Ellagic Acid Recovery by Solid State Fermentation of Pomegranate Wastes by Aspergillus niger and Saccharomyces cerevisiae: A Comparison
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solid State Fermentation
2.2. EA Recovery
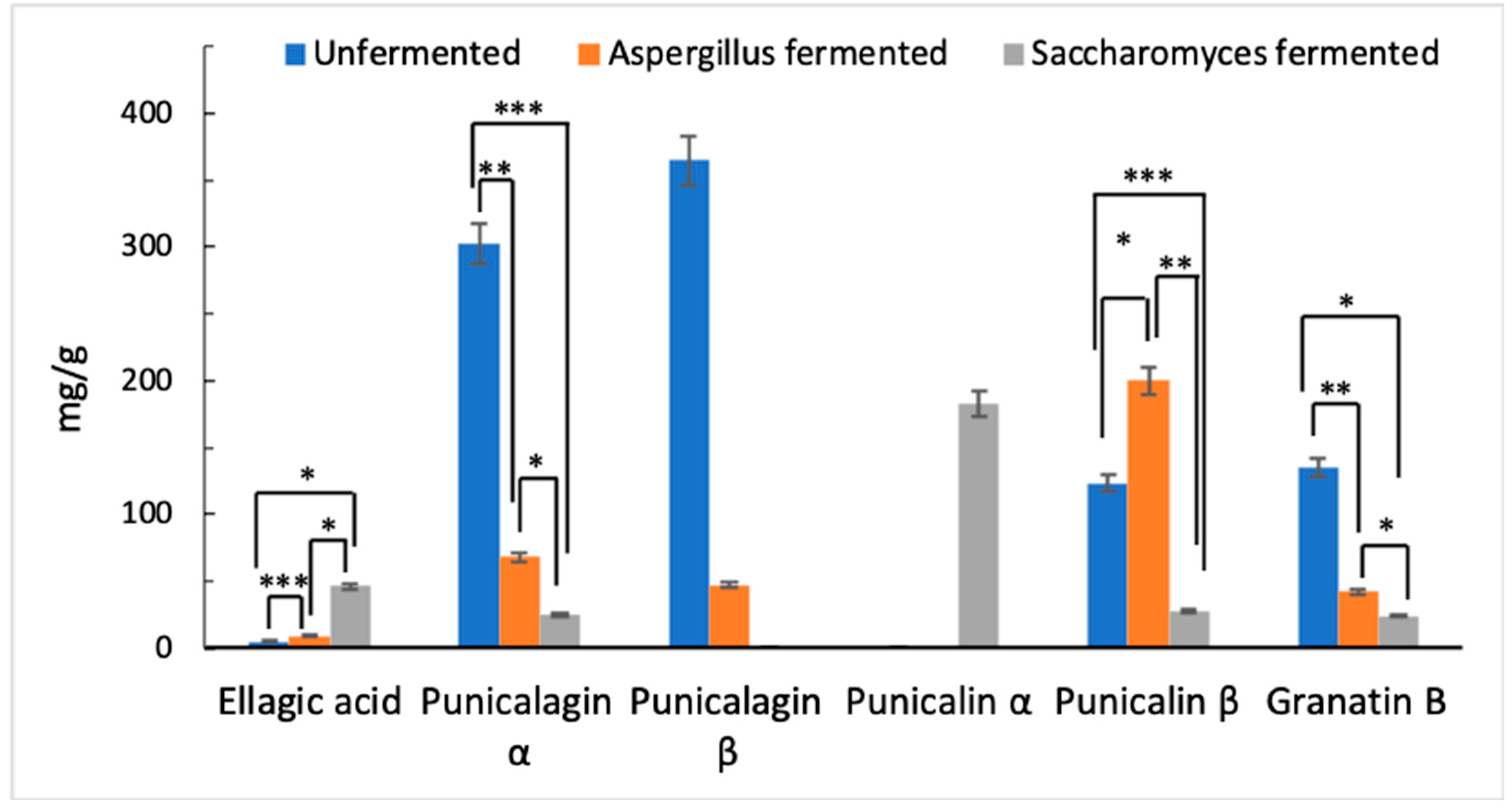
2.3. Identification and Quantitative Analysis of Ellagitannins
- Punicalagins α/β represent the main target of the hydrolase activity of the microorganisms suggesting a likely pathway of formation of EA.
- The occurrence of punicalin further confirms the hydrolytic cleavage of punicalagin during fermentation as a reaction pathway responsible at least in part for the generation of EA.
- Hydrolysis of granatin B may have a central role in the generation of EA during fermentation.
- Besides A. niger fungus, S. cerevisiae yeast is also capable of affecting the hydrolysis of ellagitannins to EA leading to even higher yields of the compound.
2.4. Evaluation of Different Extraction Conditions
2.5. Effects of Acid Hydrolysis on EA Recovery
3. Materials and Methods
3.1. Microorganisms
3.2. Raw Material
3.3. SSC with Aspergillus Niger
3.4. SSC with Saccharomyces Cerevisiae
3.5. Extraction Conditions
3.6. Acid Hydrolysis
3.7. HPLC Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: a boon for production of bioactive compounds by processing of food industries wastes (by-products). Molecules 2018, 23, 2560. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Wu, J.; Wang, X.; Sun, X.; Hackman, R.M.; Li, Z.; Feng, X. Evaluation of antioxidant capacity and flavor profile change of pomegranate wine during fermentation and aging process. Food Chem. 2017, 232, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar]
- Martinez-Avila, G.C.G.; Aguilera, A.F.; Saucedo, S.; Rojas, R.; Rodriguez, R.; Aguilar, C.N. Fruit wastes fermentation for phenolic antioxidants edible coatings and films fruit wastes fermentation for phenolic antioxidants production and their application in manufacture. Crit. Rev. Food Sci. Nutr. 2014, 54, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Kim, Y.; Choi, I.; Kim, G. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Dessie, W.; Zhang, W.; Xin, F.; Dong, W.; Zhang, M.; Ma, J.; Jiang, M. Succinic acid production from fruit and vegetable wastes hydrolyzed by on-site enzyme mixtures through solid state fermentation. Bioresour. Technol. 2018, 247, 1177–1180. [Google Scholar] [CrossRef]
- Sadh, P.K.; Chawla, P.; Duhan, J.S. Fermentation approach on phenolic, antioxidants and functional properties of peanut press cake. Food Biosci. 2018, 22, 113–120. [Google Scholar] [CrossRef]
- Doriya, K.; Jose, N.; Gowda, M.; Kumar, D.S. Solid-state fermentation vs. submerged fermentation for the production of l-asparaginase. In Advances in Food and Nutrition Research, 1st ed.; Elsevier Inc.: Philadelphia, PA, USA, 2016; Volume 78, pp. 115–135. [Google Scholar]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef]
- De La Cruz Quiroz, R.; Roussos, S.; Hernández, D.; Rodríguez, R.; Castillo, F.; Aguilar, C.N. Challenges and opportunities of the bio-pesticides production by solid-state fermentation: Filamentous fungi as a model. Crit. Rev. Biotechnol. 2015, 35, 326–333. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Phenolic compounds of pomegranate byproducts (outer skin, mesocarp, divider membrane) and their antioxidant activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef]
- Garcia-Villalba, R.; Espín, J.C.; Kroon, P.A.; Alasalvar, C.; Heinonen, M.; Voorspoels, S.; Tomas-Barberan, F. A validated method for the characterization and quantification of extractable and non-extractable ellagitannins after acid hydrolysis in pomegranate fruits, juices, and extracts. J. Agric. Food Chem. 2015, 63, 6555–6566. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, X.; Li, M.; Zhao, W.; Liu, L.; Kong, X. Chemical fingerprint and quantitative analysis for quality control of polyphenols extracted from pomegranate peel by HPLC. Food Chem. 2015, 176, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Verotta, L.; Macchi, M.P.; Venkatasubramanian, P. Monograph on pomegranate. In Connecting Indian Wisdom and Western Science: Plant Usage for Nutrition and Health; CRC Press: Boca Raton, FL, USA, 2015; pp. 303–307. [Google Scholar]
- Seeram, N.; Lee, R.; Hardy, M.; Heber, D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep. Purif. Technol. 2005, 41, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Kalaycıoğlu, Z.; Erim, F.B. Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem. 2017, 221, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Gurban, C.; Serban, A.; Andrica, F.; Serban, M. Effects of supplementation with pomegranate juice on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine 2016, 23, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Amigo-benavent, M.; Mesias, M.; Baeza, G.; Bravo, L.; Morales, F.J. An aqueous pomegranate seed extract ameliorates oxidative stress of human hepatoma HepG2 cells. J. Sci. Food Agric. 2014, 94, 1622–1627. [Google Scholar] [CrossRef]
- Sharma, P.; McClees, S.F.; Afaq, F. Pomegranate for prevention and treatment of cancer: An update. Molecules 2017, 22, 177. [Google Scholar] [CrossRef]
- García-Niño, W.R.; Zazueta, C. Ellagic acid: Pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol. Res. 2015, 97, 84–103. [Google Scholar] [CrossRef]
- Bae, J.; Choi, J.; Kang, S.; Lee, Y.; Park, J.; Kang, Y. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp. Dermatol. 2010, 182–190. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1–36. [Google Scholar] [CrossRef]
- Mele, L.; Mena, P.; Piemontese, A.; Marino, V.; López-Gutiérrez, N.; Bernini, F.; Brighenti, F.; Zanotti, I.; Del Rio, D. Antiatherogenic effects of ellagic acid and urolithins in vitro. Arch. Biochem. Biophys. 2016, 599, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Ascacio-Valdés, J.A.; Gironés-Vilaplana, A.; Del, D.; Moreno, D.A.; García-Viguera, C. Assessment of pomegranate wine lees as a valuable source for the recovery of (poly) phenolic compounds. Food Chem. 2014, 145, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; García-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and comprehensive evaluation of (Poly)phenolic compounds in pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Breksa, A.P.; Pan, Z.; Ma, H. Quantitative determination of major polyphenol constituents in pomegranate products. Food Chem. 2012, 132, 1585–1591. [Google Scholar] [CrossRef]
- Aguilar-Zarate, P.; Wong-Paz, J.E.; Buenrostro-Figueroa, J.J.; Ascacio, J.A.; Contreras-Esquivel, J.C.; Aguilar, C.N. Ellagitannins: Bioavailability, Purification and Biotechnological Degradation. Mini-Rev. Med. Chem. 2018, 18, 1244–1252. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.F.; Buenrostro, J.J.; Prado-Barragán, A.; Rodríguez-Herrera, R.; Aguilar, C.N. The complete biodegradation pathway of ellagitannins by Aspergillus niger in solid-state fermentation. J. Basic Microbiol. 2016, 56, 329–336. [Google Scholar] [CrossRef]
- Vattem, D.A.; Shetty, K. Ellagic acid production and phenolic antioxidant acti v ity in cranberry pomace (Vaccinium macrocarpon) mediated by Lentinus edodes using a solid-state system. Process Biochem. 2003, 39, 367–379. [Google Scholar] [CrossRef]
- Vattem, D.A.; Shetty, K. Solid-state Pproduction of phenolic antioxidants from cranberry pomace by Rhizopus Oligosporus. Food Biotechnol. 2002, 16, 189–210. [Google Scholar] [CrossRef]
- Puupponen-pimiä, R.; Nohynek, L.; Juvonen, R.; Kössö, T.; Truchado, P.; Westerlund-Wikstrom, B.; Leppanen, T.; Moilanen, E.; Oksman-Caldentey, K.-M. Fermentation and dry fractionation increase bioactivity of cloudberry (Rubus chamaemorus). Food Chem. 2016, 197, 950–958. [Google Scholar] [CrossRef]
- Huang, W.; Ni, J.; Borthwick, A.G.L. Biosynthesis of valonia tannin hydrolase and hydrolysis of valonia tannin to ellagic acid by Aspergillus SHL 6. Process Biochem. 2005, 40, 1245–1249. [Google Scholar] [CrossRef]
- Shi, B.; He, Q.; Yao, K.; Huang, W.; Li, Q. Production of ellagic acid from degradation of valonea tannins by Aspergillus niger and Candida utilis. J. Chem. Technol. Biotechnol. 2005, 80, 1154–1159. [Google Scholar] [CrossRef]
- Aguilera-Carbo, A.; Hernández, J.S.; Augur, C.; Prado-Barragan, L.A.; Favela-Torres, E.; Aguilar, C.N. Ellagic acid production from biodegradation of creosote bush ellagitannins by Aspergillus niger in solid state culture. Food Bioprocess Technol. 2009, 2, 208–212. [Google Scholar] [CrossRef]
- Verotta, L.; Panzella, L.; Antenucci, S.; Calvenzani, V.; Tomay, F.; Petroni, K.; Caneva, E.; Napolitano, A. Fermented pomegranate wastes as sustainable source of ellagic acid: Antioxidant properties, anti-inflammatory action, and controlled release under simulated digestion conditions. Food Chem. 2018, 246, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, L.; Aguilera-carbó, A.; Ascacio-valdés, J.A.; Rodríguez-herrera, R.; Martínez-hernández, J.L.; Aguilar, C.N. Optimization of ellagic acid accumulation by Aspergillus niger GH1 in solid state culture using pomegranate shell powder as a support. Process Biochem. 2012, 47, 2199–2203. [Google Scholar] [CrossRef]
- Robledo, A.; Aguilera-Carbó, A.; Rodriguez, R.; Martinez, J.L.; Garza, Y.; Aguilar, C.N. Ellagic acid production by Aspergillus niger in solid state fermentation of pomegranate residues. J. Ind. Microbiol. Biotechnol. 2008, 35, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, L.; Buenrostro-Figueroa, J.J.; Ascacio-Valdés, J.A.; Aguilera-Carbó, A.F.; Rodríguez-Herrera, R.; Contreras-Esquivel, J.C.; Aguilar, C.N. Submerged culture for production of ellagic acid from pomegranate husk by Aspergillus niger GH1. Micol. Apl. Int. 2014, 26, 27–35. [Google Scholar]
- Wu, S.; Tian, L. Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef]
- De Melo Lopes, L.M.; Costa Batista, L.H.; Gouveia, M.J.; Leite, T.C.C.; de Mello, M.R.F.; de Assis, S.A.; de Sena, A.R. Kinetic and thermodynamic parameters, and partial characterization of the crude extract of tannase produced by Saccharomyces cerevisiae CCMB 520. Nat. Prod. Res. 2018, 32, 1068–1075. [Google Scholar] [CrossRef]
- Panzella, L.; Cerruti, P.; Ambrogi, V.; Agustin-Salazar, S.; D’Errico, G.; Carfagna, C.; Goya, L.; Ramos, S.; Martín, M.A.; Napolitano, A.; et al. A Superior All-Natural Antioxidant Biomaterial from Spent Coffee Grounds for Polymer Stabilization, Cell Protection, and Food Lipid Preservation. ACS Sustain. Chem. Eng. 2016, 4, 1169–1179. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Compound | ||||
|---|---|---|---|---|
| Retention Time (min) | [M−H]− | Unfermented Material | Fungal Fermentation | Yeast Fermentation |
| 4.55 | 377.1 | 3,4-DHPEA-EA a | 3,4-DHPEA-EA a | |
| 4.75 | 781.1 | Punicalin α | ||
| 15.47 | 781.1 | Punicalin β | Punicalin β | Punicalin β |
| 18.07 | 783.1 | Terflavin B | Terflavin B | |
| 21.93 | 1083 | Punicalagin α | Punicalagin α | Punicalagin α |
| 22.58 | 1083 | Punicalagin β | Punicalagin β | |
| 27.99 | 633.1 | Galloyl-HHDP-hexoside b | ||
| 28.67 | 463.1 | Ellagic acid-hexoside | ||
| 29.83 | 784.1 | Pedunculagin II | Pedunculagin II | |
| 30.27 | 934.9 | Casuarinin | ||
| 31.22 | 951 | Granatin B | Granatin B | Granatin B |
| 32.74 | 433 | Ellagic acid pentoside | ||
| 33.76 | 301 | Ellagic acid | Ellagic acid | Ellagic acid |
| Treatment | T (°C) | Humidity (%) | Inoculum (cells/g) | pH | Peptone (g/L) | Extract of Yeast (g/L) | NaCl (g/L) | EA (mg/g) |
|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 0.15 |
| 2 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | 0.03 |
| 3 | −1 | 1 | −1 | −1 | 1 | −1 | 1 | 0.65 |
| 4 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 0.08 |
| 5 | −1 | −1 | 1 | 1 | −1 | −1 | 1 | 0.11 |
| 6 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | 0.14 |
| 7 | −1 | 1 | 1 | −1 | −1 | 1 | −1 | 0.09 |
| 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.11 |
| 9 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 0.26 |
| 10 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | 0.27 |
| 11 | −1 | 1 | −1 | −1 | 1 | −1 | 1 | 0.33 |
| 12 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 0.21 |
| 13 | −1 | −1 | 1 | 1 | −1 | −1 | 1 | 0.06 |
| 14 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | 0.15 |
| 15 | −1 | 1 | 1 | −1 | −1 | 1 | −1 | 0.07 |
| 16 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.31 |
| 17 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 0.20 |
| 18 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | 0.29 |
| 19 | −1 | 1 | −1 | −1 | 1 | −1 | 1 | 0.26 |
| 20 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 0.37 |
| 21 | −1 | −1 | 1 | 1 | −1 | −1 | 1 | 0.26 |
| 22 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | 0.27 |
| 23 | −1 | 1 | 1 | −1 | −1 | 1 | −1 | 0.18 |
| 24 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moccia, F.; Flores-Gallegos, A.C.; Chávez-González, M.L.; Sepúlveda, L.; Marzorati, S.; Verotta, L.; Panzella, L.; Ascacio-Valdes, J.A.; Aguilar, C.N.; Napolitano, A. Ellagic Acid Recovery by Solid State Fermentation of Pomegranate Wastes by Aspergillus niger and Saccharomyces cerevisiae: A Comparison. Molecules 2019, 24, 3689. https://doi.org/10.3390/molecules24203689
Moccia F, Flores-Gallegos AC, Chávez-González ML, Sepúlveda L, Marzorati S, Verotta L, Panzella L, Ascacio-Valdes JA, Aguilar CN, Napolitano A. Ellagic Acid Recovery by Solid State Fermentation of Pomegranate Wastes by Aspergillus niger and Saccharomyces cerevisiae: A Comparison. Molecules. 2019; 24(20):3689. https://doi.org/10.3390/molecules24203689
Chicago/Turabian StyleMoccia, Federica, Adriana C. Flores-Gallegos, Mónica L. Chávez-González, Leonardo Sepúlveda, Stefania Marzorati, Luisella Verotta, Lucia Panzella, Juan A. Ascacio-Valdes, Cristobal N. Aguilar, and Alessandra Napolitano. 2019. "Ellagic Acid Recovery by Solid State Fermentation of Pomegranate Wastes by Aspergillus niger and Saccharomyces cerevisiae: A Comparison" Molecules 24, no. 20: 3689. https://doi.org/10.3390/molecules24203689