Quality Evaluation of Mahonia bealei (Fort.) Carr. Using Supercritical Fluid Chromatography with Chemical Pattern Recognition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Chromatographic Conditions
2.2. Repeatability and Stability Evaluation
2.3. SFC Fingerprint Analysis and Similarity Evaluation
2.4. CPR Analysis
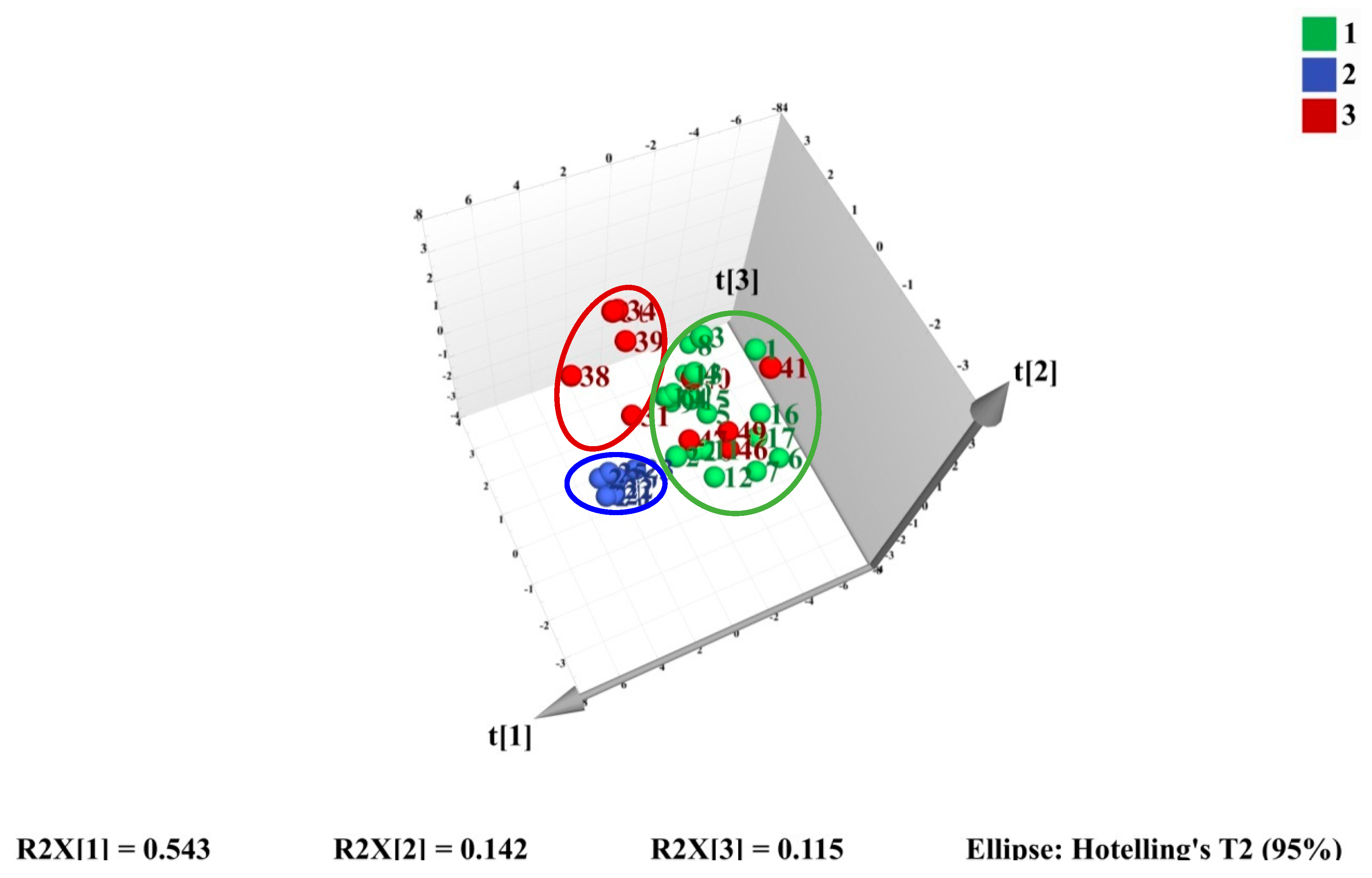
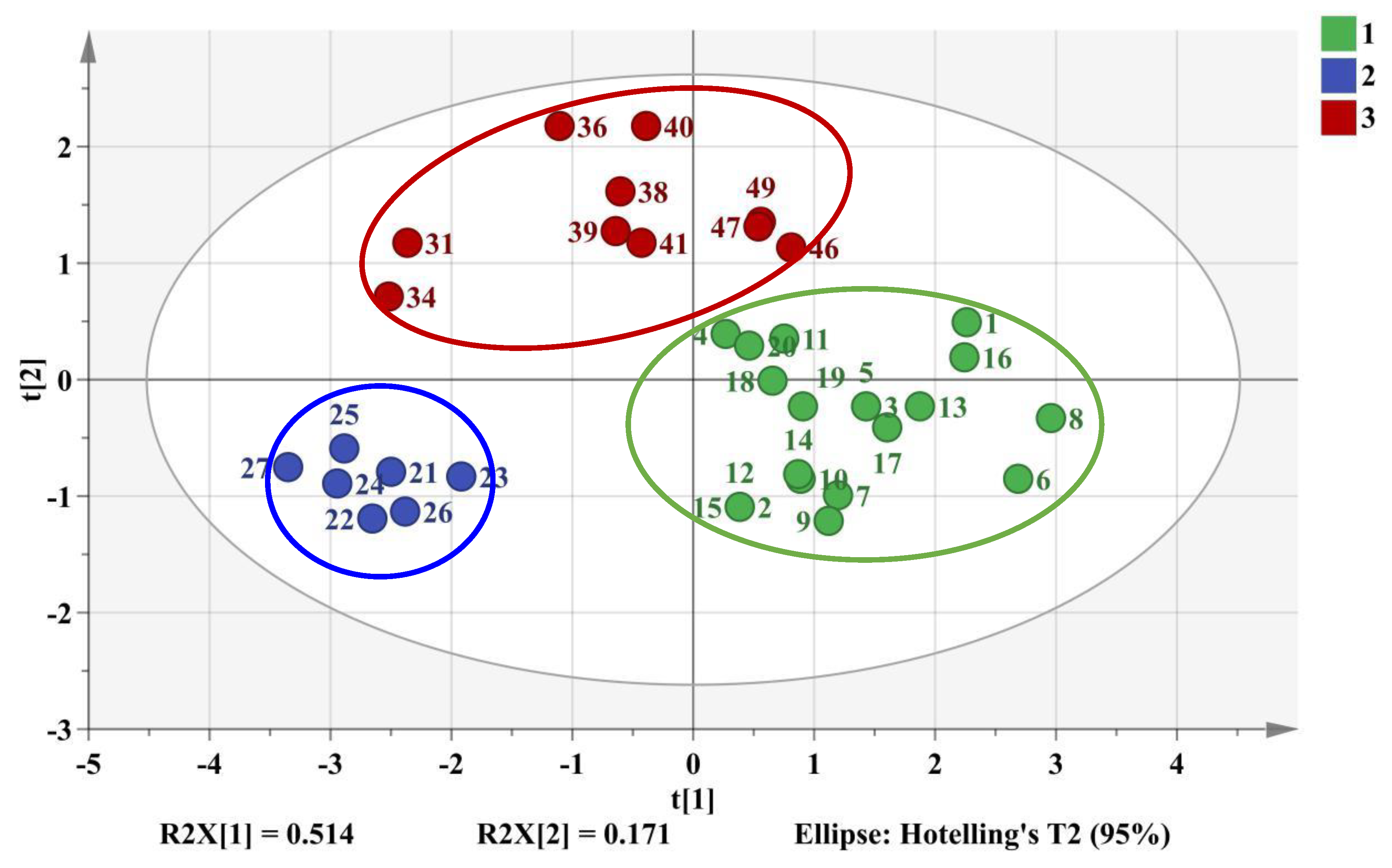
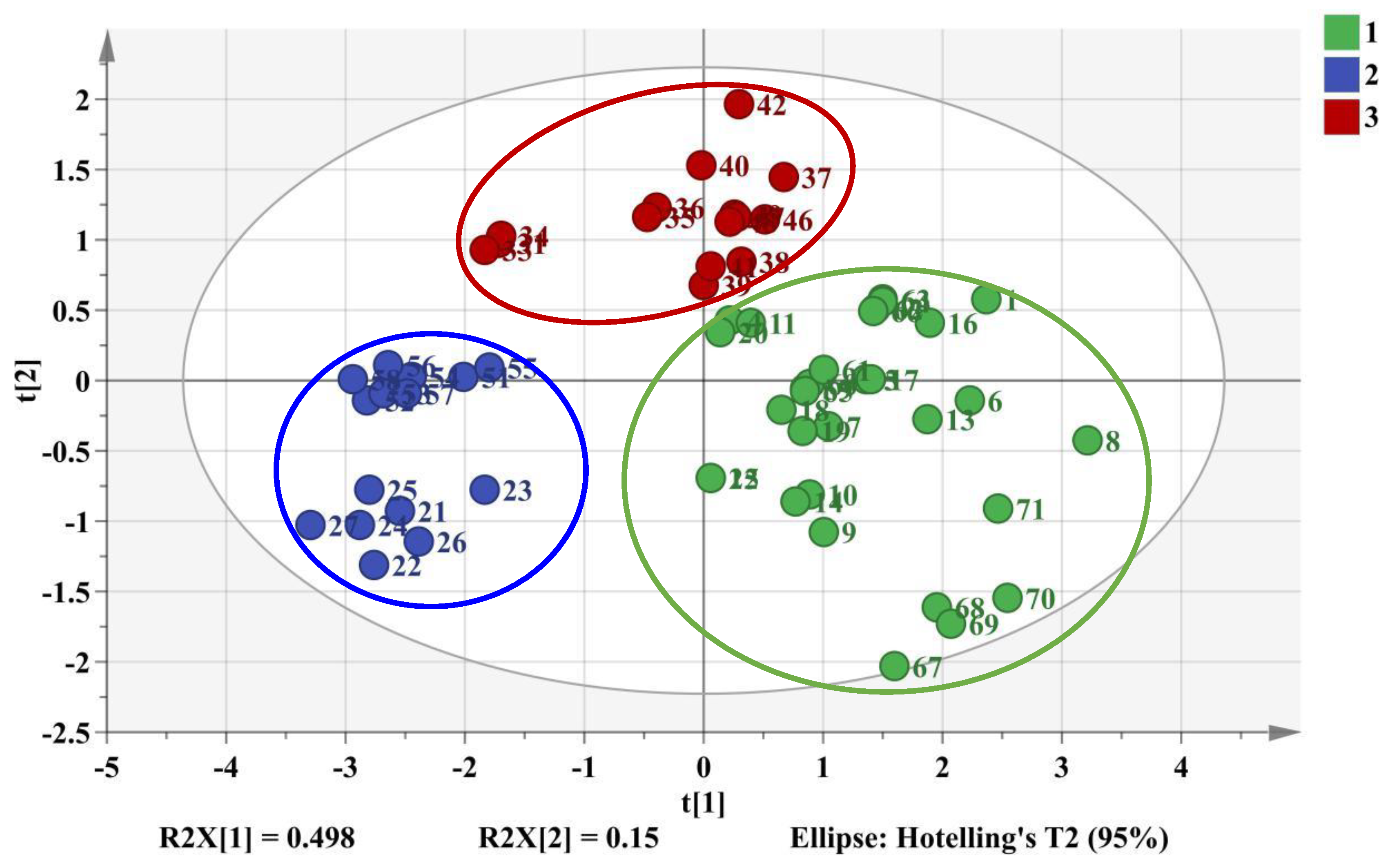
2.4.1. HCA and PCA Analysis
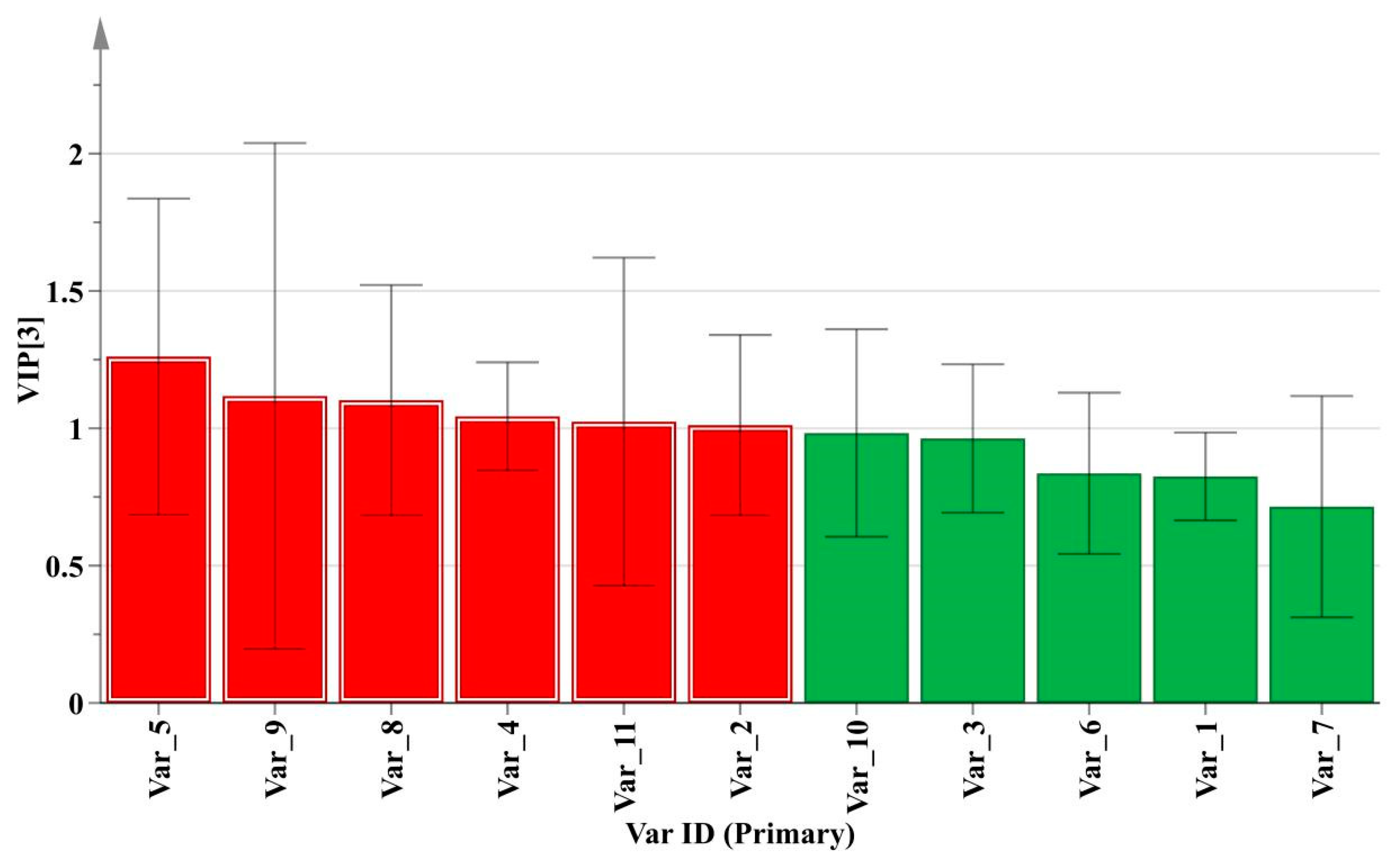
2.4.2. PLS-DA Analysis
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Instrumentation
3.3. Preparation of Sample Solutions
3.4. Chromatographic Conditions
3.5. Data Analysis
3.5.1. Similarity Analysis (SA)
3.5.2. CPR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China; Chinese Pharmacopoeia Commission: Beijing, China, 2015; Volume I, pp. 85–86. [Google Scholar]
- Guizhou Provincial Health Department. Quality Standard of National Chinese Herbal Medicines in Guizhou Province; DB52/YC005-2003; Guizhou Provincial Health Department: Guizhou, China, 2003; p. 7.
- Guangxi Medical Products Administration. Quality Standard of Zhuang Medicine in Guangxi Zhuang Autonomous Region; DYB45-GXZYC0047-2011; Guangxi Medical Products Administration: Guangxi, China, 2011; Volume 2, pp. 75–76.
- Abu-Qaoud, H.; Shawarb, N.; Hussen, F.; Jaradat, N.; Shtaya, M. Comparison of qualitative, quantitative analysis and antioxidant potential between wild and cultivated Borago officinalis leaves from palestine. Pak. J. Pharm. Sci. 2018, 31, 953–959. [Google Scholar] [PubMed]
- He, J.M.; Mu, Q. The medicinal uses of the genus Mahonia in traditional Chinese medicine: An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 175, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Kong, L. Automatic authentication and distinction of Epimedium koreanum and Epimedium wushanense with HPLC fi ngerprint analysis assisted by pattern recognition techniques. Biochem. Syst. Ecol. 2012, 40, 138–145. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Shao, C.; Li, C.; Liu, S. Chemical Fingerprint Analysis for the Quality Evaluation of Deepure Instant Pu-erh Tea by HPLC Combined with Chemometrics. Food Anal. Methods 2016, 9, 3298–3309. [Google Scholar] [CrossRef]
- Esteki, M.; Farajmand, B.; Amanifar, S.; Barkhordari, R.; Ahadiyan, Z.; Dashtaki, E.; Mohammadlou, M.; Vander Heyden, Y. Classification and authentication of Iranian walnuts according to their geographical origin based on gas chromatographic fatty acid fingerprint analysis using pattern recognition methods. Chemom. Intell. Lab. Syst. 2017, 171, 251–258. [Google Scholar] [CrossRef]
- Wang, L.; Hui, Y.; Jiang, K.; Yin, G.; Wang, J.; Yan, Y.; Wang, Y.; Li, J.; Wang, P.; Bi, K.; et al. Potential of near infrared spectroscopy and pattern recognition for rapid discrimination and quantification of Gleditsia sinensis thorn powder with adulterants. J. Pharm. Biomed. Anal. 2018, 160, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Pedan, V.; Weber, C.; Do, T.; Fischer, N.; Reich, E.; Rohn, S. HPTLC fingerprint profile analysis of cocoa proanthocyanidins depending on origin and genotype. Food Chem. 2018, 267, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Chen, Y.; Wei, Z.; Yang, J. A method based on covariance and pattern recognition for improving resolutions of spatially encoded NMR spectra. Magn. Reson. Chem. 2015, 53, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, W.; Su, M.; Sun, Y.; Liu, H.; Nie, L.; Zang, H. Quality evaluation of traditional Chinese medicines based on fingerprinting. J. Sep. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, P.; Chau, F. Chromatographic fingerprinting and related chemometric techniques for quality control of traditional Chinese medicines. J. Sep. Sci. 2010, 33, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tang, G.; Zhang, T.; Fillet, M.; Crommen, J.; Jiang, Z. Supercritical fluid chromatography in traditional Chinese medicine analysis. J. Pharm. Biomed. Anal. 2018, 147, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, T.; Zhou, H.; Feng, Y.; Fan, C.; Chen, W.; Crommen, J.; Jiang, Z. Fast separation of triterpenoid saponins using supercritical fluid chromatography coupled with single quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 2016, 121, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, D.; Dogra, A.K.; Tahrani, A.; Herrmann, F.; Wink, M. Extracts from traditional Chinese medicinal plants inhibit acetylcholinesterase, a known Alzheimer’s Disease target. Molecules 2016, 21, 1161. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Sun, L.; Li, D.; You, G.; Wang, M.; Ren, X. Quality Evaluation of Phellodendri Chinensis Cortex by Fingerprint–Chemical Pattern Recognition. Molecules 2018, 23, 2307. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Qi, H.; Shi, Y. Fingerprint Analysis of Codonopsis Radix by HPLC Coupled with Chemometrics Analysis. Chin. Herb. Med. 2013, 5, 307–312. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, H.; Yu, X.; Luo, H.; Lu, Y.; Yang, H.; Li, X.; Li, Z.; Tang, L.; Wang, Z. Determination of Bitterness of Andrographis Herba Based on Electronic Tongue Technology and Discovery of the Key Compounds of Bitter Substances. Molecules 2018, 23, 3362. [Google Scholar] [CrossRef] [PubMed]
- Beale, D.J.; Morrison, P.D.; Karpe, A.V.; Dunn, M.S. Chemometric analysis of lavender essential oils using targeted and untargeted GC-MS acquired data for the rapid identification and characterization of oil quality. Molecules 2017, 22, 1339. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
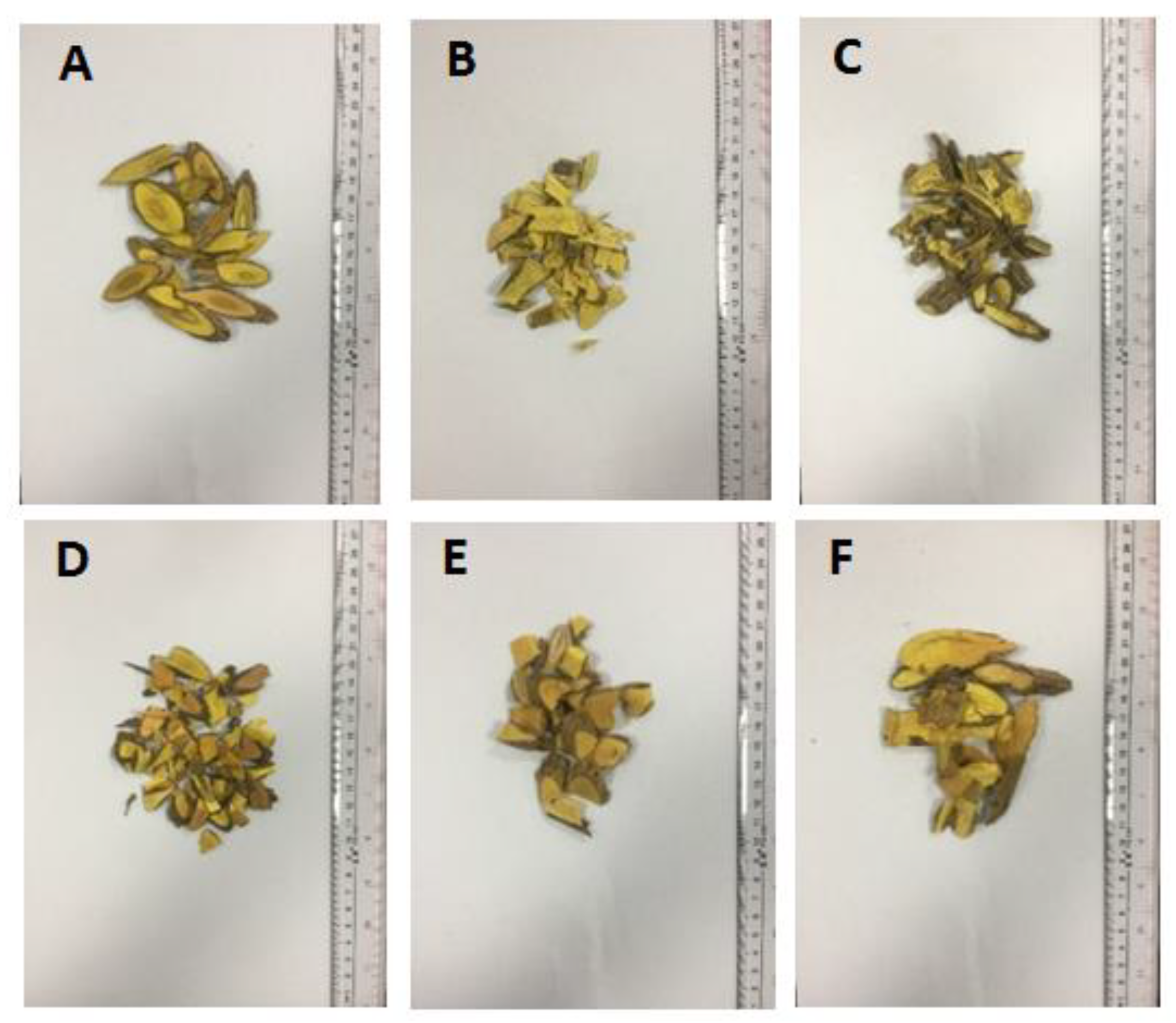


| Sample | Similarity | Sample | Similarity |
|---|---|---|---|
| S1 | 0.942 | S20 | 0.936 |
| S2 | 0.942 | S21 | 0.870 |
| S3 | 0.955 | S22 | 0.871 |
| S4 | 0.952 | S23 | 0.898 |
| S5 | 0.957 | S24 | 0.861 |
| S6 | 0.964 | S25 | 0.862 |
| S7 | 0.976 | S26 | 0.875 |
| S8 | 0.862 | S27 | 0.850 |
| S9 | 0.909 | S31 | 0.912 |
| S10 | 0.910 | S34 | 0.913 |
| S11 | 0.941 | S36 | 0.841 |
| S12 | 0.968 | S38 | 0.912 |
| S13 | 0.830 | S39 | 0.896 |
| S14 | 0.913 | S40 | 0.952 |
| S15 | 0.956 | S41 | 0.980 |
| S16 | 0.973 | S46 | 0.954 |
| S17 | 0.982 | S47 | 0.973 |
| S18 | 0.928 | S49 | 0.955 |
| S19 | 0.929 |
| Members | Correct | Cultivated M. bealei | Wild M. bealei | Substitutes | |
|---|---|---|---|---|---|
| Cultivated M. bealei | 18 | 100% | 18 | 0 | 0 |
| Wild M. bealei | 2 | 100% | 0 | 2 | 0 |
| Substitutes | 6 | 100% | 0 | 0 | 6 |
| No class | 0 | / | 0 | 0 | 0 |
| Total | 26 | 100% | 18 | 2 | 6 |
| Fisher’s probability | 5.6 × 10−6 |
| Sample No. | Species | Origin | Specification |
|---|---|---|---|
| 1–12 | M. bealei (Fort.) Carr. | Guangxi | Crude drugs (cultivated) |
| 13 | M. bealei (Fort.) Carr. | Yunnan | Crude drugs (cultivated) |
| 14 | M. bealei (Fort.) Carr. | Anhui | Crude drugs (cultivated) |
| 15 | M. bealei (Fort.) Carr. | Zhejiang | Crude drugs (cultivated) |
| 16 | M. bealei (Fort.) Carr. | Jiangxi | Crude drugs (cultivated) |
| 17 | M. bealei (Fort.) Carr. | Sichuan | Crude drugs (cultivated) |
| 18–20 | M. bealei (Fort.) Carr. | Guizhou | Crude drugs (cultivated) |
| 21–29 | M. bealei (Fort.) Carr. | Guangxi | Crude drugs (wild) |
| 30–34 | M. breviracema Y. S. Wang et Hsiao | Guangxi | Crude drugs (wild) |
| 35–39 | M. duclouxiana Gagnep | Guangxi | Crude drugs (wild) |
| 40–45 | M. bodinieri Gagnep | Guangxi | Crude drugs (wild) |
| 46–50 | M. fordii Schneid | Guangxi | Crude drugs (wild) |
| 51–58 | M. bealei (Fort.) Carr. | Guangdong | Crude drugs (wild) |
| 59–65 | M. bealei (Fort.) Carr. | Anhui | Crude drugs (cultivated) |
| 66–72, 74–75, 79 | M. bealei (Fort.) Carr. | Jiangxi | Crude drugs (cultivated) |
| 73 | M. bealei (Fort.) Carr. | Anhui | Crude drugs (cultivated) |
| 76–78, 80–83 | M. bealei (Fort.) Carr. | Guangdong | Crude drugs (cultivated) |
| 84 | M. bealei (Fort.) Carr. | Jiangxi | Crude drugs (cultivated) |
| 85–86 | M. bealei (Fort.) Carr. | Anhui | Crude drugs (cultivated) |
| 87–89 | M. bealei (Fort.) Carr. | Guangxi | Crude drugs (cultivated) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Jiang, Z.; Wang, J.; Yin, G.; Jiang, K.; Tu, J.; Wang, T. Quality Evaluation of Mahonia bealei (Fort.) Carr. Using Supercritical Fluid Chromatography with Chemical Pattern Recognition. Molecules 2019, 24, 3684. https://doi.org/10.3390/molecules24203684
Huang Y, Jiang Z, Wang J, Yin G, Jiang K, Tu J, Wang T. Quality Evaluation of Mahonia bealei (Fort.) Carr. Using Supercritical Fluid Chromatography with Chemical Pattern Recognition. Molecules. 2019; 24(20):3684. https://doi.org/10.3390/molecules24203684
Chicago/Turabian StyleHuang, Yang, Zhengjin Jiang, Jue Wang, Guo Yin, Kun Jiang, Jiasheng Tu, and Tiejie Wang. 2019. "Quality Evaluation of Mahonia bealei (Fort.) Carr. Using Supercritical Fluid Chromatography with Chemical Pattern Recognition" Molecules 24, no. 20: 3684. https://doi.org/10.3390/molecules24203684
APA StyleHuang, Y., Jiang, Z., Wang, J., Yin, G., Jiang, K., Tu, J., & Wang, T. (2019). Quality Evaluation of Mahonia bealei (Fort.) Carr. Using Supercritical Fluid Chromatography with Chemical Pattern Recognition. Molecules, 24(20), 3684. https://doi.org/10.3390/molecules24203684





