20(S)-Ginsenoside Rg3 Promotes HeLa Cell Apoptosis by Regulating Autophagy
Abstract
1. Introduction
2. Results
2.1. 20(S)-GRg3 Inhibits the Proliferation of Serum-Starved HeLa Cells
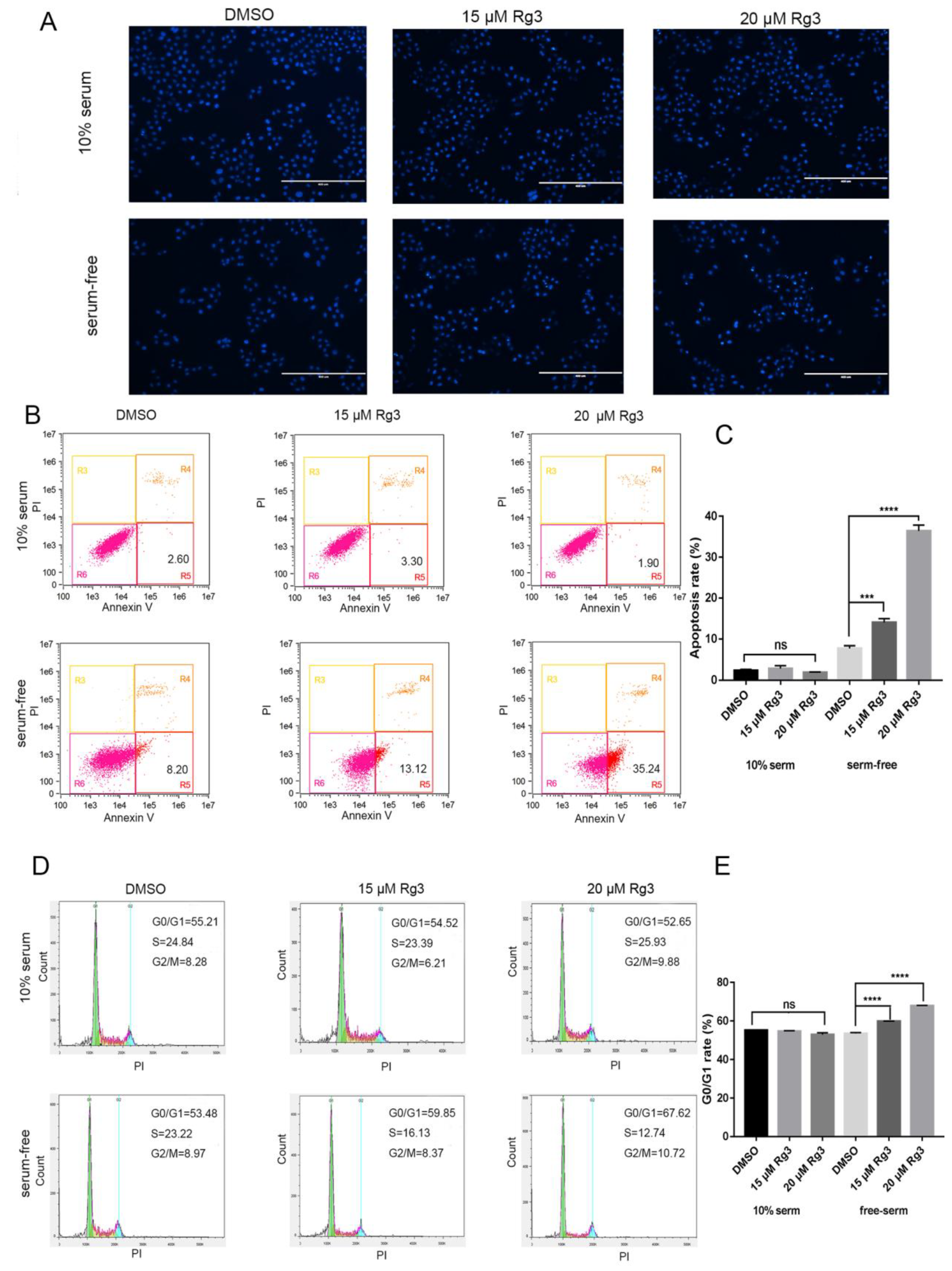
2.2. 20(S)-GRg3 Increases Apoptosis and Regulates the Cell Cycle
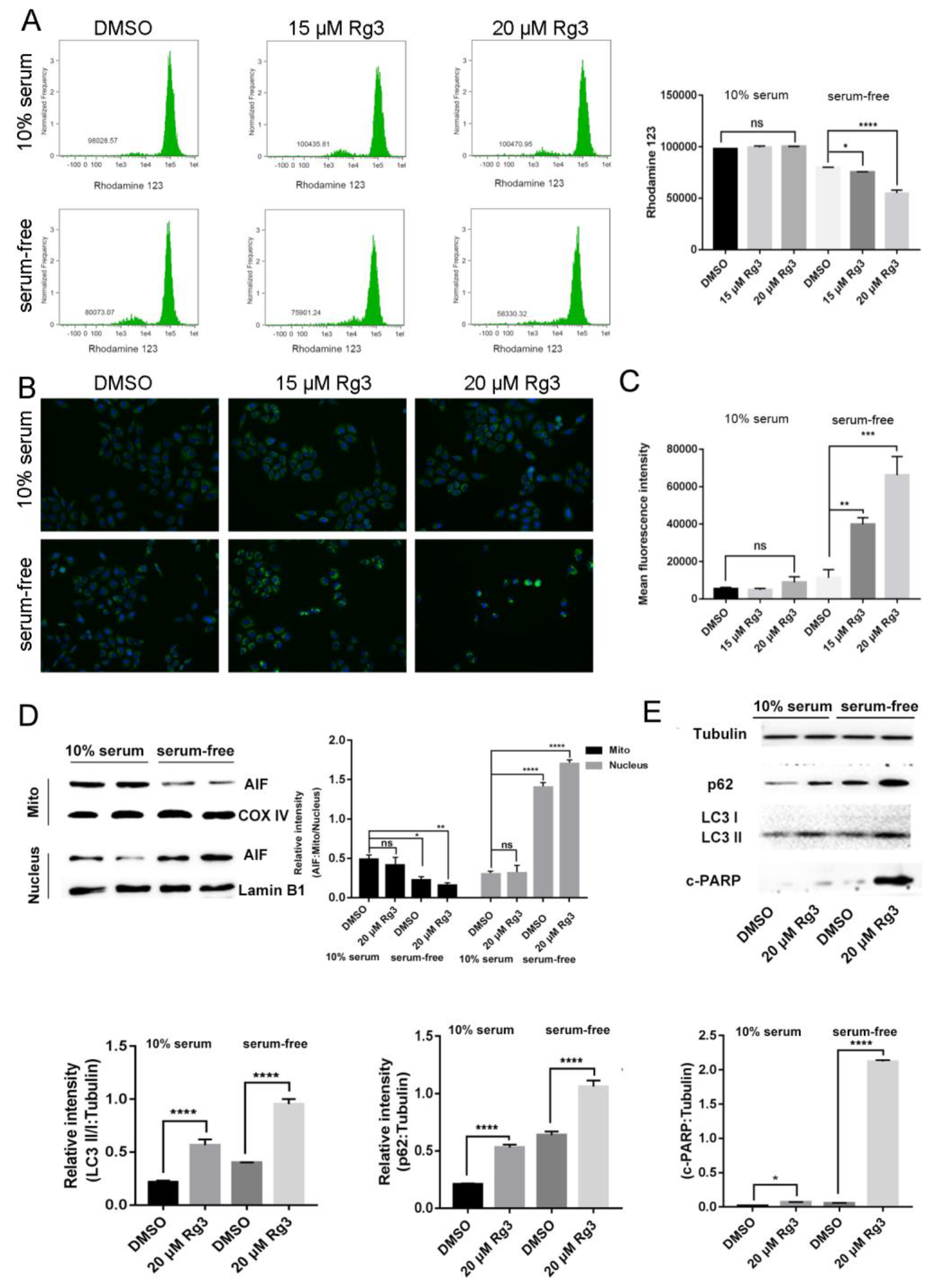
2.3. 20(S)-GRg3 Induces Apoptosis through the Mitochondrion-AIF Pathway
2.4. 20(S)-GRg3 Represses Starvation-Induced Autophagic Flux
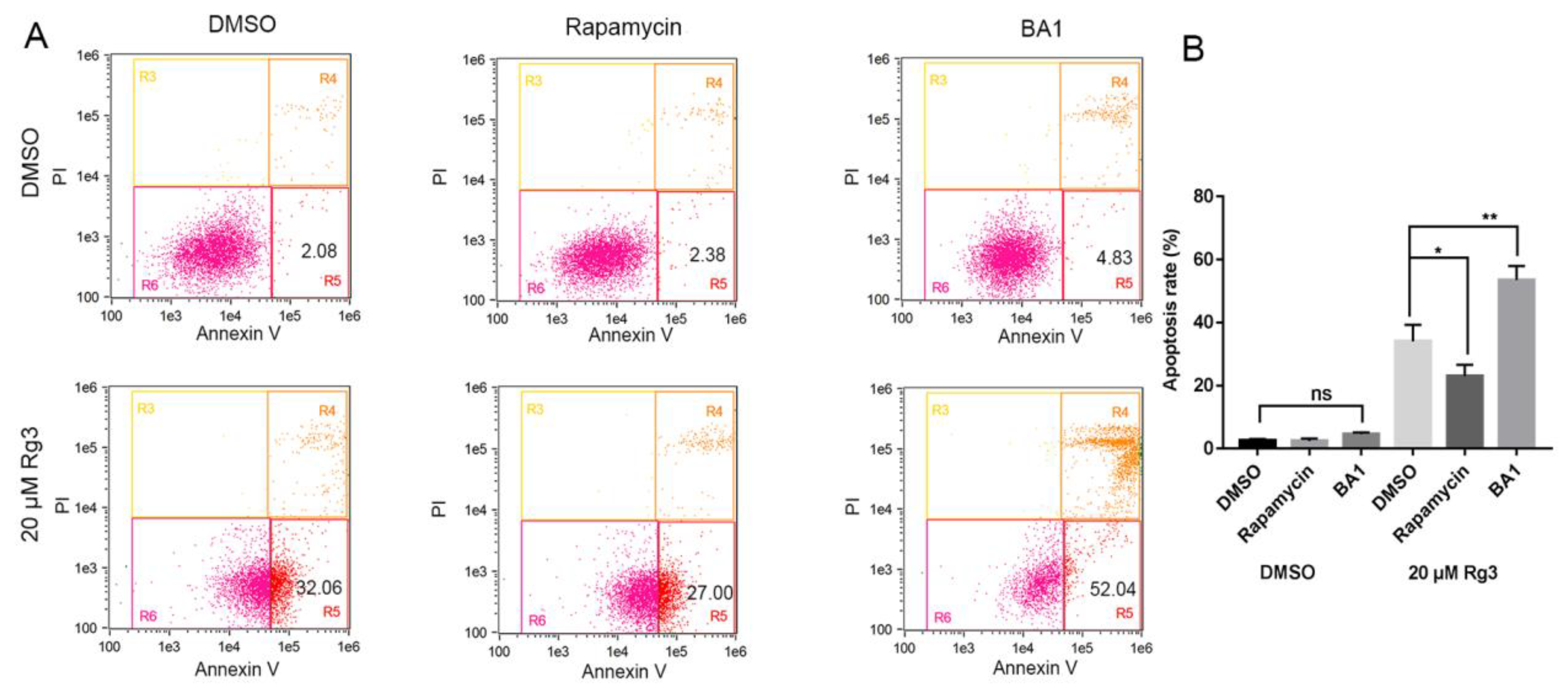
2.5. Apoptosis Induced by 20(S)-GRg3 is Associated with Autophagy
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Cell Culture and Transfection
4.3. Hoechst 33342 Staining Assay
4.4. CCK-8 Assay
4.5. Flow Cytometric Analysis of Apoptosis
4.6. ROS Determination
4.7. Rhodamine 123 Assay for Measurement of Mitochondrial Membrane Potentials
4.8. Acidic Vesicular Organelle Staining
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pandey, R.A.; Karmacharya, E. Cervical cancer screening behavior and associated factors among women of Ugrachandi Nala, Kavre, Nepal. Eur. J. Med Res. 2017, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Vora, C.; Gupta, S. Targeted therapy in cervical cancer. ESMO Open 2018, 3, e000462. [Google Scholar] [CrossRef] [PubMed]
- Mesafint, Z.; Berhane, Y.; Desalegn, D. Health Seeking Behavior of Patients Diagnosed with Cervical Cancer in Addis Ababa, Ethiopia. Ethiop. J. Heal. Sci. 2018, 28, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Tsikouras, P.; Zervoudis, S.; Manav, B.; Tomara, E.; Iatrakis, G.; Romanidis, C.; Bothou, A.; Galazios, G. Cervical cancer: Screening, diagnosis and staging. J. B.U.ON. Off. J. Balk. Union Oncol. 2016, 21, 320–325. [Google Scholar]
- Liang, L.D.; He, T.; Du, T.W.; Fan, Y.G.; Chen, D.S.; Wang, Y. Ginsenoside-Rg5 induces apoptosis and DNA damage in human cervical cancer cells. Mol. Med. Rep. 2015, 11, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Gkretsi, V.; Stylianopoulos, T. Cell Adhesion and Matrix Stiffness: Coordinating Cancer Cell Invasion and Metastasis. Front. Oncol. 2018, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lo, H.L.; Tang, W.C.; Hsiao, H.H.; Yang, P.M. A gene expression signature-based approach reveals the mechanisms of action of the Chinese herbal medicine berberine. Sci. Rep. 2014, 4, 6394. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jeon, J.N.; Jang, M.G.; Oh, J.Y.; Kwon, W.S.; Jung, S.K.; Yang, D.C. Ginsenoside profiles and related gene expression during foliation in Panax ginseng Meyer. J. Ginseng Res. 2014, 38, 66–72. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nature Reviews Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef]
- Son, K.J.; Choi, K.R.; Lee, S.J.; Lee, H. Immunogenic Cell Death Induced by Ginsenoside Rg3: Significance in Dendritic Cell-based Anti-tumor Immunotherapy. Immune Netw. 2016, 16, 75–84. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, X.; Lu, J.; Chen, W.; Li, X.; Zhao, L. Ginsenoside 20(S)-Rg3 Inhibits the Warburg Effect Via Modulating DNMT3A/ MiR-532-3p/HK2 Pathway in Ovarian Cancer Cells. Cell. Physiol. Biochem. 2018, 45, 2548–2559. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Wang, Y.; Li, P.; Liu, J.; Wei, A.; Wang, H.; Meng, X.; Pan, D.; Zhang, X. Effects of R type and S type ginsenoside Rg3 on DNA methylation in human hepatocarcinoma cells. Mol. Med. Rep. 2017, 15, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, J.; Bai, F.; Xiao, Y.; Guo, Y.; Dong, Z. Ginsenoside Rg3 Suppresses Proliferation and Induces Apoptosis in Human Osteosarcoma. BioMed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed]
- White, E.; Mehnert, J.M.; Chan, C.S. Autophagy, metabolism, and cancer. Clin. Cancer Res. 2015, 21, 5037–5046. [Google Scholar] [CrossRef]
- Xia, T.; Wang, J.; Wang, Y.; Wang, Y.; Cai, J.; Wang, M.; Chen, Q.; Song, J.; Yu, Z.; Huang, W.; et al. Inhibition of autophagy potentiates anticancer property of 20(S)-ginsenoside Rh2 by promoting mitochondria-dependent apoptosis in human acute lymphoblastic leukaemia cells. Oncotarget 2016, 7, 27336–27349. [Google Scholar] [CrossRef]
- Chen, L.; Meng, Y.; Sun, Q.; Zhang, Z.; Guo, X.; Sheng, X.; Tai, G.; Cheng, H.; Zhou, Y. Ginsenoside compound K sensitizes human colon cancer cells to TRAIL-induced apoptosis via autophagy-dependent and -independent DR5 upregulation. Cell Death Dis. 2016, 7, e2334. [Google Scholar] [CrossRef]
- Moon, J.H.; Lee, J.H.; Lee, Y.J.; Park, S.Y. Autophagy flux induced by ginsenoside-Rg3 attenuates human prion protein-mediated neurotoxicity and mitochondrial dysfunction. Oncotarget 2016, 7, 85697–85708. [Google Scholar] [CrossRef][Green Version]
- Wu, Y.-T.; Tan, H.-L.; Huang, Q.; Kim, Y.-S.; Pan, N.; Ong, W.-Y.; Liu, Z.-G.; Ong, C.-N.; Shen, H.-M. Autophagy plays a protective role during zVAD-induced necrotic cell death. Autophagy 2008, 4, 457–466. [Google Scholar] [CrossRef]
- Mizushima, N. Methods for monitoring autophagy. Int. J. Biochem. Cell. Biol. 2004, 36, 2491–2502. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T. How to interpret LC3immunoblotting. Autophagy 2007, 3, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abeliovich, H.; Agostinis, P.; Agrawal, D.K.; Aliev, G.; Askew, D.S.; Baba, M.; Baehrecke, E.H.; Bahr, B.A.; Ballabio, A. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008, 4, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.-A.; Outzen, H.; Overvatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 Binds Directly to Atg8/LC3 to Facilitate Degradation of Ubiquitinated Protein Aggregates by Autophagy. J. Boil. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [PubMed]
- Bjørkøy, G.; Lamark, T.; Brech, A.; Outzen, H.; Perander, M.; Øvervatn, A.; Stenmark, H.; Johansen, T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Boil. 2005, 171, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Im, K.; Kim, J.; Min, H. Ginseng, the natural effectual antiviral: Protective effects of Korean Red Ginseng against viral infection. J. Ginseng Res. 2016, 40, 309–314. [Google Scholar] [CrossRef]
- Kim, Y.R.; Yang, C.S. Protective roles of ginseng against bacterial infection. Microb. Cell 2018, 5, 472–481. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Wachtel-Galor, S. Herbal Medicine: Biomolecular and Clinical Aspects; CRC press: Boca Raton, FL, USA, 2011; pp. 1–11. [Google Scholar]
- Lee, J.S.; Ko, E.-J.; Hwang, H.S.; Lee, Y.-N.; Kwon, Y.-M.; Kim, M.-C.; Kang, S.-M. Antiviral activity of ginseng extract against respiratory syncytial virus infection. Int. J. Mol. Med. 2014, 34, 183–190. [Google Scholar] [CrossRef]
- Radad, K.; Gille, G.; Liu, L.; Rausch, W.-D. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J. Pharmacol. Sci. 2006, 100, 175–186. [Google Scholar] [CrossRef]
- Yoo, D.-G.; Kim, M.-C.; Park, M.-K.; Song, J.-M.; Quan, F.-S.; Park, K.-M.; Cho, Y.-K.; Kang, S.-M. Protective Effect of Korean Red Ginseng Extract on the Infections by H1N1 and H3N2 Influenza Viruses in Mice. J. Med. Food 2012, 15, 855–862. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Cho, J. Korean Red Ginseng Extract Exhibits Neuroprotective Effects through Inhibition of Apoptotic Cell Death. Boil. Pharm. Bull. 2014, 37, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wide-ranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kim, B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Panigrahi, D.P.; Patil, S.; Bhutia, S.K. Autophagy in health and disease: A comprehensive review. Biomed. Pharmacother. 2018, 104, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Jung, K.H.; Lee, D.G.; Yoon, J.H.; Choi, K.S.; Kwon, S.W.; Shen, H.M.; Morgan, M.J.; Hong, S.S.; Kim, Y.S. 20(S)-Ginsenoside Rg(3) is a novel inhibitor of autophagy and sensitizes hepatocellular carcinoma to doxorubicin. Oncotarget 2014, 5, 4438–4451. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.W.; Chen, X.M.; Chen, X.H.; Zheng, S.S. Ginsenoside Rg3 inhibit hepatocellular carcinoma growth via intrinsic apoptotic pathway. World J. Gastroenterol. 2011, 17, 3605–3613. [Google Scholar] [CrossRef]
- Kumar, L.; Harish, P.; Malik, P.S.; Khurana, S. Chemotherapy and targeted therapy in the management of cervical cancer. Curr. Probl. Cancer 2018, 42, 120–128. [Google Scholar] [CrossRef]
- Sun, M.; Ye, Y.; Xiao, L.; Duan, X.; Zhang, Y.; Zhang, H. Anticancer effects of ginsenoside Rg3 (Review). Int. J. Mol. Med. 2017, 39, 507–518. [Google Scholar] [CrossRef]
- Wang, S.-G.; Mu, N.; Sun, H.-Y. Effect of Interventional Therapy on the Expression of Survivin mRNA in Cervical Cancer. Anticancer. Res. 2017, 37, 4707–4710. [Google Scholar]
Sample Availability: Samples of the compounds 20(S)-ginsenoside Rg3 are available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, S.; Zhao, Y.; Li, F.; Lu, S.; Wang, S.; Bai, X.; Liu, M.; Zhao, D.; Wang, J.; Guo, D. 20(S)-Ginsenoside Rg3 Promotes HeLa Cell Apoptosis by Regulating Autophagy. Molecules 2019, 24, 3655. https://doi.org/10.3390/molecules24203655
Bian S, Zhao Y, Li F, Lu S, Wang S, Bai X, Liu M, Zhao D, Wang J, Guo D. 20(S)-Ginsenoside Rg3 Promotes HeLa Cell Apoptosis by Regulating Autophagy. Molecules. 2019; 24(20):3655. https://doi.org/10.3390/molecules24203655
Chicago/Turabian StyleBian, Shuai, Yue Zhao, Fangyu Li, Shuyan Lu, Siming Wang, Xueyuan Bai, Meichen Liu, Daqing Zhao, Jiawen Wang, and Dean Guo. 2019. "20(S)-Ginsenoside Rg3 Promotes HeLa Cell Apoptosis by Regulating Autophagy" Molecules 24, no. 20: 3655. https://doi.org/10.3390/molecules24203655
APA StyleBian, S., Zhao, Y., Li, F., Lu, S., Wang, S., Bai, X., Liu, M., Zhao, D., Wang, J., & Guo, D. (2019). 20(S)-Ginsenoside Rg3 Promotes HeLa Cell Apoptosis by Regulating Autophagy. Molecules, 24(20), 3655. https://doi.org/10.3390/molecules24203655




