Distribution Pattern, Emission Characteristics and Environmental Impact of Polycyclic Aromatic Hydrocarbons (PAHs) in Download Ash and Dust from Iron and Steel Enterprise
Abstract
1. Introduction
2. Results and Discussion
2.1. Particle Size Distribution of Download Ashes in Different Production Processes
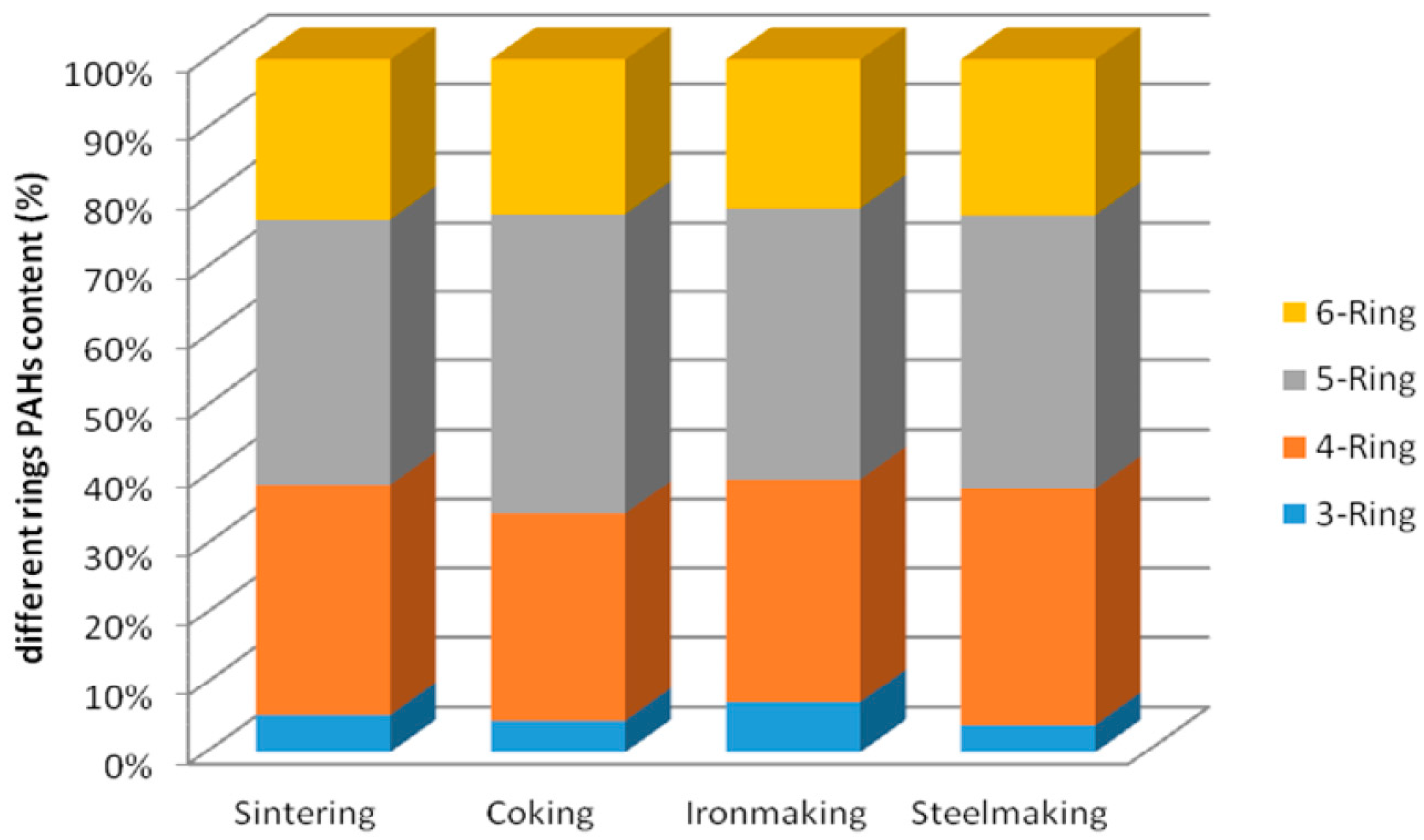
2.2. Characteristics and Level of PAHs Pollution in Download Ash from Different Processes
2.3. Concentration Distribution of PAHs in Dust Emission from Different Processes
2.4. Environmental Impact of PAHs in the Surrounding Air of the Steel Plant
3. Materials and Methods
3.1. Data and Sample Collection
3.2. Pretreatment and Determination of Samples
3.3. Quality Control
3.4. Particle Size Analysis Method
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsapakis, M.; Stephanou, E.G. Occurrence of gaseous and particulate polycyclic aromatic hydrocarbons in the urban atmosphere: Study of sources and ambient temperature effect on the gas/particle concentration and distribution. Environ. Pollut. 2005, 133, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Ma, J.H.; Duan, H.J.; Wei, L.H. Compositions, Sources and Health Risks of Polycyclic Aromatic Hydrocarbons (PAHs) in Surface Dusts from Driving-schools in a City of Henan Province, China. Environ. Sci. 2017, 38, 711–720. [Google Scholar]
- Zhang, Y.; Tao, S.; Shen, H.; Jiamin, M. Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. Proc. Nati. Acad. Sci. USA 2009, 106, 21063–21067. [Google Scholar] [CrossRef] [PubMed]
- Dat, N.D.; Chang, M.B. Review on characteristics of PAHs in atmosphere, anthropogenic sources and control technologies. Sci. Total Environ. 2017, 609, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, C.; Shen, H. Concentrations and origins of nitro-polycyclic aromatic hydrocarbons and oxy-polycyclic aromatic hydrocarbons in ambient air in urban and rural areas in northern China. Environ. Pollut. 2015, 197, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Bi, C.; Wang, D.; Yu, Z.; Chen, Z. Atmospheric deposition of polycyclic aromatic hydrocarbons (PAHs) in Shanghai: the spatio-temporal variation and source identification. Front Earth Sci. 2018, 12, 63–71. [Google Scholar] [CrossRef]
- Viglianti, C.; Hanna, K.; Brauer, C.D.; Germain, P. Removal of polycyclic aromatic hydrocarbons from aged-contaminated soil using cyclodextrins: Experimental study. Environ. Pollut. 2006, 140, 427–435. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.H.; Li, H.L.; Liu, X.H.; Wang, W.X. PAHs distribution in precipitation at Mount Taishan: China. Identification of sources and meteorological influences. Atmos. Res. 2010, 95, 0–7. [Google Scholar] [CrossRef]
- Liu, G.R.; Zheng, M.H.; Liu, W.B.; Wang, C.Z.; Zhang, B.; Gao, L.R.; Su, G.J.; Xiao, K.; Lv, P. Atmospheric emission of PCDD/Fs, PCBs, hexachlorobenzene, and pentachlorobenzene from the coking industry. Environ. Sci. Technol. 2009, 43, 9196–9201. [Google Scholar] [CrossRef]
- Liu, G.R.; Shi, G.L.; Zhang, P.; Zhou, L.D.; Wu, J.H.; Feng, Y.C. Source identification and toxicity assessment of polycyclic aromatic hydrocarbons in particulate matter of Chengdu, China. China Environ. Sci. 2014, 34, 2479–2484. [Google Scholar]
- Li, C.C.; Zhang, X.; Gao, X.B.; Qi, S.H.; Wang, Y.X. The Potential Environmental Impact of PAHs on Soil and Water Resources in Air Deposited Coal Refuse Sites in Niangziguan Karst Catchment, Northern China. Int. J. Environ. Res. Pub. Health 2019, 16, 1368. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.W.F.; Takeda, K.; Sakugawa, H. Polycyclic aromatic hydrocarbons (PAHs) associated with atmospheric particles in Higashi Hiroshima, Japan: Influence of meteorological conditions and seasonal variations. Atmos. Res. 2008, 88, 224–233. [Google Scholar] [CrossRef]
- Guo, H.; Lee, S.C.; Ho, K.F.; Wang, S.M.; Zou, S.C. Particle-associated polycyclic aromatic hydrocarbons in urban air of Hong Kong. Atmos. Environ. 2003, 37, 5307–5317. [Google Scholar] [CrossRef]
- Gilio, A.D.; Ventrella, G.; Giungato, P.; Tutino, M.; Giua, R.; Assennato, G.; Gennaro, G.D. An intensive monitoring campaign of PAHs for assessing the impact of a steel plant. Chemosphere 2017, 168, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Ooi, T.C.; Thompson, D.; Anderson, D.R. The effect of charcoal combustion on iron-ore sintering performance and emission of persistent organic pollutants. Combust. Flame 2011, 158, 979–987. [Google Scholar] [CrossRef]
- Wang, F.; Xing, S.; Hou, X. Study on the Distribution Regularity of PAHs in the Coking Dust from the Coking Environment. Procedia Eng. 2012, 45, 959–961. [Google Scholar] [CrossRef][Green Version]
- Ma, J.H. Emission Characteristies of Partieulates from TyPical Produetion Proeesso fIron and Steel EnterPrises. Southwest Univ. 2009, 15, 24–30. [Google Scholar]
- Mastral, A.M.; Callén, M.S. A Review on Polycyclic Aromatic Hydrocarbon (PAH) Emissions from Energy Generation. Environ. Sci. Technol. 2000, 34, 66–69. [Google Scholar] [CrossRef]
- Yu, T.G.; Wang, T.G.; Zhu, X.P.; Wu, D.P. Source apportionment of PAHs in aerosol of northwest of Beijing. Environ. Chem. 2008, 27, 245–250. [Google Scholar]
- Xu, G.L. Study on the Emisson Characteristic of Polycyclic Aromatic Hydrocarbons in Coking Process. Taiyuan Univ. Technol. 2011, 30–34. [Google Scholar]
- Zhu, X.L.; Liu, W.L. A Comparison of PAHs source profiles of domestic coal combustion, coke plant and petroleum asphalt industry. Acta Sci. Circumst. 2002, 22, 199–203. [Google Scholar]
- Ciaparra, D.; Aries, E.; Booth, M.J.; Anderson, D.R.; Almerida, S.M.; Harrad, S. Characterisation of volatile organic compounds and polycyclic aromatic hydrocarbons in the ambient air of steelworks. Atmos. Environ. 2009, 43, 2070–2079. [Google Scholar] [CrossRef]
- Larsen, R.K.; Baker, J.E. Source Apportionment of Polycyclic Aromatic Hydrocarbons in the Urban Atmosphere: A Comparison of Three Methods. Environl. Sci. Technol. 2003, 37, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.L.; Shi, M.J.; Liang, J.J. Concentration and origin of polycyclic aromatic hydrocarbons in the soil of Dagang Oil Field. Environ. Sci. Technol. 2018, 41, 151–157. [Google Scholar]
- Wang, J.; Zhu, L.Z.; Shen, X.Y. PAHs Pollution in Air of Coke Plant and Health Risk Assessment. Environ. Sci. 2003, 24, 135–138. [Google Scholar]
- Zhou, B.H.; Zhang, C.Z.; Wang, G.H. Seasonal variation and health risk assessment of atmospheric polycyclic aromatic hydrocarbons (PAHs) in the urban area of Xi’an. Acta Sci. Circumst. 2012, 32, 2324–2331. [Google Scholar]
- Wang, C.; Dao, X.; Zhang, L.L.; LV, Y.B. Characteristics and toxicity assessment of airborne particulate polycyclic aromatic hydrocarbons of four background sites in China. Chin. Environ. Sci. 2015, 35, 3543–3549. [Google Scholar] [CrossRef]
- Wise, S.A.; Sander, L.C.; Schantz, M.M. Analytical Methods for Determination of Polycyclic Aromatic Hydrocarbons (PAHs)—A Historical Perspective on the 16 U.S. EPA Priority Pollutant PAHs. Polycyclic Aromat. Compd. 2015, 35, 187–247. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Diameter | Sinter Tank | Coking | Taphole of Ironmaking | Steelmaking Refining |
|---|---|---|---|---|
| 0–2.5 μm | 22.57 | 10.48 | 29.86 | 72.62 |
| 2.5–10 μm | 15.70 | 12.71 | 23.06 | 27.38 |
| 10–100 μm | 40.76 | 68.18 | 38.38 | 0 |
| 100–300 μm | 20.97 | 7.63 | 8.70 | 0 |
| Coking | Ironmaking | Steelmaking | |
|---|---|---|---|
| NaP | 0.41 ± 0.03 | 0.30 ± 0.03 | N.D. |
| Ace | 0.43 ± 0.05 | 0.06 ± 0.02 | N.D. |
| Acy | 0.24 ± 0.03 | 0.18 ± 0.02 | N.D. |
| Flu | 1.43 ± 0.12 | 0.66 ± 0.06 | N.D. |
| Phe | 6.34 ± 0.05 | 2.68 ± 0.21 | 0.05 ± 0.01 |
| Ant | 2.03 ± 0.17 | 0.13 ± 0.03 | 0.01 ± 0.01 |
| Flua | 7.58 ± 0.68 | 1.40 ± 0.12 | 0.08 ± 0.03 |
| Pyr | 5.41 ± 0.65 | 0.49 ± 0.04 | 0.04 ± 0.02 |
| BaA | 5.63 ± 0.62 | 0.17 ± 0.02 | 0.08 ± 0.01 |
| Chry | 6.21 ± 0.75 | 0.18 ± 0.03 | 0.07 ± 0.02 |
| BbF | 9.80 ± 0.83 | 0.05 ± 0.02 | 0.04 ± 0.01 |
| BkF | 9.80 ± 1.17 | 0.05 ± 0.01 | 0.04 ± 0.02 |
| BaP | 4.80 ± 0.42 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| IcdP | 3.10 ± 0.40 | 0.03 ± 0.02 | 0.03 ± 0.01 |
| DahA | 1.75 ± 0.21 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| BghiP | 4.67 ± 0.42 | 0.03 ± 0.02 | 0.02 ± 0.01 |
| ∑16PAHs | 69.63 ± 5.57 | 6.49 ± 0.62 | 0.49 ± 0.06 |
| BaP/∑16PAHs (%) | 6.89 ± 0.61 | 0.31 ± 0.03 | 0.99 ± 0.09 |
| Sintering | Coking | Ironmaking | Steelmaking | ∑PAHs | |
|---|---|---|---|---|---|
| NaP | N.D. | N.D. | N.D. | N.D. | N.D. |
| Ace | N.D. | N.D. | N.D. | N.D. | N.D. |
| Acy | 0.006 ± 0.001 | N.D. | N.D. | N.D. | 0.006 |
| Flu | N.D. | N.D. | N.D. | N.D. | N.D. |
| Phe | 0.148 ± 0.155 | 0.603 ± 0.054 | 0.149 ± 0.042 | 0.065 ± 0.008 | 0.966 ± 0.077 |
| Ant | 0.083 ± 0.010 | 0.280 ± 0.031 | 0.054 ± 0.007 | 0.047 ± 0.005 | 0.465 ± 0.042 |
| Flua | 0.137 ± 0.018 | 0.503 ± 0.040 | 0.111 ± 0.009 | 0.099 ± 0.008 | 0.852 ± 0.094 |
| Pyr | 0.263 ± 0.034 | 0.963 ± 0.125 | 0.166 ± 0.023 | 0.168 ± 0.022 | 1.561 ± 0.142 |
| BaA | 0.622 ± 0.055 | 2.472 ± 0.222 | 0.366 ± 0.029 | 0.428 ± 0.048 | 3.889 ± 0.311 |
| Chry | 0.451 ± 0.059 | 1.869 ± 0.241 | 0.260 ± 0.032 | 0.286 ± 0.022 | 2.868 ± 0.261 |
| BbF | 0.407 ± 0.036 | 2.482 ± 0.298 | 0.289 ± 0.023 | 0.280 ± 0.029 | 3.460 ± 0.311 |
| BkF | 0.407 ± 0.053 | 2.483 ± 0.276 | 0.289 ± 0.038 | 0.281 ± 0.023 | 3.462 ± 0.318 |
| BaP | 0.390 ± 0.046 | 1.742 ± 0.156 | 0.240 ± 0.032 | 0.258 ± 0.033 | 2.631 ± 0.292 |
| IcdP | 0.556 ± 0.072 | 2.244 ± 0.179 | 0.332 ± 0.049 | 0.356 ± 0.004 | 3.489 ± 0.314 |
| DahA | 0.495 ± 0.058 | 1.701 ± 0.189 | 0.283 ± 0.022 | 0.317 ± 0.042 | 2.797 ± 0.223 |
| BghiP | 0.465 ± 0.051 | 2.081 ± 0.169 | 0.271 ± 0.030 | 0.287 ± 0.025 | 3.105 ± 0.292 |
| ∑16PAHs | 4.435 ± 0.355 | 19.429 ± 2.545 | 2.815 ± 0.253 | 2.876 ± 0.377 | 29.556 ± 3.281 |
| BaP/∑16PAHs (%) | 8.81 ± 1.13 | 8.97 ± 0.83 | 8.53 ± 0.68 | 8.97 ± 1.15 | 8.90 ± 0.71 |
| Serial Number | Name | Abbreviations | Number of Benzene Ring | Limit of Detection (μg/mL) | Limit of Quantification (μg/mL) |
|---|---|---|---|---|---|
| 1 | naphthalene | NaP | 2 | 0.002 | 0.005 |
| 2 | acenaphthene | Ace | 3 | 0.004 | 0.010 |
| 3 | acenaphthylene | Acy | 3 | 0.005 | 0.012 |
| 4 | fluorine | Flu | 3 | 0.005 | 0.013 |
| 5 | phenanthrene | Phe | 3 | 0.003 | 0.008 |
| 6 | anthracene | Ant | 3 | 0.004 | 0.011 |
| 7 | fluoranthene | Flua | 4 | 0.007 | 0.124 |
| 8 | pyrene | Pyr | 4 | 0.004 | 0.013 |
| 9 | benzo[a]anthracene | BaA | 4 | 0.002 | 0.007 |
| 10 | chrysene | Chry | 4 | 0.007 | 0.013 |
| 11 | benzo[b]fluoranthene | BbF | 5 | 0.003 | 0.006 |
| 12 | benzo[k]fluoranthene | BkF | 5 | 0.004 | 0.013 |
| 13 | benzo[a]pyrene | BaP | 5 | 0.004 | 0.007 |
| 14 | indeno[1,2,3-cd] pyrene | IcdP | 6 | 0.005 | 0.015 |
| 15 | dibenz[ah]anthracene | DahA | 5 | 0.006 | 0.013 |
| 16 | benzo[ghi]perylene | BghiP | 6 | 0.004 | 0.013 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Chen, C.; Ding, C.; Liu, G.; Zhang, G. Distribution Pattern, Emission Characteristics and Environmental Impact of Polycyclic Aromatic Hydrocarbons (PAHs) in Download Ash and Dust from Iron and Steel Enterprise. Molecules 2019, 24, 3646. https://doi.org/10.3390/molecules24203646
Sun Y, Chen C, Ding C, Liu G, Zhang G. Distribution Pattern, Emission Characteristics and Environmental Impact of Polycyclic Aromatic Hydrocarbons (PAHs) in Download Ash and Dust from Iron and Steel Enterprise. Molecules. 2019; 24(20):3646. https://doi.org/10.3390/molecules24203646
Chicago/Turabian StyleSun, Youmin, Chunzhu Chen, Chun Ding, Guanghui Liu, and Guiqin Zhang. 2019. "Distribution Pattern, Emission Characteristics and Environmental Impact of Polycyclic Aromatic Hydrocarbons (PAHs) in Download Ash and Dust from Iron and Steel Enterprise" Molecules 24, no. 20: 3646. https://doi.org/10.3390/molecules24203646
APA StyleSun, Y., Chen, C., Ding, C., Liu, G., & Zhang, G. (2019). Distribution Pattern, Emission Characteristics and Environmental Impact of Polycyclic Aromatic Hydrocarbons (PAHs) in Download Ash and Dust from Iron and Steel Enterprise. Molecules, 24(20), 3646. https://doi.org/10.3390/molecules24203646




