Abstract
This is the first report of a natural ligand improving the copper-catalyzed homocouplings of (hetero)arylboronic acids. Various important synthetic biaryl intermediates in organic synthesis could be assembled via this method. To gain insight into this reaction, in situ React IR technology was used to confirm the effectivity of this catalyst system. This protocol could provide important biaryl compounds in high yields within a short time.
1. Introduction
Symmetrical biaryls are ubiquitous reagents in organic synthesis and play a significant role in the pharmaceutical and chemical industries because of their unique bioactivities and chemical properties. As depicted in Figure 1, some natural symmetrical biaryl scaffolds have been discovered to display diverse biological activities, such as the antimicrobial agent Honokiol [1] and the hepatoprotective agent α-DDB [2]. The synthesis of symmetrical biaryl scaffolds mainly includes the use of metal-catalyzed homocoupling of aryl halides [3,4], boronic acids [5], aryl Grignard reagents [6], 1,2-diarylditellanes [7], and arenediazonium salts [8]. A common method for the preparation of symmetrical biaryls depends on palladium catalysis in the presence of related ligands, bases, and solvents [9]. Valiente et al. reported the aerobic homocoupling of arylboronic acids catalyzed by Pd(II) complexes, but this method requires special Pd(II)@MOF species, which limits its large-scale use [10]. Demir et al. found that copper(II) acetate could mediate the homocoupling of arylboronic acids [11]. Yamamoto and coworkers reported that 1,10-phenanthroline could accelerated the copper-catalyzed homocouplings of arylboronic acids, but this method suffered from low yields for some electron-withdrawing functionalities and substrates [12]. Singh et al. reported the ruthenium-catalyzed homocoupling of arylboronic acid in water [13]. CuII–β-Cyclodextrin was discovered to catalyze the homocoupling and cross-coupling reactions of arylboronic acids [14], but the drawbacks of this method included a long reaction time and moderate yields.
Figure 1.
Chemical structures of some symmetrical biaryls and 2-OMG.
2-O-Methyl-d-glucopyranose (2-OMG), a glucose analogue, is common in plants and contains multiple chirality centers; it was first reported as a promoter for copper catalyst. Herein we report on the development of conditions for the homocoupling of (hetero)arylboronic acids using a cheap and readily available copper catalyst with the assistance of 2-OMG. These reactions occurred at a mild temperature (40 °C), employing substituted arylboronic acids and inexpensive Cu(II) acetate, with high yields. This wide scope allows for the preparation of many symmetrical biaryls. To our delight, some substituted arylboronic acids also have good tolerance and moderate to high yields, and some coupling results could be preliminarily summarized that were advantageous for the preparation of symmetrical biaryls.
2. Results and Discussion
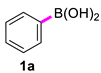
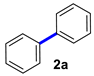
Our investigations began with the homocoupling of phenylboronic acid in the presence of copper salts, using 2-OMG as a ligand (Table 1). A screen of copper salts revealed that no biphenyl was observed when the reaction was conducted at 40 °C for 1 h with CuI, CuO, or powdered Cu as the catalyst (entries 1–3). However, CuSO4 slightly promoted the homocoupling reaction, and a lower yield of homocoupling products was observed 10% (entry 4). Replacing CuSO4 with Cu(OAc)2 offered an opportunity to implement a synthesis of symmetrical biaryls such as biphenyl directly from phenylboronic acid. Attempts to optimize the reaction through modification of the base proved unsuccessful, and we did not find any effects of bases on the reaction in terms of yield (entries 5–7). Practically, the coupling reaction could process smoothly without any assistance of bases, yielding biphenyl at 99% (entry 8). Different solvents including DMF, dioxane, H2O, and methanol were tested, with DMF being the most effective in the screening reaction (entries 9–11). Notably, the use of H2O was detrimental, as an unexpected byproduct phenol was obtained. Lowering the temperature to room temperature prolonged the reaction time without impacting the yield (entry 12). Control experiments (entry 13) confirmed that 2-OMG could indeed promote the Cu(OAc)2-catalyzed coupling reaction, forming biphenyl at a high yield within a short time at room temperature. However, under basic conditions in the absence of 2-OMG, the desired product was obtained at a 58% yield. The loading catalyst investigation showed that the 5% loading catalyst needed longer reaction time, but did not impact the yield compared to a 10% loading catalyst (entry 14). Further increasing it to a 15% loading did not significantly accelerate the reaction (entry 15). The scalability of this protocol was tested through the synthesis of biphenyl 2a on a 10-fold scale. In this case, the catalyst loading was lowered to 5 mol % and the desired biphenyl was obtained at 98% isolated yield (entry 16).

Table 1.
Identification of the reaction conditions a.
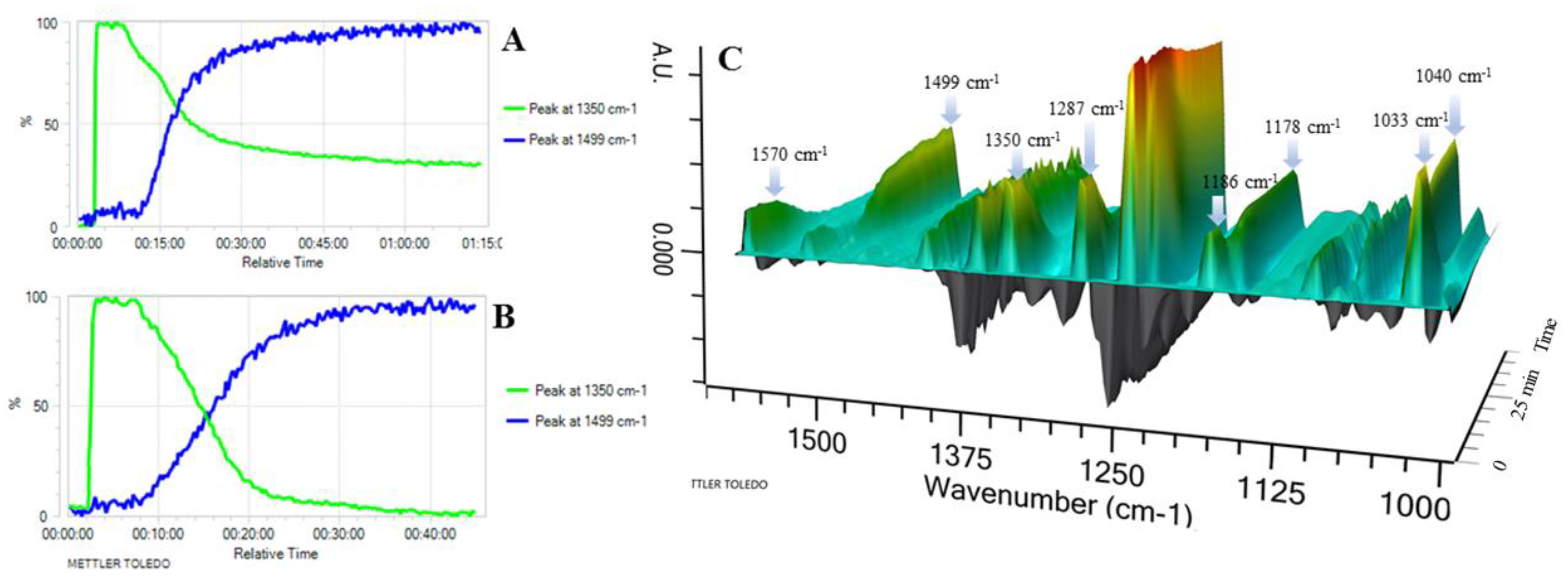
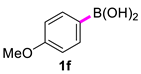
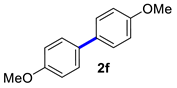
In situ React IR technology was employed to monitor the conversion of 4-methoxyphenylboronic acid (1f) to the corresponding 4,4′-dimethoxy-1,1′-bipheny (2f). As seen in Figure 2 (A and B: 2D trends; C: 3D surface), less than 5 min after the addition of 2-OMG and Cu(OAc)2 to the 4-methoxyphenylboronic acid start heating, some new, sharp peaks appeared at 1040, 1178, and 1499 cm−1, indicating the appearance of the product. The intensity of these peaks gradually increased as the reaction temperature was raised to 40 °C. Importantly, the disappearance of the 1033, 1186, 1287, 1350, and 1570 cm−1 bands was marked by the appearance of three bands at 1723, 1622, and 1611 cm−1, corresponding to 4,4′-dimethoxy-1,1′-bipheny (Figure 2C). The conversion of 4-methoxyphenylboronic acid reached 50% after only 15 min, and 100% after 40 min (Figure 2B). In contrast, a control experiment (without 2-OMG) showed that the conversion of 4-methoxyphenylboronic acid reached only 65% after 75 min; complete conversion required higher temperatures and a longer time (Figure 2A). Thus, it was concluded that the conversion of 4-methoxyphenylboronic acid to the corresponding 4,4′-dimethoxy-1,1′-bipheny underwent quick coupling to produce the target compound.
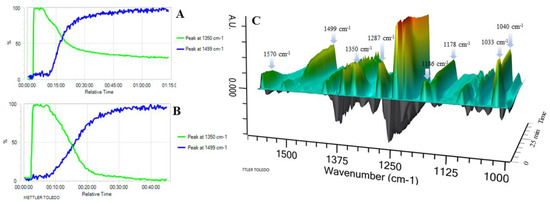
Figure 2.
2D trends and 3D surface of ReactIR experiment. (A) Reaction conducted without using 2-OMG; (B) reaction conducted with the use of 2-OMG. Y axis indicates the intensity of the signal; (C) 3D surface figure was obtained with the use of 2-OMG.
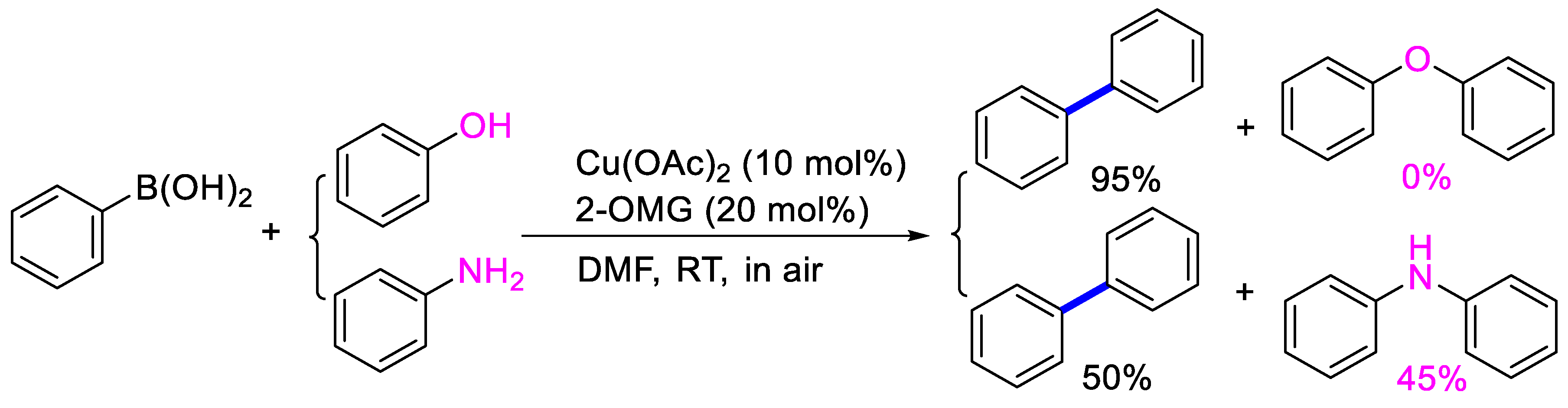
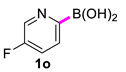
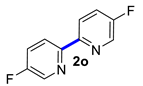
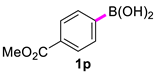
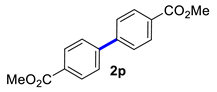
In the optimized conditions, the scope of the (hetero)arylboronic acids was investigated (Table 2). A range of functional groups on the substituted (hetero)arylboronic acids proved to be compatible, including phenyl, methyl, methoxy, hydroxyl, and ester (1b−1i, 1p). The pyridine boronic acid and substituted pyridine boronic acid could also be applied in the reaction (1m−1o). Substrates bearing either electron-withdrawing or electron-donating groups on the aromatic ring cross-coupled with universally high yields; however, substrates bearing electron-donating groups generally required a longer reaction time. The introduction of a second methoxy group at the aromatic ring was also tolerated, while the reaction stopped at 80% conversion, presumably due to the strong electron-donating effect of 1g. The presence of an ortho substituent on the aromatic ring decreased the yield of the homocoupling product (1d, 1h). Fluorine substituents were well tolerated, and excellent results were observed (1j–1l, 1n–1o). The cross-coupling reactions of phenylboronic acids with phenols for the synthesis of biaryl ethers failed to react (Scheme 1), proving that the hydroxyl group was broadly tolerant to the homocoupling (1h, 1i). However, a limitation of the reaction was that amino-substituted (hetero)arylboronic acids led to a complex reaction due to the production of biaryls and diphenylamines (Scheme 1).

Table 2.
Scope with respect to (hetero)arylboronic acids a.
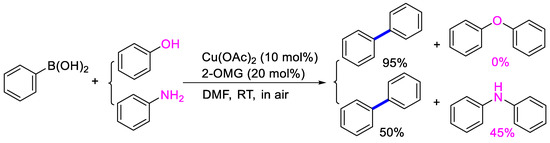
Scheme 1.
Attempted cross-coupling of phenol or aniline with phenylboronic acid.
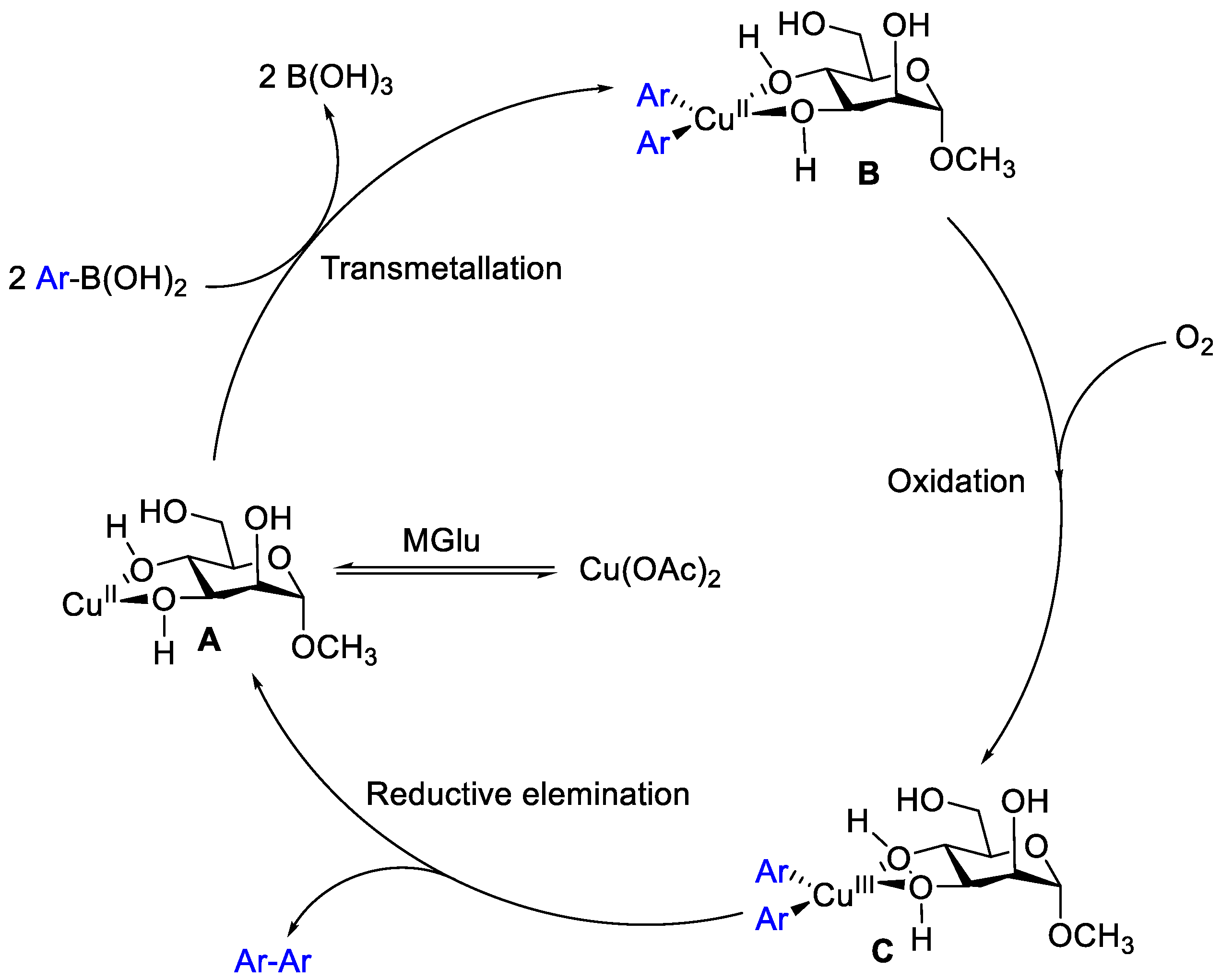
The mechanism of the homocoupling was analogous to the catalytic cycle with previous report [11,15], a plausible reaction pathway was proposed as shown in Scheme 2. The reaction was initiated by the coordination of the oxygen atoms from MGlu to the copper center, forming a catalytically active complex. Then, double transmetallation of CuII with two molecules of (hetero)arylboronic acids afforded Ar–Cu(II)–Ar, which undergoes oxidation by air to yield a CuIII intermediate, the reductive elimination of which released the homocoupled product Ar–Ar.
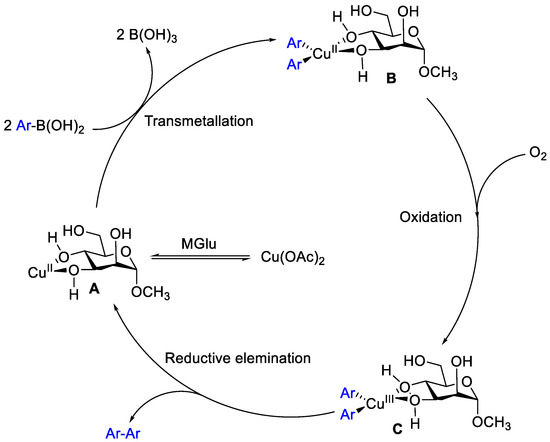
Scheme 2.
Plausible mechanism for the homocoupling of arylboronic acid.
3. Materials and Methods
All of the starting materials, reagents, and solvents are commercially available and were used without further purification. Melting points were determined with an X-4 apparatus and were uncorrected (Beijing Taike Instrument Co., Ltd, Beijing, China). The nuclear magnetic resonance (NMR) spectra were recorded on a Bruker 400 MHz spectrometer (Bruker Technology Co., Ltd., Karlsruhe, Germany) in CDCl3 or DMSO-d6 using tetramethylsilane (TMS) as an internal standard. Electrospray ionization mass spectrometry (MS (ESI)) analyses were recorded in an Agilent 1100 Series MSD Trap SL (Santa Clara, CA, USA). The reactions were monitored by thin-layer chromatography (TLC: HG/T2354-92, GF254), and compounds were visualized with UV light.
3.1. React IR Experiment
React IR experiments were conducted using a Mettler Toledo ReactIR 15 with MCT detector (Mettler Toledo International Trading Co., Ltd., Zurich, Switzerland) using HappGenzel; DiComp (Diamond) probe connected via AgX × 9.5 mm × 1.5 m fiber (silver halide). iC IR 4.3 reaction analysis software (Mettler Toledo International Trading Co., Ltd., Zurich, Switzerland.) was used during data collection and analysis.
To a 50 mL, three-neck flask equipped with a magnetic stirrer was inserted the ReactIR 15 DiComp probe. IR spectra were obtained every 15 s. Data collection began at the start of the experiment. To a solution of 4-methoxyphenylboronic acid (0.40 g, 2.63 mmol) in DMF (5 mL) and at room temperature was added 2-OMG (102 mg, 0.53 mmol) and Cu(OAc)2 (48 mg, 0.26 mmol). The resulting solution was gradually heated to 40 °C and stirred for 60 min. The workup was consistent with the general procedure for catalytic experiments.
3.2. General Procedure for Catalytic Experiments
To a solution of (hetero)arylboronic acids (2.63 mmol), and 2-OMG (20 mol %) in DMF (5 mL) was added Cu(OAc)2 (10 mol %) in air. The mixture was heated to 40 °C with stirring for the indicated time. The reactor was cooled to room temperature, and the reaction mixture was poured into water (20 mL), extracted with ethyl acetate (20 mL × 3), and the organic layer washed with water (20 mL × 2) and once with brine (25 mL), dried over magnesium sulfate, and concentrated in vacuo. The product was purified by flash column chromatography on silica gel using petroleum ether as the eluent.
Biphenyl (2a) [16]: white solid (397 mg, 98%), m.p. 69–70 °C. 1H NMR (400 MHz, CDCl3) δ: 7.60 (t, J = 1.2 Hz, 2H), 7.44 (t, J = 7.2 Hz, 2H), 7.34 (t, J = 7.4 Hz, 1H). 13C NMR (150 MHz, CDCl3) δ: 141.2, 128.7, 127.2, 127.1.
4-Quaterphenyl (2b) [17]: white solid (780 mg, 97%), m.p. > 300 °C. 1H NMR (400 MHz, CDCl3) δ: 7.77–7.37 (m, 18H). 13C NMR (150 MHz, CDCl3) δ: 140.7, 140.3, 139.6, 128.8, 127.6, 127.5, 127.1.
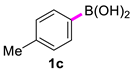
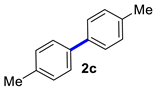
4,4′-Dimethyl-1,1′-biphenyl (2c) [18]: white solid (450 mg, 94%), m.p. 121–123 °C. 1H NMR (600 MHz, CDCl3) δ: 7.45 (d, J = 7.7 Hz, 2H), 7.24 (d, J = 8.2 Hz, 2H), 2.38 (s, 6H). 13C NMR (150 MHz, CDCl3) δ: 138.3, 136.7, 129.4, 126.8, 21.1.
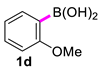
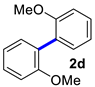
2,2′-Dimethoxy-1,1′-biphenyl (2d) [18]: white solid (473 mg, 84%). m.p. 178–179 °C. 1H NMR (400 MHz, CDCl3) δ: 7.35–7.31 (m, 2H), 7.26–7.02 (m, 2H), 7.00 (q, J = 7.4, 6.6 Hz, 4H), 3.77 (s, 6H). 13C NMR (150 MHz, CDCl3) δ: 157.0, 131.4, 128.6, 127.8, 120.3, 111.1, 55.7.
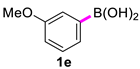
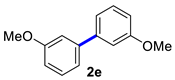
3,3′-Dimethoxy-1,1′-biphenyl (2e) [18]: colorless liquid (507 mg, 90%). 1H NMR (600 MHz, CDCl3) δ: 7.32 (t, J = 7.9 Hz, 2H), 7.17–7.15 (m, 2H), 7.11 (t, J = 2.2 Hz, 2H), 6.88–6.86 (m, 2H), 3.81 (s, 6H). 13C NMR (150 MHz, CDCl3) δ: 159.9, 142.6, 129.7, 119.7, 112.9, 112.8, 55.2.
4,4′-Dimethoxy-1,1′-biphenyl (2f) [18]: white solid (512 mg, 91%), m.p. 177–179 °C. 1H NMR (400 MHz, CDCl3) δ: 7.49–7.45 (m, 4H), 6.97–6.93 (m, 4H), 3.84 (s, 6H). 13C NMR (100 MHz, CDCl3) δ: 158.7, 133.5, 127.7, 114.2, 55.4.
3,3′,5,5′-Tetramethoxy-1,1’-biphenyl (2g) [11]: colorless liquid (641 mg, 89%). 1H NMR (400 MHz, CDCl3) δ: 7.18 (t, J = 8.3 Hz, 2H), 6.52 (d, J = 2.4 Hz, 2H), 6.50 (d, J = 2.4 Hz, 2H), 6.47 (t, J = 2.3 Hz, 2H), 3.79 (s, 12H). 13C NMR (100 MHz, CDCl3) δ: 160.9, 129.9, 106.2, 100.5, 55.3.
[1,1′-Biphenyl]-2,2′-diol (2h) [19]: white solid (445 mg, 91%), m.p. 108–109 °C. 1H NMR (400 MHz, CDCl3) δ: 7.38–7.33 (m, 2H), 7.30 (d, J = 1.4 Hz, 1H), 7.10–7.05 (q, J = 8.6, 1.0 Hz, 4H), 5.40 (br, 2H). 13C NMR (150 MHz, CDCl3) δ: 152.0, 130.6, 129.2, 122.9, 120.9, 115.9.
[1,1′-Biphenyl]-4,4′-diol (2i) [20]: white solid (455 mg, 93%), m.p. 229–230 °C. 1H NMR (400 MHz, DMSO-d6) δ: 9.38 (s, 2H), 7.37 (d, J = 8.5 Hz, 4H), 6.79 (d, J = 8.5 Hz, 4H). 13C NMR (150 MHz, DMSO-d6) δ: 155.7, 130.5, 126.3, 115.0.
4,4′-Difluoro-1,1′-biphenyl (2j) [18]: white solid (490 mg, 98%), m.p. 86–88 °C. 1H NMR (400 MHz, CDCl3) δ: 7.43–7.38 (m, 2H), 7.36–7.33 (m, 2H), 7.28–7.27 (m, 1H), 7.26–7.25 (m, 1H), 7.09–7.04 (m, 2H). 13C NMR (100 MHz, CDCl3) δ: 163.2 (J = 244.5 Hz), 142.2 (J = 7.6 Hz), 130.4 (J = 8.4 Hz), 122.7 (J = 2.8 Hz), 114.8, 114.6, 114.2, 113.9.
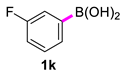
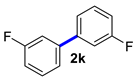
3,3′-Difluoro-1,1′-biphenyl (2k) [18]: colorless liquid (490, 98%). 1H NMR (400 MHz, CDCl3) δ: 7.50–7.47 (m, 4H), 7.14–7.09 (m, 2H). 13C NMR (100 MHz, CDCl3) δ: 162.4 (J = 244.9 Hz), 136.4 (J = 3.1 Hz), 128.6 (J = 8.4 Hz), 115.6 (J = 21.3 Hz).
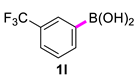
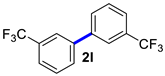
3,3′-Bis(trifluoromethyl)-1,1′-biphenyl (2l) [21]: white solid (755 mg, 99%). 1H NMR (400 MHz, CDCl3) δ: 7.83 (s, 2H), 7.77 (d, J = 7.6 Hz, 2H), 7.66 (d, J = 7.8 Hz, 2H), 7.60 (t, J = 7.7 Hz, 2H). 13C NMR (150 MHz, CDCl3) δ: 140.6, 131.3 (J = 64.3 Hz), 130.5, 129.5, 124.7 (J = 7.5 Hz), 124.0 (J = 270.0 Hz), 123.9 (J = 7.6 Hz).
2,2′-Bipyridine (2m) [18]: white solid (386 mg, 94%), m.p. 71 - 72 °C. 1H NMR (600 MHz, CDCl3) δ: 8.71 (d, J = 4.3 Hz, 2H), 8.44 (d, J = 7.9 Hz, 2H), 7.85 (t, J = 7.6 Hz, 2H), 7.34 (t, J = 5.4 Hz, 2H). 13C NMR (150 MHz, CDCl3) δ: 156.0, 149.1, 137.1, 123.8, 121.2.
6,6′-Difluoro-3,3′-bipyridine (2n): white solid (480 mg, 95%), m.p. 106–108 °C. 1H NMR (400 MHz, CDCl3) δ: 8.40 (d, J = 1.7 Hz, 2H), 7.98–7.93 (m, 2H), 7.07 (dd, J = 8.4, 3.0 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ: 163.6 (J = 239.6 Hz), 145.9 (J = 15.1 Hz), 139.7 (J = 8.1 Hz), 130.6 (J = 4.6 Hz), 110.2 (J = 4.6 Hz), 110.2, 109.8.
5,5′-Difluoro-2,2′-bipyridine (2o) [22]: white solid (470 mg, 93%), m.p. 151–152 °C. 1H NMR (600 MHz, CDCl3) δ: 8.50 (d, J = 2.3 Hz, 2H), 8.39 (dd, J = 8.8, 4.4 Hz, 2H), 7.54–7.51 (m, 2H). 13C NMR (150 MHz, CDCl3) δ: 160.7, 159.0, 151.4, 151.42, 137.3, 137.1, 123.9, 123.8, 122.3, 122.2.
Dimethyl [1,1′-biphenyl]-4,4′-dicarboxylate (2p) [23]: white solid (639 mg, 90%), m.p. 211–213 °C. 1H NMR (400 MHz, CDCl3) δ: 8.15 (d, J = 8.4 Hz, 4H), 7.72 (q, J = 8.4, 1.6Hz, 4H), 3.97 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ: 167.4, 143.7, 130.4, 129.1, 127.8, 52.7.
Copies of 1H NMR and 13C NMR are available in supplementary materials.
4. Conclusions
In summary, we have developed a catalyst system for the homocoupling of (hetero)arylboronic acids that utilizes readily available (hetero)arylboronic acids and inexpensive Cu(OAc)2 in the presence of 2-OMG, with a moderate to high yield. The natural ligand could indeed promote copper-catalyzed homocouplings through an in situ IR reactor. The naturally available ligand (2-OMG) contains chiral centers, which could pave the way for the enantioselective synthesis of many important optically active molecules. Efforts to understand the crucial factors responsible for the improved reactivity and to expand the scope of the homocoupling of (hetero)arylboronic acids to other classes of aromatic compounds are currently underway.
Supplementary Materials
The following are available online. Copies of 1H NMR and 13C NMR for all compounds were placed in supporting information.
Author Contributions
Conceptualization, C.Y.; Methodology, C.Y. and L.Z.; Software, C.Y. and L.Z.; Formal Analysis, Y.Z. and L.Z.; Writing—Original Draft Preparation, C.Y.; Writing—Review and Editing, C.Y. and Y.Z.; Project Administration, C.Y.
Funding
This research was funded by the Natural Science Foundation of Liaoning Province, grant number 2019-BS-102; and National Training Programs of Innovation and Entrepreneurship for Undergraduates of Liaoning Province, grant number 201910160017.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Agharahimi, M.R.; Lebel, N.A. Synthesis of (−)-monoterpenylmagnolol and magnolol. J. Org. Chem. 1995, 60, 1856–1863. [Google Scholar] [CrossRef]
- Wu, G.; Guo, H.; Gao, K.; Liu, Y.; Bastow, K.F.; Morris-Natschke, S.L.; Lee, K.H.; Xie, L. Synthesis of unsymmetrical biphenyls as potent cytotoxic agents. Bioorg. Med. Chem. Lett. 2008, 18, 5272–5276. [Google Scholar] [CrossRef]
- Ullmann, F. Ueber eine neue Darstellungsweise von Phenyläthersalicylsäure. Ber. Dtsch. Chem. Ges. 1904, 37, 853–857. [Google Scholar] [CrossRef]
- Miyake, Y.; Wu, M.; Rahman, M.J.; Kuwatani, Y.; Iyoda, M. Efficient construction of biaryls and macrocyclic cyclophanes via electron-transfer oxidation of lipshutz cuprates. J. Org. Chem. 2006, 71, 6110–6117. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cheng, D.; Pei, W. Iron-catalyzed homocoupling of bromide compounds. J. Org. Chem. 2006, 71, 6637–6639. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Mao, J.; Zhang, Y. Pd (OAc) 2-catalyzed room temperature homocoupling reaction of arylboronic acids under air without ligand. Catal. Commun. 2008, 9, 97–100. [Google Scholar] [CrossRef]
- Cahiez, G.; Moyeux, A.; Buendia, J.; Duplais, C. Manganese- or iron-catalyzed homocoupling of grignard reagents using atmospheric oxygen as an oxidant. J. Am. Chem. Soc. 2007, 129, 13788–13789. [Google Scholar] [CrossRef]
- Robinson, M.K.; Kochurina, V.S.; Hanna, J.M. Palladium-catalyzed homocoupling of arenediazonium salts: An operationally simple synthesis of symmetrical biaryls. Tetrahedron Lett. 2007, 48, 7687–7690. [Google Scholar] [CrossRef]
- Ostrowska, S.; Rogalski, S.; Lorkowski, J.; Walkowiak, J.; Pietraszuk, C. Efficient homocoupling of aryl- and alkenylboronic acids in the presence of low loadings of [{Pd (μ-OH) Cl (IPr)} 2]. Synlett 2018, 29, 1735–1740. [Google Scholar]
- Valiente, A.; Carrasco, S.; Sanz-Marco, A.; Tai, C.W. Aerobic homocoupling of arylboronic acids catalyzed by regenerable Pd (II) @MIL-88B-NH 2 (Cr). ChemCatChem 2019, 11, 3933–3940. [Google Scholar] [CrossRef]
- Demir, A.S.; Reis, O.; Emrullahoglu, M. Role of copper species in the oxidative dimerization of arylboronic acids: Synthesis of symmetrical biaryls. J. Org. Chem. 2003, 68, 10130–10134. [Google Scholar] [CrossRef] [PubMed]
- Kirai, N.; Yamamoto, Y. Homocoupling of arylboronic acids catalyzed by 1, 10-phenanthroline-ligated copper complexes in air. Eur. J. Org. Chem. 2009, 12, 1864–1867. [Google Scholar] [CrossRef]
- Tyagi, D.; Binnani, C.; Rai, R.K.; Dwivedi, A.D.; Gupta, K.; Li, P.; Zhao, Y.; Singh, S.K. Ruthenium-catalyzed oxidative homocoupling of arylboronic acids in water: Ligand tuned reactivity and mechanistic study. Inorg. Chem. 2016, 55, 6332–6343. [Google Scholar] [CrossRef] [PubMed]
- Kaboudin, B.; Abedi, Y.; Yokomatsu, T. Cu-II–β-cyclodextrin complex as a nanocatalyst for the homo- and crosscoupling of arylboronic acids under ligand-and base-free conditions in air: Chemoselective cross-coupling of arylboronic acids in water. Eur. J. Org. Chem. 2011, 2011, 6656–6662. [Google Scholar] [CrossRef]
- Kaboudin, B.; Mostafalu, R.; Yokomatsu, T. Fe3O4 nanoparticle-supported Cu(II)-β-cyclodextrin complex as a magnetically recoverable and reusable catalyst for the synthesis of symmetrical biaryls and 1,2,3-triazoles from aryl boronic acids. Green Chem. 2013, 15, 2266–2274. [Google Scholar] [CrossRef]
- Modak, A.; Mondal, J.; Sasidharan, M.; Bhaumik, A. Triazine functionalized ordered mesoporous polymer: A novel solid support for Pd-mediated C-C cross-coupling reactions in water. Green Chem. 2011, 13, 1317–1331. [Google Scholar] [CrossRef]
- Cheng, J.W.; Luo, F.T. Coupling of aryl Grignard reagents by electron transfer to 2,3-dichloropropene. Tetrahedron Lett. 1988, 29, 1293–1294. [Google Scholar] [CrossRef]
- Zeng, M.; Du, Y.; Qi, C.; Zuo, S.; Li, X.; Shao, L.; Zhang, X. An efficient and recyclable heterogeneous palladiumcatalyst utilizing naturally abundant pearl shell waste. Green Chem. 2011, 13, 350–356. [Google Scholar] [CrossRef]
- Endo, Y.; Shudo, K.; Okamoto, T. Acid-catalyzed solvolysis of N-sulfonyl- and N-acyl-O-arylhydroxylamines. Phenoxenium ions. J. Am. Chem. Soc. 1982, 104, 6393–6397. [Google Scholar] [CrossRef]
- Allen, C.F.H.; Burnss, D.M. The chemistry of o-terphenyl. III. Sulfonic acids. J. Org. Chem. 1949, 14, 163–169. [Google Scholar] [CrossRef]
- Gilman, H.; Ingham, R.K. Some tetraorganosilanes. J. Am. Chem. Soc. 1955, 77, 1680–1681. [Google Scholar] [CrossRef]
- Lee, K.; Lee, P.H. Efficient homo-coupling reactions of heterocyclic aromatic bromides catalyzed by Pd (OAc) 2 using indium. Tetrahedron Lett. 2008, 49, 4302–4305. [Google Scholar] [CrossRef]
- Leowanawat, P.; Zhang, N.; Resmerita, A.M.; Rosen, B.M.; Percec, V. Ni(COD)2/PCy3 catalyzed cross-coupling of aryl and heteroaryl neopentylglycolboronates with aryl and heteroaryl mesylates and sulfamates in THF at room temperature. J. Org. Chem. 2011, 76, 9946–9955. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).