIA (CD32A): Biochemical, biological and functional characterization. Immunology 2007, 121, 392–404. [Google Scholar] [CrossRef]
- Yuasa, T.; Kubo, S.; Yoshino, T.; Ujike, A.; Matsumura, K.; Ono, M.; Ravetch, J.V.; Takai, T. Deletion of fcgamma receptor IIB renders H-2(b) mice susceptible to collagen-induced arthritis. J. Exp. Med. 1999, 189, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Fas, S.C.; Sven, B.; Yun, Z.J.; Marco, G.; Treiber, M.K.; Ulrich, M.; Krammer, P.H.; Min, L.W. Wogonin sensitizes resistant malignant cells to TNFalpha- and TRAIL-induced apoptosis. Blood 2006, 108, 3700–3706. [Google Scholar] [CrossRef]
- Saranya, E.; Kavin, F.; Vikram, S.; Huiqing, F.; Li, R.; Shalini, G.; Brenda, R.; Xiaokui, M.; Carolyn, C.; Edward, B. Granzyme B expression is enhanced in human monocytes by TLR8 agonists and contributes to antibody-dependent cellular cytotoxicity. J. Immunol. 2015, 194, 2786–2795. [Google Scholar]
- Ji, Z.S.; Mahley, R.W. Lactoferrin binding to heparan sulfate proteoglycans and the LDL receptor-related protein. Further evidence supporting the importance of direct binding of remnant lipoproteins to HSPG. Arter. Thromb. A J. Vasc. Biol. 1994, 14, 2025. [Google Scholar] [CrossRef]
- Duchardt, F.; Ruttekolk, I.W. A cell-penetrating peptide derived from human lactoferrin with conformation-dependent uptake efficiency. J. Biol. Chem. 2010, 284, 36099–36108. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |




| Forward (5′-3′) | Reverse (5′-3′) | |
|---|---|---|
| hGAPDH | GAGTCAACGGATTTGGTCGT | TTGATTTTGGAGGGATCTCG |
| hGzA | AACCAGGAACCATGTGCCAA | GGCTTCCAGAATCTCCATTGC |
| hGzB | GAGCAAGGAGGAAACAACAGC | GGCCCCCAAGGTGACATTTA |
| hGzH | ATGCTACTGCAGGGGGACT | TCAGGCCCAGAGGAAGGTTA |
| hGzK | CCCTGCGAGAAGTCACTGTT | CCCCCTGAGTCACCCTTACA |
| hGzM | GTCAGTAGCTCCTTTGGGACC | GGCTGTTGTTACACATGCGG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, J.; Gong, Z.; Pan, X.-H.; Ma, Z.-H.; Ma, S.-Y.; Wang, H.-M.; Dong, H.-L.; Gong, F.-Y.; Gao, X.-M. M860, a Monoclonal Antibody against Human Lactoferrin, Enhances Tumoricidal Activity of Low Dosage Lactoferrin via Granzyme B Induction. Molecules 2019, 24, 3640. https://doi.org/10.3390/molecules24203640
Li Y, Li J, Gong Z, Pan X-H, Ma Z-H, Ma S-Y, Wang H-M, Dong H-L, Gong F-Y, Gao X-M. M860, a Monoclonal Antibody against Human Lactoferrin, Enhances Tumoricidal Activity of Low Dosage Lactoferrin via Granzyme B Induction. Molecules. 2019; 24(20):3640. https://doi.org/10.3390/molecules24203640
Chicago/Turabian StyleLi, Ya, Jie Li, Zheng Gong, Xiao-Hua Pan, Zi-Han Ma, Shu-Yan Ma, Hong-Min Wang, Hong-Liang Dong, Fang-Yuan Gong, and Xiao-Ming Gao. 2019. "M860, a Monoclonal Antibody against Human Lactoferrin, Enhances Tumoricidal Activity of Low Dosage Lactoferrin via Granzyme B Induction" Molecules 24, no. 20: 3640. https://doi.org/10.3390/molecules24203640
APA StyleLi, Y., Li, J., Gong, Z., Pan, X. -H., Ma, Z. -H., Ma, S. -Y., Wang, H. -M., Dong, H. -L., Gong, F. -Y., & Gao, X. -M. (2019). M860, a Monoclonal Antibody against Human Lactoferrin, Enhances Tumoricidal Activity of Low Dosage Lactoferrin via Granzyme B Induction. Molecules, 24(20), 3640. https://doi.org/10.3390/molecules24203640




