In-Vitro Antioxidant, Hypoglycemic Activity, and Identification of Bioactive Compounds in Phenol-Rich Extract from the Marine Red Algae Gracilaria edulis (Gmelin) Silva
Abstract
:1. Introduction
2. Results
2.1. Quantification of Total Phenolic, Flavonoid, and Alkaloid Contents of G. edulis
2.2. In Vitro Antioxidant Activity
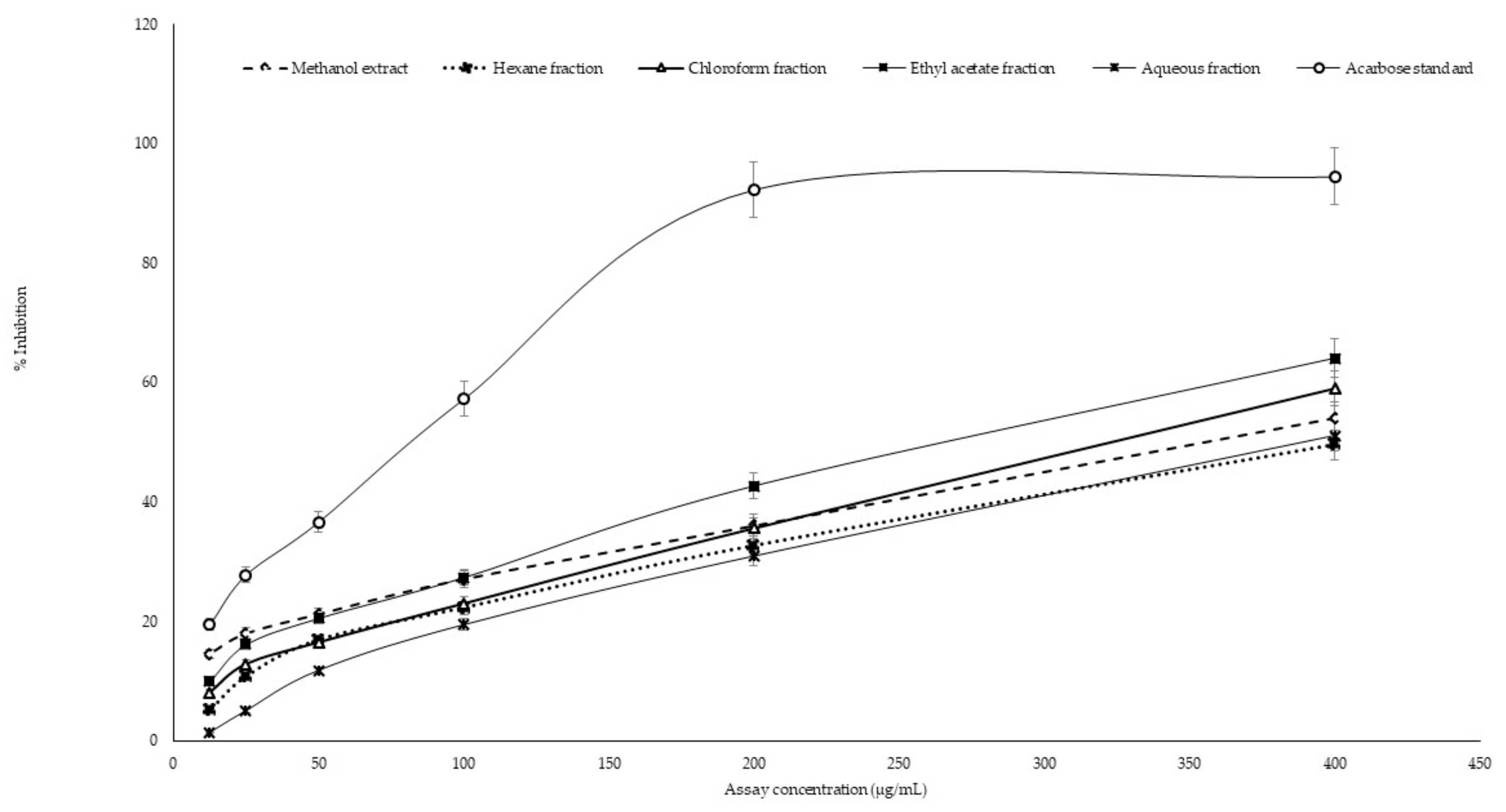
2.3. In Vitro α-Amylase Inhibitory Assay
2.4. In Vitro α-Glucosidase Inhibitory Assay
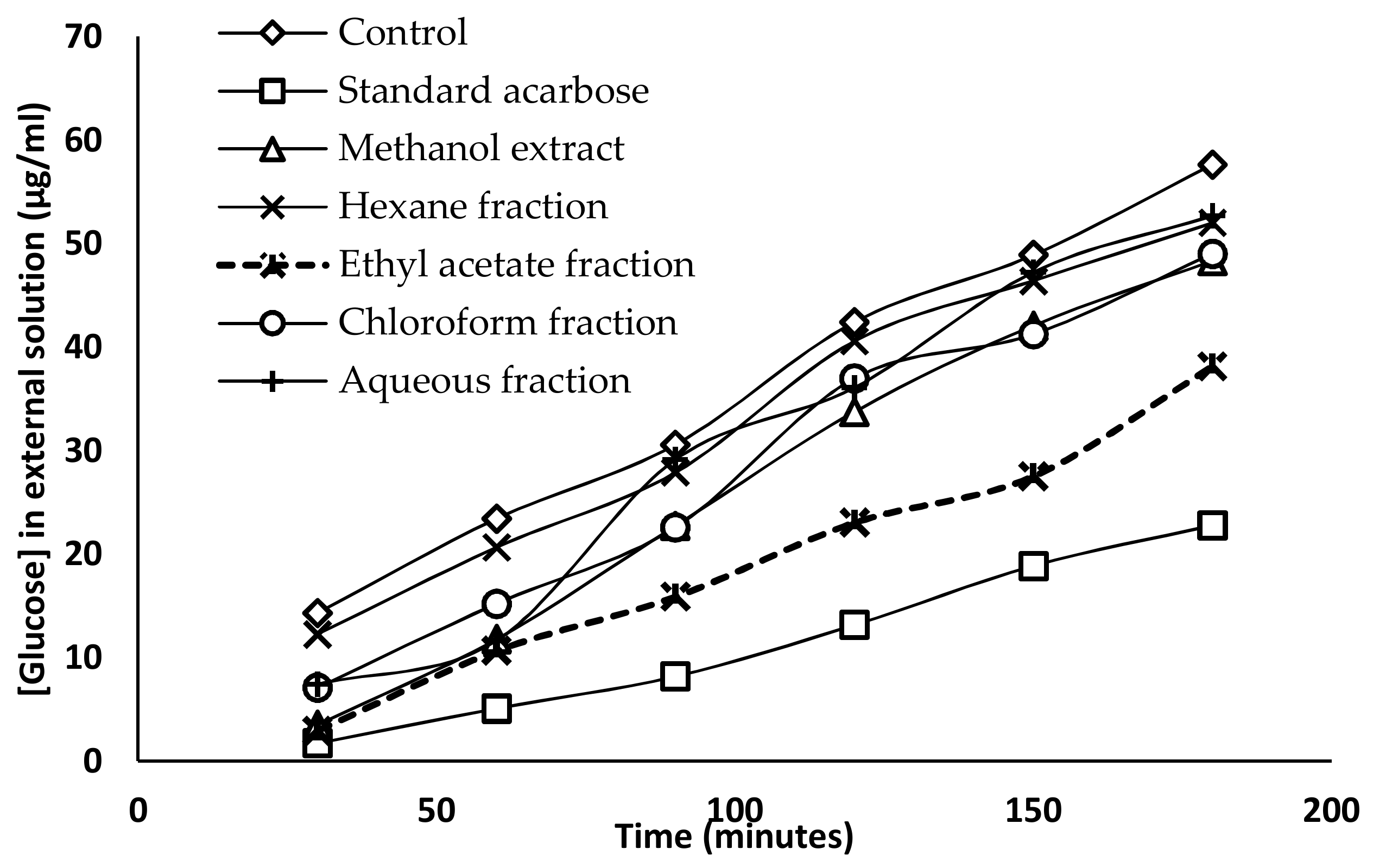
2.5. Glucose Diffusion Inhibitory Activity
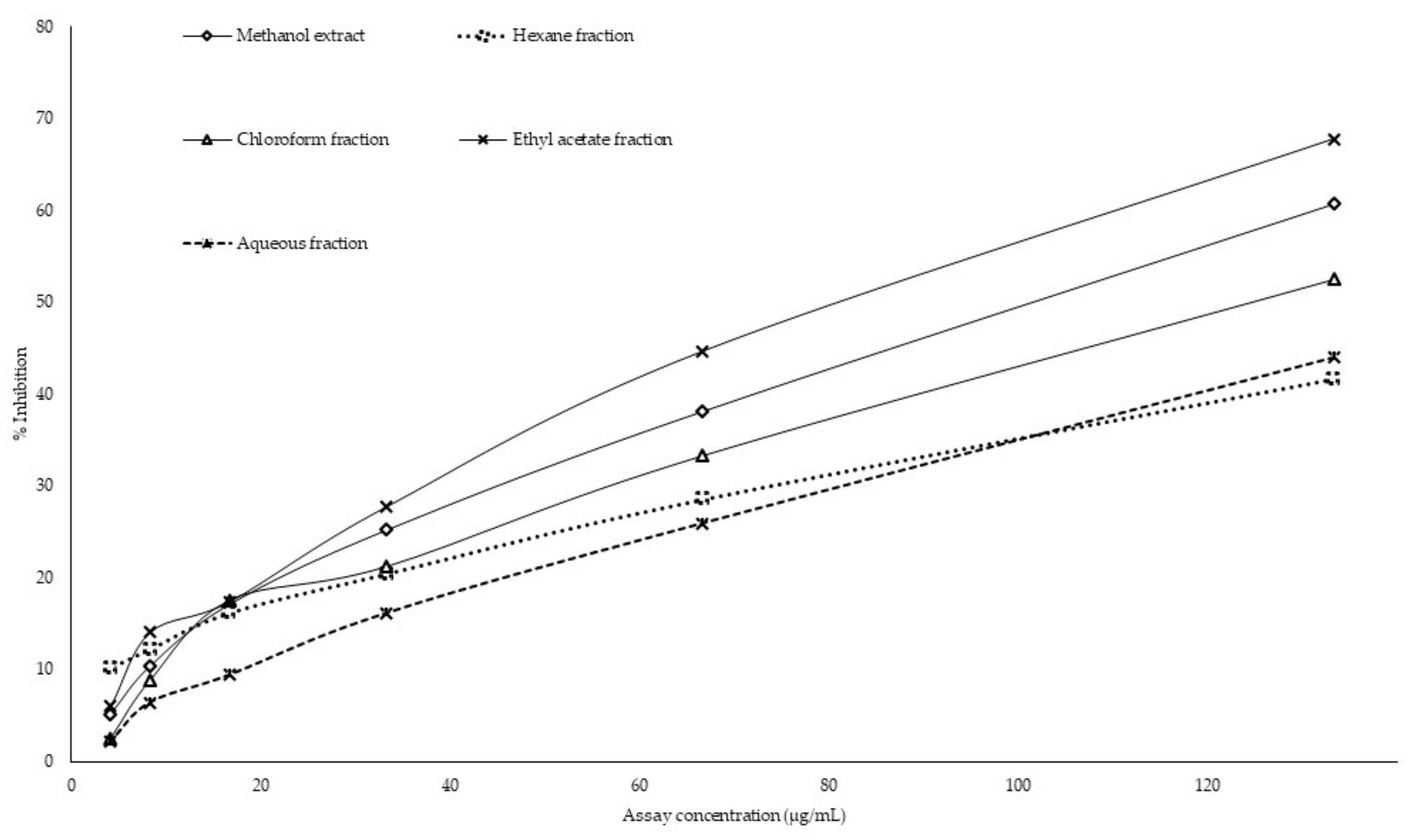
2.6. Antiglycation Activity
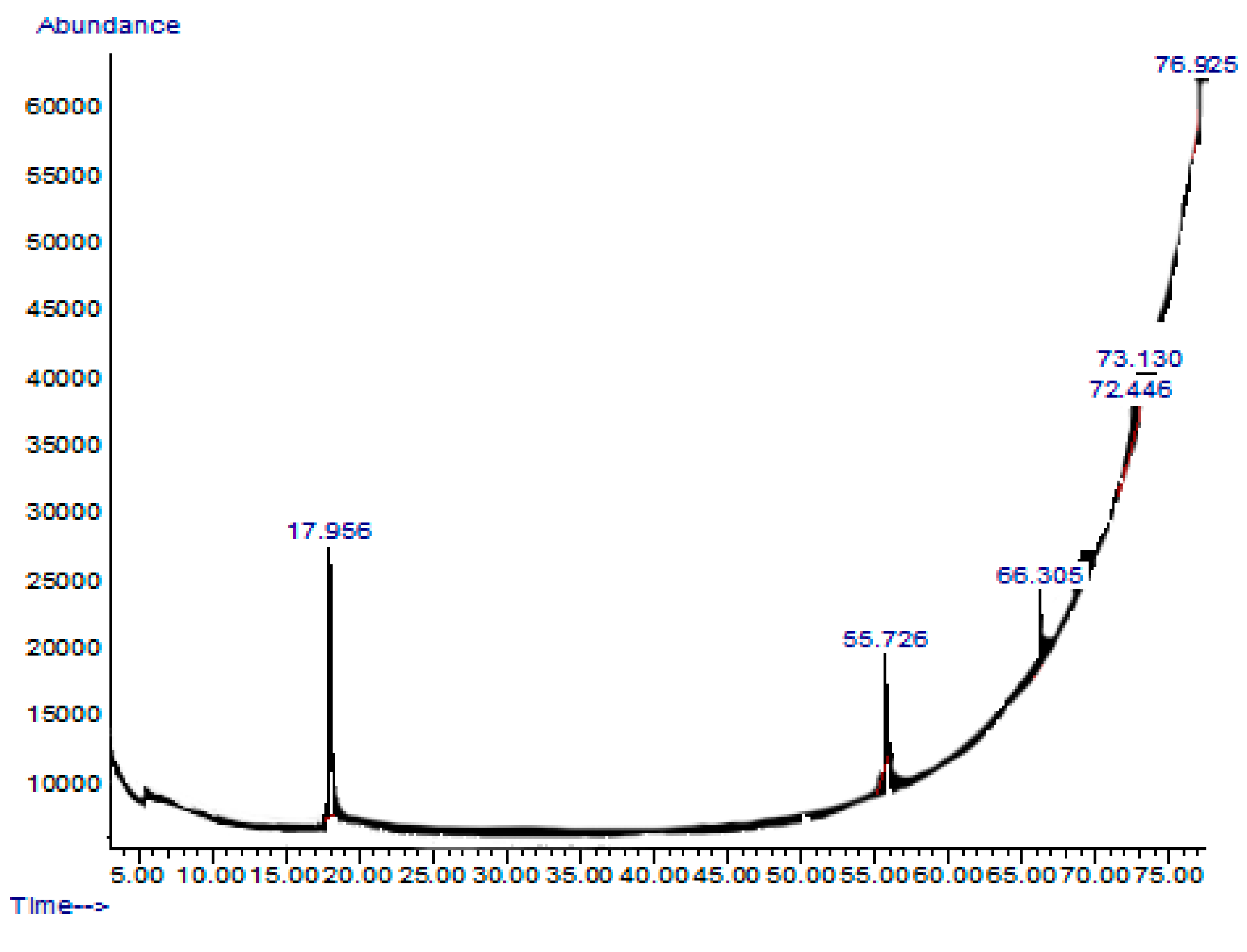
2.7. GC-MS or Gas Chromatography-Mass Spectrometry Analysis of Extract and Solvent Fractions
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection of Algae Sample
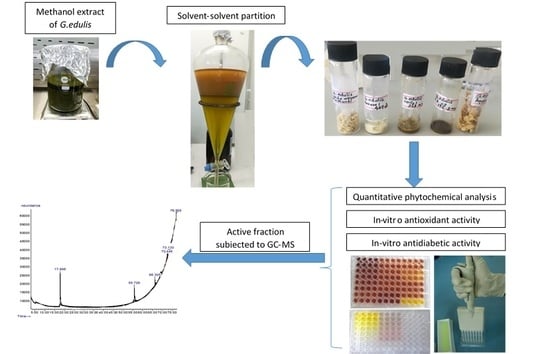
4.3. Preparation of Gracillaria edulis Extract and Its Solvent Fractions
4.4. Quantification of Phenol, Flavonoid, and Alkaloid Contents
4.5. In Vitro Antioxidant Activity
4.5.1. DPPH Radical Scavenging Activity
4.5.2. ABTS+ Radical Scavenging Activity
4.5.3. Ferric Reducing Antioxidant Power (FRAP)
4.5.4. Ferrous Iron Chelating Capacity (FICC)
4.5.5. Oxygen Radical Absorbance Capacity (ORAC)
4.6. In Vitro α-amylase and α-glucosidase Inhibitory Assay
4.6.1. Alpha-Amylase Inhibitory Activity
4.6.2. Alpha-Glucosidase Inhibitory Activity
4.6.3. Glucose Diffusion Inhibitory Activity
4.6.4. Antiglycation Activity
4.7. GC-MS Analysis of Extract and Solvent Fractions
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

| Time (min) | Glucose Concentration (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Control | Standard (Acarbose) | Methanol Extract | Hexane Fraction | Chloroform Fraction | Ethyl Acetate Fraction | Aqueous Fraction | |
| 30 | 14.32 ± 0.77 a | 1.72 ± 0.34 b | 3.51 ± 0.47 b | 12.26 ± 1.96 a | 7.09 ± 0.82 c | 2.96 ± 0.47 b | 7.43 ± 0.59 c |
| 60 | 23.41 ± 1.01 a | 5.09 ± 0.72 b | 11.77 ± 0.47 c | 20.66 ± 1.97 a | 15.22 ± 1.13 d | 10.67 ± 0.75 c | 11.64 ± 1.62 c |
| 90 | 30.58 ± 1.14 a | 8.19 ± 0.47 b | 22.73 ± 0.57 c | 27.89 ± 1.13 d | 22.59 ± 1.12 c | 15.91 ± 0.61 e | 29.13 ± 0.79 a,d |
| 120 | 42.43 ± 1.54 a | 13.15 ± 0.61 b | 33.75 ± 0.79 c | 40.57 ± 1.44 a | 36.98 ± 1.19 e | 23.07 ± 2.02 d | 36.09 ± 1.54 c,e |
| 150 | 48.90 ± 1.22 a | 18.87 ± 0.57 b | 42.08 ± 1.37 c | 46.42 ± 0.91 d | 41.25 ± 0.61 c | 27.55 ± 0.59 e | 47.25 ± 1.35 a,d |
| 180 | 57.65 ± 1.67 a | 22.79 ± 0.47 b | 48.36 ± 1.04 c | 52.01 ± 0.96 d | 49.04 ± 0.67 c | 38.15 ± 1.11 e | 52.69 ± 1.31 d |
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diab. Care 2013, 36, 67–74. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diabetes. 2016. Available online: http://www.who.int/mediacentre/factsheets/fs312/en/ (accessed on 31 January 2019).
- Jenum, A.K.; Diep, L.M.; Holmboe-Ottesen, G.; Holme, I.M.; Kumar, B.N.; Birkeland, K.I. Diabetes susceptibility in ethnic minority groups from Turkey, Vietnam, Sri Lanka and Pakistan compared with Norwegians - the association with adiposity is strongest for ethnic minority women. BMC Pub. Heal. 2012, 12, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum. Indian J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Eleazu, C.O. The concept of low glycemic index and glycemic load foods as panacea for type 2 diabetes mellitus. Afr. Health Sci. 2016, 16, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Kavrekar, V.; Mishra, A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur. J. Exp. Biol. 2013, 3, 128–132. [Google Scholar]
- Ferná1ndez-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [Green Version]
- Wright, E., Jr.; Glass, L.C. Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int. J. Clin. Pract. 2006, 60, 308–314. [Google Scholar]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive Compounds and Properties of Seaweeds—A Review. Biotechnol. Bioinform. 2014, 1, 1–11. [Google Scholar]
- De Souza, M.D.F.V.; Barbosa-filho, J.M.; Batista, L.M. Bioactivities from Marine Algae of the Genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar]
- Fernando, S.; Sanjeewa, A. Preliminary screening of two marine algae and sea grass harvested from Sri Lankan waters against the LPS-induced inflammatory responses in RAW 264.7 macrophages and in vivo Zebra fish embryo model. J. Natl. Sci. Found. 2018, 46, 117–124. [Google Scholar] [CrossRef]
- Koneri, R.; Jha, D.K. A Study on the Type II Antidiabetic Activity of Methanolic Extract of Marine Algae, Gracilaria edulis and Sargassum polycystum. Int. J. Pharm. Sci. Rev. Res. 2017, 47, 154–159. [Google Scholar]
- Patra, S.; Muthuraman, M.S. Gracilaria edulis extract induces apoptosis and inhibits tumor in Ehrlich Ascites tumor cells in vivo. BMC Complementary Altern. Med. 2013, 13, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Al-Marzoqi, A.H.; Hameed, I.M.; Ali Idan, S. Analysis of bioactive chemical components of two medicinal plants (Coriandrum sativum and Melia azedarach) leaves using gas chromatography-mass spectrometry (GC-MS). Afr. J. Biotchenol. 2015, 14, 2812–2830. [Google Scholar]
- Villalva, M.; Jaime, L.; Aguado, E.; Nieto, J.A.; Reglero, G.; Santoyo, S. Anti-Inflammatory and Antioxidant Activities from the Basolateral Fraction of Caco-2 Cells Exposed to a Rosmarinic Acid Enriched Extract. J. Agric. Food Chem. 2018, 66, 1167–1174. [Google Scholar] [CrossRef]
- Jalill, A.; Dh, R.; Jalill, A. GC-MS analysis of Calendula officinalis and cytotoxic effects of its flower crude extract on human epidermoid larynx carcinoma (hep-2). World J. Pharm. Pharm. Sci. 2014, 3, 237–275. [Google Scholar]
- Ali, N.; Dar, B.; Farooqui, M. Chemistry and Biology of Indoles and Indazoles: A Mini-Review. Med. Chem. 2012, 13, 1–16. [Google Scholar]
- World Intellectual Property Organization. Indole and Indazole derivatives as glycogen synthase activators. Int. Appl. Publ. Under Pat. Coop. Treaty 2011, 74, 1–10. [Google Scholar]
- Zeng, H.; Chen, X.; Liang, J. In vitro antifungal activity and mechanism of essential oil from fennel (Foeniculum vulgare L.) on dermatophyte species. J. Med. Microbiol. 2015, 64, 93–103. [Google Scholar] [CrossRef]
- Agirbas, H.; Budak, F. Synthesis and structure-antibacterial activity relationship studies of 4-substituted phenyl -4, 5-dihydrobenzo [f] [1, 4] oxazepin-3 (2H)-thiones. Med. Chem. Res. 2011, 20, 1170–1180. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Ohkoshi, M. Characteristics of antibacterial molecular activities in poplar wood extractives. Saudi J. Biol. Sci. 2017, 24, 399–404. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. J. Clin. Investig. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Ganesan, P.; Kumar, C.S.; Bhaskar, N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour. Technol. 2008, 99, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Fellah, F.; Louaileche, H.; Touati, N. Seasonal variations in the phenolic compound content and antioxidant activities of three selected species of seaweeds from Tiskerth islet, Bejaia, Algeria. J. Mater. Environ. Sci. 2017, 8, 4451–4456. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; Angelo, G.D.; Nicotera, A.; Parisi, E.; Rosa, G.D.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2015, 16, 378–400. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.G.; Dheeba, K.P.; Sivakumar, R. In vitro anti-diabetic, antioxidant and anti- inflammatory activity of Clitoria Ternatea, L. Artic. Int. J. Pharm. Pharm. Sci. 2014, 6, 342–347. [Google Scholar]
- Francavilla, M. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P. Antioxidant Activity of Caryota urens L. (Kithul) Sap. Trop. Agric. Res. 2012, 23, 117–125. [Google Scholar] [CrossRef]
- Bernaert, N. Antioxidant capacity, total phenolic and ascorbate content as a function of the genetic diversity of leek (Allium ampeloprasum var porrum). Food Chem. 2012, 134, 669–677. [Google Scholar] [CrossRef]
- Gulati, V.; Harding, I.H.; Palombo, E.A. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: Potential application in the management of hyperglycemia. Bmc Complementary Altern Med. 2012, 12, 2–9. [Google Scholar]
- Unnikrishnan, P.S.; Suthindhiran, K.; Jayasri, M.A. Antidiabetic potential of marine algae by inhibiting key metabolic enzymes. J. Front. Life Sci. 2015, 8, 148–159. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Sudha, S. Evaluation of Alpha-Amylase and Alpha-Glucosidase Inhibitory Properties of Selected Seaweeds from Gulf of Mannar. Irjponline. Com. 2012, 3, 128–130. [Google Scholar]
- Gallagher, A.M.; Flatt, P.R.; Duffy, G.; Abdel-Wahab, Y.H.A. The effects of traditional antidiabetic plants on in vitro glucose diffusion. Nutr. Res. 2003, 23, 413–424. [Google Scholar] [CrossRef]
- Sudasinghe, H.P.; Peiris, D.C.P. Hypoglycemic and hypolipidemic activity of aqueous leaf extract of Passiflora suberosa L. Peer J. 2018, 6, e4389. [Google Scholar]
- Navale, A.M.; Paranjape, A.N. Glucose transporters: physiological and pathological roles. Biophys Rev. 2016, 8, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk-Karolak, I.; Goła, G.; Gburek, J.; Wysokin, H.; Matkowski, A. Inhibition of Advanced Glycation End-Product Formation and Antioxidant Activity by Extracts and Polyphenols from Scutellaria alpina L. and S. altissima L. Molecules 2016, 21, 739. [Google Scholar] [CrossRef] [PubMed]
- Lakmal, H.; Samarakoon, K.; Lee, W.; Lee, J.; Abeytunga, D.; Lee, H.; Jeon, Y. Anticancer and antioxidant effects of selected Sri Lankan marine algae. J. Natl. Sci. Found. Sri Lanka 2014, 42, 117–124. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocaltue reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Ucen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by complementary colorimetric method. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Sreevidya, N.; Mehrotra, S. Spectrometric method for estimation of precipitable with Dragendorff’s reagent in plant material. J. Aoac Int. 2003, 36, 1124–1127. [Google Scholar]
- Blois, M.S. Antioxidant determination by use of stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization Assay. Free Rad. Biol. Med 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Carter, P. Spectrophotometric determination of serum iron at the sub-microgram level with a new reagent ferrozine. Annu. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Bernfeld, P. Amylases, alpha and beta. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1955; Volume 1, pp. 149–158. [Google Scholar]
- Matsui, T.; Ueda, T.; Oki, T.; Sugita, K.; Terahara, N.; Matsumoto, K. α-Glucosidase inhibitory action of natural acylated anthocyanins. J. Agric. Food Chem. 2001, 49, 1948–1951. [Google Scholar] [CrossRef]
- Roy, A.; Mahalingam, M.G. The in-vitro antidiabetic activity of Phoenix roebelenii leaf extract. Int. J. Green Pharm. 2017, 11, 884–890. [Google Scholar]
- Matsuura, N.; Aradate, T.; Sasaki, C.; Kojima, H.; Ohara, M.; Hasegawa, J.; Ubukata, M. Screening system for the Maillard reaction inhibitor from natural product extracts. J. Health Sci. 2002, 48, 520–526. [Google Scholar] [CrossRef]
- Jerkovi, I. Phytochemical study of the headspace volatile organic compounds of fresh algae and sea grass from the Adriatic Sea (single point collection). PLoS ONE 2008, 13, e0196462. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

| Extract/Fraction | TPC (µg GAE/g) | TFC (µg QE/g) | Total Alkaloids (µg PE/g) |
|---|---|---|---|
| Crude methanol extract | 1007.81 ± 54.21 a | 541.02 ± 51.84 a | 7177.72 ± 63.04 a |
| Hexane fraction | 760.85 ± 37.75 b | 688.60 ± 9.55 a | 1656.97 ± 45.80 b |
| Chloroform fraction | 560.85 ± 55.08 c | 289.39 ± 9.55 b | 2875.54 ± 22.29 c |
| Ethyl acetate fraction | 2414.51 ± 50.34 d | 1461.49 ± 75.22 c | 1073.75 ± 45.88 b |
| Aqueous fraction | 1704.69 ± 43.16 e | 786.95 ± 62.04 d | 522.34 ± 67.13 d |
| Extract/ Fraction | IC50 (mg/mL) | Activity Equivalent to Standard (mg TE/g) | |||
|---|---|---|---|---|---|
| DPPH | ABTS | FICA | FRAP | ORAC | |
| Crude methanol extract | 3.19 ± 0.02 a | 0.56 ± 0.01 a | 9.23 ± 0.19 a | 0.26 ± 0.03 a | 1.61 ± 0.19 a |
| Hexane fraction | 6.22 ± 0.01 b | 0.54 ± 0.01 b | 2.58 ± 0.03 bc | 1.93 ± 0.35 b | 0.57 ± 0.07 bc |
| Chloroform fraction | 3.29 ± 0.02 c | 0.44 ± 0.01 c | 2.43 ± 0.01 c | 2.19 ± 0.23 b | 0.77 ± 0.05 b |
| Ethyl acetate fraction | 3.17 ± 0.04 a | 0.41 ± 0.02 d | 2.22 ± 0.01 bc | 8.51 ± 0.09 c | 1.44 ± 0.29 a |
| Aqueous fraction | 3.91 ± 0.03 d | 0.45 ± 0.03 c | 2.71 ± 0.02 b | 1.23 ± 0.21 d | 0.44 ± 0.09 c |
| Trolox (standard) | 0.011 ± 0.00 e | 0.008 ± 0.00 e | - | - | - |
| EDTA (standard) | - | - | 0.019 ± 00 d | - | - |
| Extract/Fraction | Alpha-Amylase (µg/mL) | Alpha-Glucosidase (µg/mL) | Anti-Glycation (µg/mL) |
|---|---|---|---|
| Crude methanol extract | 349.59 ± 2.44 a | 102.24 ± 0.89 a | 702.33 ± 12.72 a |
| Hexane fraction | 393.04 ± 4.73 b | 163.90 ± 5.23 b | 637.53 ± 6.21 b |
| Chloroform fraction | 322.71 ± 4.80 c | 122.65 ± 2.37 c | 258.23 ± 3.24 c |
| Ethyl acetate fraction | 279.48 ± 5.62 d | 87.92 ± 1.62 d | 586.54 ± 4.37 b |
| Aqueous fraction | 376.49 ± 12.14 e | 148.57 ± 1.87 e | 723.78 ± 12.81 d |
| Acarbose (standard) | 87.43 ± 0.59 f | 0.38 ± 0.06 f | - |
| Rutin (standard) | - | - | 11.55 ± 0.82 e |
| Retention Time and % | Name | Molecular Formula | Compound Class | Reported Biological Activity |
|---|---|---|---|---|
| 17.956 (54.27%) | 2,5-Dimethylhexane-2,5-dihydroperoxide | C8H18O4 | Organic compound | Anti-inflammatory Antioxidant [14,15] |
| 55.726 (11.46%) | Phthalic acid-6-ethyloct-3-yl 2-ethylhexyl ester | C26H42O4 | Phthalic acid derivative | Anticancer Antimicrobial [16] |
| 66.305 (15.39%) | 1H-Indole-2-carboxylic acid,6-(4-ethoxyphenyl)-3-methyl-4-oxo-4,5,6,7-tetrahydro-isopropyl ester | C19H20FNO3 | Indole derivative | Antidiabetic [17,18] |
| 72.446 (10.93%) | 1,2-dimethoxy-4 (1,3-dimethoxy-1-propenyl) benzene | C13H18O4 | Benzene derivatives | Antifungal [19] |
| 73.130 (1.52%) | Benz(b)1,4-oxazepine-4 (5H)-thione, 2,3-dihydro-2,8-dimethyl | C11H13NOS | Benzoxazepine derivatives | Anti-inflammatory Antimicrobial [20] |
| 76.925 (6.49%) | 2-acetoxymethyl-3-(methoxycarbonyl)biphenylene | C17H14O4 | Biphenyl derivatives | Antibacterial [21] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunathilaka, T.L.; Samarakoon, K.W.; Ranasinghe, P.; Peiris, L.D.C. In-Vitro Antioxidant, Hypoglycemic Activity, and Identification of Bioactive Compounds in Phenol-Rich Extract from the Marine Red Algae Gracilaria edulis (Gmelin) Silva. Molecules 2019, 24, 3708. https://doi.org/10.3390/molecules24203708
Gunathilaka TL, Samarakoon KW, Ranasinghe P, Peiris LDC. In-Vitro Antioxidant, Hypoglycemic Activity, and Identification of Bioactive Compounds in Phenol-Rich Extract from the Marine Red Algae Gracilaria edulis (Gmelin) Silva. Molecules. 2019; 24(20):3708. https://doi.org/10.3390/molecules24203708
Chicago/Turabian StyleGunathilaka, Thilina L., Kalpa W. Samarakoon, P. Ranasinghe, and L. Dinithi C. Peiris. 2019. "In-Vitro Antioxidant, Hypoglycemic Activity, and Identification of Bioactive Compounds in Phenol-Rich Extract from the Marine Red Algae Gracilaria edulis (Gmelin) Silva" Molecules 24, no. 20: 3708. https://doi.org/10.3390/molecules24203708
APA StyleGunathilaka, T. L., Samarakoon, K. W., Ranasinghe, P., & Peiris, L. D. C. (2019). In-Vitro Antioxidant, Hypoglycemic Activity, and Identification of Bioactive Compounds in Phenol-Rich Extract from the Marine Red Algae Gracilaria edulis (Gmelin) Silva. Molecules, 24(20), 3708. https://doi.org/10.3390/molecules24203708