Synthesis and Glycosidase Inhibition of Broussonetine M and Its Analogues
Abstract
1. Introduction
2. Results and Discussion
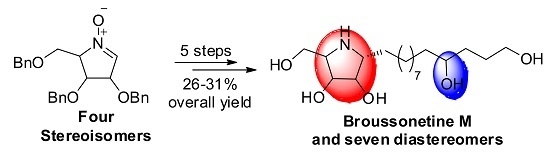
2.1. Synthesis of Broussonetine M
2.2. Synthesis of Analogues of Broussonetine M
2.3. Glycosidase Inhibition
3. Materials and Methods
3.1. General Methods
3.2. Material and Methods for the Enzyme Inhibition Assay
3.3. Chemistry
3.3.1. Synthesis of 4-(benzyloxy)butan-1-ol (21)
3.3.2. Synthesis of 4-(benzyloxy)butanal (16)
3.3.3. General Procedure for Synthesis of (R)-7-(benzyloxy)hept-1-en-4-ol (15) and (S)-7-(benzyloxy)hept-1-en-4-ol (ent-15), with Alcohol 15 as an Example
3.3.4. General Procedure for Synthesis of Compounds 19, ent-19, ent-3-epi-19, and 2-epi-19, with 19 as an Example
3.3.5. General Procedure for Synthesis of Compounds 13, ent-13, 3-epi-ent-13, and 2-epi-13, with 13 as an Example
3.3.6. General Procedure for Synthesis of Compounds 12, 10’-epi-12, ent-10’-epi-12, ent-12, ent-3,10’-di-epi-12, ent-3-epi-12, 2-epi-12, and 2,10’-di-epi-12, with 12 as an Example
3.3.7. General Procedure for Synthesis of Compounds 3, 10’-epi-3, ent-10’-epi-3, ent-3, ent-3,10’-di-epi-3, ent-3-epi-3, 2-epi-3, 2, and 10’-di-epi-3, with Broussonetine M (3) as an Axample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Compain, P.; Martin, O.R. Iminosugars: From Synthesis to Therapeutic Applications, 1st ed.; Wiley-VCH: Chichester, UK, 2007; pp. 328–446. [Google Scholar]
- Wrodnigg, T.M. From Lianas to Glycobiology Tools: Twenty-Five Years of 2,5-dideoxy-2,5-imino-d-mannitol. Mon. Chem. 2002, 133, 393–426. [Google Scholar] [CrossRef]
- Asano, N.; Nash, R.J.; Molyneux, R.J.; Fleet, G.W.J. Sugar-mimic glycosidase inhibitors: Natural occurrence, biological activity and prospects for therapeutic application. Tetrahedron Asymmetry 2000, 11, 1645–1680. [Google Scholar] [CrossRef]
- Watson, A.A.; Fleet, G.W.J.; Asano, N.; Molyneux, R.J.; Nash, R.J. Polyhydroxylated alkaloids—Natural occurrence and therapeutic applications. Phytochemistry 2001, 56, 265–295. [Google Scholar] [CrossRef]
- Horne, G.; Wilson, F.X.; Tinsley, J.; Williams, D.H.; Storer, R. Iminosugars past, present and future: Medicines for tomorrow. Drug Discov. Today 2010, 16, 107–118. [Google Scholar] [CrossRef]
- Nash, R.J.; Kato, A.; Yu, C.-Y.; Fleet, G.W.J. Iminosugars as Therapeutic Agents: Recent Advances and Promising Trends. Future Med. Chem. 2011, 3, 1513–1521. [Google Scholar]
- Davis, B.G. A silver-lined anniversary of Fleet iminosugars: 1984–2009, from DIM to DRAM to LABNAc. Tetrahedron Asymmetry 2009, 20, 652–671. [Google Scholar] [CrossRef]
- Butters, T.D.; Dwek, R.A.; Platt, F.M. Inhibition of Glycosphingolipid Biosynthesis: Application to Lysosomal Storage Disorders. Chem. Rev. 2000, 100, 4683–4696. [Google Scholar] [CrossRef]
- Nash, R.J.; Bell, E.A.; Williams, J.M. 2-Hydroxymethyl-3,4-dihydroxypyrrolidine in fruits of angylocalyx boutiqueanus. Phytochemistry 1985, 24, 1620–1622. [Google Scholar] [CrossRef]
- Welter, A.; Jadot, J.; Dardenne, G.; Marlier, M.; Casimir, J. 2,5-Dihydroxymethyl 3,4-dihydroxypyrrolidine dans les feuilles de Derris elliptica. Phytochemistry 1976, 15, 747–749. [Google Scholar] [CrossRef]
- Jones, D.W.C.; Nash, R.J.; Bell, E.A.; Williams, J.M. Identification of the 2-hydroxymethyl-3,4-dihydroxypyrrolidine (or 1,4-dideoxy-1,4-iminopentitol) from angylocalyx boutiqueanus and from arachniodes standishii as the (2R, 3R, 4S)-isomer by the synthesis of its enantiomer. Tetrahedron Lett. 1985, 26, 3125–3126. [Google Scholar] [CrossRef]
- Fleet, G.W.J.; Nicholas, S.J.; Smith, P.W.; Evans, S.V.; Fellows, L.E.; Nash, R.J. Potent competitive inhibition of α-galactosidase and α-glucosidase activity by 1,4-dideoxy-1,4-iminopentitols: Syntheses of 1,4-dideoxy-1,4-imino-d-lyxitol and of both enantiomers of 1,4-dideoxy-1,4-iminoarabinitol. Tetrahedron Lett. 1985, 26, 3127–3130. [Google Scholar] [CrossRef]
- Asano, N.; Kizu, H.; Oseki, K.; Tomioka, E.; Matsui, K.; Okamoto, M.; Baba, M. N-Alkylated Nitrogen-in-the-Ring Sugars: Conformational Basis of Inhibition of Glycosidases and HIV-1 Replication. J. Med. Chem. 1995, 38, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Merino, P.; Delso, I.; Tejero, T.; Cardona, F.; Marradi, M.; Faggi, E.; Parmeggiani, C.; Goti, A. Nucleophilic additions to cyclic nitrones en route to iminocyclitols - Total syntheses of DMDP, 6-deoxy-DMDP, DAB-1, CYB-3, nectrisine, and radicamine B. Eur. J. Org. Chem. 2008, 2929–2947. [Google Scholar] [CrossRef]
- Cruz, F.P.d.; Newberry, S.; Jenkinson, S.F.; Wormald, M.R.; Butters, T.D.; Alonzi, D.S.; Nakagawa, S.; Becq, F.; Norez, C.; Nash, R.J.; et al. 4-C-Me-DAB and 4-C-Me-LAB—enantiomeric alkyl-branched pyrrolidine iminosugars—are specific and potent α-glucosidase inhibitors; acetone as the sole protecting group. Tetrahedron Lett. 2011, 52, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Ayers, B.J.; Ngo, N.; Jenkinson, S.F.; Martínez, R.F.; Shimada, Y.; Adachi, I.; Weymouth-Wilson, A.C.; Kato, A.; Fleet, G.W.J. Glycosidase Inhibition by All 10 Stereoisomeric 2,5-Dideoxy-2,5-iminohexitols Prepared from the Enantiomers of Glucuronolactone. J. Org. Chem. 2012, 77, 7777–7792. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Westergaard, N.; Quistorff, B.; Grunnet, N.; Kristiansen, M.; Lundgren, K. Kinetic and Functional Characterization of 1,4-Dideoxy-1,4-imino-d-arabinitol: A Potent Inhibitor of Glycogen Phosphorylase with Anti-hyperglyceamic Effect in ob/ob Mice. Arch. Biochem. Biophys. 2000, 380, 274–284. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Asano, N.; Ikeda, K.; Wang, M.-X.; Butters, T.D.; Wormald, M.R.; Dwek, R.A.; Winters, A.L.; Nash, R.J.; Fleet, G.W.J. Looking glass inhibitors: l-DMDP, a more potent and specific inhibitor of alpha-glucosidases than the enantiomeric natural product DMDP. Chem. Commun. 2004, 1936–1937. [Google Scholar] [CrossRef]
- Wang, G.-N.; Yang, L.; Zhang, L.-H.; Ye, X.-S. A Versatile Approach to N-Alkylated 1,4-Dideoxy-1,4-imino-d-arabinitols and 1,4-Dideoxy-1,4-imino-l-xylitols. J. Org. Chem. 2011, 76, 2001–2009. [Google Scholar] [CrossRef]
- Bouillon, M.E.; Pyne, S.G. Diastereoselective concise syntheses of the polyhydroxylated alkaloids DMDP and DAB. Tetrahedron Lett. 2014, 55, 475–478. [Google Scholar] [CrossRef]
- Li, Y.-X.; Kinami, K.; Hirokami, Y.; Kato, A.; Su, J.-K.; Jia, Y.-M.; Fleet, G.W.J.; Yu, C.-Y. Gem-difluoromethylated and trifluoromethylated derivatives of DMDP-related iminosugars: Synthesis and glycosidase inhibition. Org. Biomol. Chem. 2016, 14, 2249–2263. [Google Scholar] [CrossRef]
- Jenkinson, S.F.; Best, D.; Saville, A.W.; Mui, J.; Martínez, R.F.; Nakagawa, S.; Kunimatsu, T.; Alonzi, D.S.; Butters, T.D.; Norez, C.; et al. C-Branched Iminosugars: α-Glucosidase Inhibition by Enantiomers of isoDMDP, isoDGDP, and isoDAB–l-isoDMDP Compared to Miglitol and Miglustat. J. Org. Chem. 2013, 78, 7380–7397. [Google Scholar] [CrossRef] [PubMed]
- Best, D.; Jenkinson, S.F.; Saville, A.W.; Alonzi, D.S.; Wormald, M.R.; Butters, T.D.; Norez, C.; Becq, F.; Blériot, Y.; Adachi, I.; et al. Cystic fibrosis and diabetes: isoLAB and isoDAB, enantiomeric carbon-branched pyrrolidine iminosugars. Tetrahedron Lett. 2010, 51, 4170–4174. [Google Scholar] [CrossRef]
- Matassini, C.; Vanni, C.; Goti, A.; Morrone, A.; Marradi, M.; Cardona, F. Multimerization of DAB-1 onto Au GNPs affords new potent and selective N-acetylgalactosamine-6-sulfatase (GALNS) inhibitors. Org. Biomol. Chem. 2018, 16, 8604–8612. [Google Scholar] [CrossRef] [PubMed]
- D’Adamio, G.; Forcella, M.; Fusi, P.; Parenti, P.; Matassini, C.; Ferhati, X.; Vanni, C.; Cardona, F. Probing the Influence of Linker Length and Flexibility in the Design and Synthesis of New Trehalase Inhibitors. Molecules 2018, 23, 436. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, S. Recent Advances in Synthetic -Glucosidase Inhibitors. Chemmedchem 2017, 12, 819–829. [Google Scholar] [CrossRef]
- Li, Y.-X.; Shimada, Y.; Adachi, I.; Kato, A.; Jia, Y.-M.; Fleet, G.W.J.; Xiao, M.; Yu, C.-Y. Fluorinated and Conformationally Fixed Derivatives of l-HomoDMDP: Synthesis and Glycosidase Inhibition. J. Org. Chem. 2015, 80, 5151–5158. [Google Scholar] [CrossRef]
- Ghavami, A.; Johnston, B.D.; Jensen, M.T.; Svensson, B.; Pinto, B.M. Synthesis of Nitrogen Analogues of Salacinol and Their Evaluation as Glycosidase Inhibitors. J. Am. Chem. Soc. 2001, 123, 6268–6271. [Google Scholar] [CrossRef]
- Kato, A.; Nakagome, I.; Sato, K.; Yamamoto, A.; Adachi, I.; Nash, R.J.; Fleet, G.W.J.; Natori, Y.; Watanabe, Y.; Imahori, T.; et al. Docking study and biological evaluation of pyrrolidine-based iminosugars as pharmacological chaperones for Gaucher disease. Org. Biomol. Chem. 2016, 14, 1039–1048. [Google Scholar] [CrossRef]
- Shibano, M.; Kitagawa, S.; Kusano, G. Studies on the constituents of Broussonetia species. 1. Two new pyrrolidine alkaloids, broussonetines C and D, as beta-galactosidase and beta-mannosidase inhibitors from Broussonetia kazinoki SIEB. Chem. Pharm. Bull. 1997, 45, 505–508. [Google Scholar] [CrossRef]
- Shibano, M.; Kitagawa, S.; Nakamura, S.; Akazawa, N.; Kusano, G. Studies on the Constituents of Broussonetia Species. II. Six New Pyrrolidine Alkaloids, Broussonetine A, B, E, F and Broussonetinine A and B, as Inhibitors of Glycosidases from Broussonetia kazinoki SIEB. Chem. Pharm. Bull. 1997, 45, 700–705. [Google Scholar] [CrossRef]
- Shibano, M.; Nakamura, S.; Akazawa, N.; Kusano, G. Studies on the Constituents of Broussonetia Species. III. Two New Pyrrolidine Alkaloids, Broussonetines G and H, as Inhibitors of Glycosidase, from Broussonetia kazinoki SIEB. Chem. Pharm. Bull. 1998, 46, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Shibano, M.; Nakamura, S.; Kubori, M.; Minoura, K.; Kusano, G. Studies on the constituents of Broussonetia species. IV. Two new pyrrolidinyl piperidine alkaloids, broussonetines I and J, from Broussonetia kazinoki SIEB. Chem. Pharm. Bull. 1998, 46, 1416–1420. [Google Scholar] [CrossRef]
- Shibano, M.; Nakamura, S.; Motoya, N.; Kusano, G. Studies on the constituents of Broussonetia species. V. Two new pyrrolidine alkaloids, broussonetines K and L, as inhibitors of glycosidase, from Broussonetia kazinoki SIEB. Chem. Pharm. Bull. 1999, 47, 472–476. [Google Scholar] [CrossRef]
- Shibano, M.; Tsukamoto, D.; Kusano, G. A new pyrrolizidine alkaloid, Broussonetine N, as an inhibitor of glycosidase, from Broussonetia kazinoki Sieb. and absolute stereostructures of Broussonetines A and B. Chem. Pharm. Bull. 1999, 47, 907–908. [Google Scholar] [CrossRef]
- Shibano, M.; Tsukamoto, D.; Fujimoto, R.; Masui, Y.; Sugimoto, H.; Kusano, G. Studies on the Constituents of Broussonetia Species. VII. Four New Pyrrolidine Alkaloids, Broussonetines M, O, P, and Q, as Inhibitors of Glycosidase, from Broussonetia kazinoki SIEB. Chem. Pharm. Bull. 2000, 48, 1281–1285. [Google Scholar] [CrossRef]
- Tsukamoto, D.; Shibano, M.; Okamoto, R.; Kusano, G. Studies on the constituents of Broussonetia species VIII. Four new pyrrolidine alkaloids, broussonetines R, S, T, and V and a new pyrroline alkaloid, broussonetine U, from Broussonetia kazinoki Sieb. Chem. Pharm. Bull. 2001, 49, 492–496. [Google Scholar] [CrossRef]
- Shibano, M.; Tsukamoto, D.; Kusano, G. Polyhydroxylated Alkaloids with Lipophilic Moieties as Glycosidase Inhibitors from Higher Plants. Heterocycles 2002, 57, 1539–1553. [Google Scholar]
- Zhao, H.; Kato, A.; Sato, K.; Jia, Y.-M.; Yu, C.-Y. Total synthesis and glycosidase inhibition of broussonetine I and J2. J. Org. Chem. 2013, 78, 7896–7902. [Google Scholar] [CrossRef]
- Song, Y.-Y.; Kinami, K.; Kato, A.; Jia, Y.-M.; Li, Y.-X.; Fleet, G.W.J.; Yu, C.-Y. First total synthesis of (+)-broussonetine W: Glycosidase inhibition of natural product & analogs. Org. Biomol. Chem. 2016, 14, 5157–5174. [Google Scholar]
- D’Alonzo, D.; Guaragna, A.; Palumbo, G. Glycomimetics at the mirror: Medicinal chemistry of l-iminosugars. Curr. Med. Chem. 2009, 16, 473–505. [Google Scholar] [CrossRef]
- Best, D.; Wang, C.; Weymouth-Wilson, A.C.; Clarkson, R.A.; Wilson, F.X.; Nash, R.J.; Miyauchi, S.; Kato, A.; Fleet, G.W.J. Looking glass inhibitors: Scalable syntheses of DNJ, DMDP, and (3R)-3-hydroxy- l-bulgecinine from d-glucuronolactone and of l-DNJ, l-DMDP, and (3S)-3-hydroxy-d-bulgecinine from l-glucuronolactone. DMDP inhibits β-glucosidases and β-galactosidases whereas l-DMDP is a potent and specific inhibitor of α-glucosidases. Tetrahedron Asymmetry 2010, 21, 311–319. [Google Scholar]
- Mohan, S.; Eskandari, R.; Pinto, B.M. Naturally Occurring Sulfonium-Ion Glucosidase Inhibitors and Their Derivatives: A Promising Class of Potential Antidiabetic Agents. Acc. Chem. Res. 2014, 47, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Boisson, J.; Thomasset, A.; Racine, E.; Cividino, P.; Sainte-Luce, T.B.; Poisson, J.-F.; Behr, J.-B.; Py, S. Hydroxymethyl-Branched Polyhydroxylated Indolizidines: Novel Selective alpha-Glucosidase Inhibitors. Org. Lett. 2015, 17, 3662–3665. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Zhang, Z.-L.; Wang, H.-Y.; Jia, Y.-M.; Yu, C.-Y.; Kinami, K.; Hirokami, Y.; Tsuji, Y.; Adachi, I.; Nash, R.J.; et al. Design and Synthesis of Labystegines, Hybrid Iminosugars from LAB and Calystegine, as Inhibitors of Intestinal α-Glucosidases: Binding Conformation and Interaction for ntSI. J. Org. Chem. 2015, 80, 4501–4515. [Google Scholar] [CrossRef] [PubMed]
- Jackova, D.; Martinkova, M.; Gonda, J.; Vilkova, M.; Pilatova, M.B.; Takac, P. Stereoselective synthesis and anticancer activity of broussonetine analogues. Tetrahedron Asymmetry 2017, 28, 1175–1182. [Google Scholar] [CrossRef]
- Ribes, C.; Falomir, E.; Murga, J.; Carda, M.; Alberto Marco, J. Convergent, stereoselective syntheses of the glycosidase inhibitors broussonetines D and M. Org. Biomol. Chem. 2009, 7, 1355–1360. [Google Scholar] [CrossRef]
- D’Adamio, G.; Parmeggiani, C.; Goti, A.; Moreno-Vargas, A.J.; Moreno-Clavijo, E.; Robina, I.; Cardona, F. 6-Azido hyacinthacine A(2) gives a straightforward access to the first multivalent pyrrolizidine architectures. Org. Biomol. Chem. 2014, 12, 6250–6266. [Google Scholar] [CrossRef]
- Lahiri, R.; Ansari, A.A.; Vankar, Y.D. Recent developments in design and synthesis of bicyclic azasugars, carbasugars and related molecules as glycosidase inhibitors. Chem. Soc. Rev. 2013, 42, 5102–5118. [Google Scholar] [CrossRef]
- Lahiri, R.; Palanivel, A.; Kulkarni, S.A.; Vankar, Y.D. Synthesis of Isofagomine-Pyrrolidine Hybrid Sugars and Analogues of (-)-Steviamine and (+)-Hyacinthacine C-5 Using 1,3-Dipolar Cycloaddition Reactions. J. Org. Chem. 2014, 79, 10786–10800. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Choi, T.L.; Sanders, D.P.; Grubbs, R.H. A general model for selectivity in olefin cross metathesis. J. Am. Chem. Soc. 2003, 125, 11360–11370. [Google Scholar] [CrossRef]
- Wennekes, T.; van den Berg, R.J.B.H.N.; Boltje, T.J.; Donker-Koopman, W.E.; Kuijper, B.; van der Marel, G.A.; Strijland, A.; Verhagen, C.P.; Aerts, J.M.F.G.; Overkleeft, H.S. Synthesis and Evaluation of Lipophilic Aza-C-glycosides as Inhibitors of Glucosylceramide Metabolism. Eur. J. Org. Chem. 2010, 1258–1283. [Google Scholar] [CrossRef]
- Wang, W.-B.; Huang, M.-H.; Li, Y.-X.; Rui, P.-X.; Hu, X.-G.; Zhang, W.; Su, J.-K.; Zhang, Z.-L.; Zhu, J.-S.; Xu, W.-H.; et al. A Practical Synthesis of Sugar-derived Cyclic Nitrones: Powerful Synthons for the Synthesis of Iminosugars. Synlett 2010, 3, 488–492. [Google Scholar]
- Cardona, F.; Faggi, E.; Liguori, F.; Cacciarini, M.; Goti, A. Total syntheses of hyacinthacine A2 and 7-deoxycasuarine by cycloaddition to a carbohydrate derived nitrone. Tetrahedron Lett. 2003, 44, 2315–2318. [Google Scholar] [CrossRef]
- Carmona, A.T.; Whightman, R.H.; Robina, I.; Vogel, P. Synthesis and glycosidase inhibitory activity of 7-deoxycasuarine. Helv. Chim. Acta. 2003, 86, 3066–3073. [Google Scholar] [CrossRef]
- Tsou, E.-L.; Chen, S.-Y.; Yang, M.-H.; Wang, S.-C.; Cheng, T.-R.-R.; Cheng, W.-C. Synthesis and biological evaluation of a 2-aryl polyhydroxylated pyrrolidine alkaloid-based library. Bioorg. Med. Chem. 2008, 16, 10198–10204. [Google Scholar] [CrossRef]
- Tsou, E.-L.; Yeh, Y.-T.; Liang, P.-H.; Cheng, W.-C. A convenient approach toward the synthesis of enantiopure isomers of DMDP and ADMDP. Tetrahedron 2009, 65, 93–100. [Google Scholar] [CrossRef]
- Keck, G.E.; Geraci, L.S. Catalytic asymmetric allylation (CAA) reactions. II. A new enantioselective allylation procedure. Tetrahedron Lett. 1993, 34, 7827–7828. [Google Scholar] [CrossRef]
- Bhoite, S.P.; Kamble, R.B.; Suryavanshi, G.M. An enantioselective synthesis of (+)-hygroline and (+)-pseudohygroline via Keck allylation and CBS reduction. Tetrahedron Lett. 2015, 56, 4704–4705. [Google Scholar] [CrossRef]
- Wormald, M.R.; Nash, R.J.; Hrnciar, P.; White, J.D.; Molyneux, R.J.; Fleet, G.W.J. Configurational and conformational analysis of highly oxygenated pyrrolizidines: Definitive identification of some naturally occurring 7a-epi-alexines. Tetrahedron Asymmetry 1998, 9, 2549–2558. [Google Scholar] [CrossRef]
- Donohoe, T.J.; Sintim, H.O.; Hollinshead, J. A Noncarbohydrate Based Approach to Polyhydroxylated Pyrrolidizines: Total Syntheses of the Natural Products Hyacinthacine A1 and 1-Epiaustraline. J. Org. Chem. 2005, 70, 7297–7304. [Google Scholar] [CrossRef]
- Calveras, J.; Egido-Gabás, M.; Gómez, L.; Casas, J.; Parella, T.; Joglar, J.; Bujons, J.; Clapés, P. Dihydroxyacetone Phosphate Aldolase Catalyzed Synthesis of Structurally Diverse Polyhydroxylated Pyrrolidine Derivatives and Evaluation of their Glycosidase Inhibitory Properties. Chem. Eur. J. 2009, 15, 7310–7328. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Acuto, O.; Storelli, C.; Murer, H.; Semenza, G.A. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of d-glucose and choline transport systems. Biochim. Biophys. Acta 1978, 506, 136–154. [Google Scholar] [CrossRef]
Sample Availability: Not available. |






| Entry | Cyclic Nitrone | Pyrrolidine | Yield a | Alcohol | Product | Yield b |
|---|---|---|---|---|---|---|
| 1 |  |  | 64% |  |  | 43% |
| 2 |  |  | 43% | |||
| 3 |  |  | 65% |  |  | 41% |
| 4 |  |  | 45% | |||
| 5 |  |  | 64% |  |  | 40% |
| 6 |  |  | 41% | |||
| 7 |  |  | 71% |  |  | 43% |
| 8 |  |  | 43% |
| Enzyme | IC50 (μM) | |||||||
|---|---|---|---|---|---|---|---|---|
 |  |  |  |  |  |  |  | |
| DAB (1) [15] | DMDP (2) [16] | 3 | 10’-epi-3 | LAB (ent-1) [15] | l-DMDP (ent-2) [16] | ent-10’-epi-3 | ent-3 | |
| α-Glucosidase | ||||||||
| Yeast | 0.15 | NI a (15.6%) b | NI (2.6%) | NI (4.6%) | 70 | NI (34.9%) | NI (1.9%) | NI (1.9%) |
| Rice | 250 | 214 | NI (0%) | NI (0%) | 3.2 | 5.8 | 1.3 | 1.2 |
| Rat intestinal maltase | 55 | 290 | NI (0%) | NI (26.4%) | 0.93 | 1.2 | 0.18 | 0.29 |
| β-Glucosidase | ||||||||
| Almond | 250 | 10 | NI (10.0%) | NI (29.1%) | NI | NI (12.1%) | NI (8.0%) | NI (9.6%) |
| Bovine liver | 638 | 9.7 | 6.3 | 0.8 | NI (13%) | NI (4.6%) | 51 | NI (46.5%) |
| α-Galactosidase | ||||||||
| Coffee beans | NI (7%) | NI (10.5%) | NI (0%) | NI (0%) | NI (2%) | NI (13.8%) | NI (12.2%) | NI (0.3%) |
| β-Galactosidase | ||||||||
| Bovine liver | NI (37%) | 3.3 | 2.3 | 0.2 | NI (16%) | NI (0%) | 9.0 | 50 |
| α-Mannosidase | ||||||||
| Jack bean | 320 | NI (31.5%) | NI (2.5%) | NI (0%) | NI (0%) | NI (17.3%) | NI (1.8%) | NI (2.2%) |
| β-Mannosidase | ||||||||
| Snail | NI (10%) | 721 | NI (1.7%) | NI (0%) | NI (1%) | NI (12.6%) | NI (2.0%) | NI (0%) |
| α-l-Fucosidase | ||||||||
| Bovine kidney | NI (11%) | NI (37.2%) | NI (0%) | NI (0%) | NI (0%) | NI (0%) | NI (6.1%) | NI (0%) |
| α,α-Trehalase | ||||||||
| Porcine kidney | 61 | 200 | NI (0%) | NI (0%) | 75 | 48 | NI (0%) | NI (2.1%) |
| Amyloglucosidase | ||||||||
| A. niger | 362 | — | NI (3.8%) | NI (36.5%) | NI (41%) | — | NI (6.1%) | NI (0%) |
| α-l-Rhamnosidase | ||||||||
| P. decumbens | NI (5%) | NI (24.1%) | NI (6.1%) | NI (4.7%) | 803 | NI (45.6%) | NI (1.8%) | NI (13.3%) |
| β-Glucuronidase | ||||||||
| E.coli | —c | NI (0.4%) | 86 | 20 | — | NI (0.8%) | NI (36.2%) | NI (44.3%) |
| Bovine liver | — | — | NI (18.4%) | NI (17.2%) | — | — | NI (14.9%) | NI (2.3%) |
| Enzyme | IC50 (μM) | |||||
|---|---|---|---|---|---|---|
 |  |  |  |  |  | |
| l-altro-DMDP [16] | ent-3,10-di-epi-3 | ent-3-epi-3 | d-gluco-DMDP [16] | 2-epi-3 | 2,10-di-epi-3 | |
| α-Glucosidase | ||||||
| Yeast | NI a (35.1%) b | NI (6.6%) | NI (0%) | 167 | NI (1.6%) | NI (3.2%) |
| Rice | NI (9.5%) | NI (9.5%) | NI (23.0%) | 131 | NI (5.6%) | 12 |
| Rat intestinal maltase | 754 | 84 | 42 | 138 | NI (16.8%) | 1.4 |
| β-Glucosidase | ||||||
| Almond | NI (15.9%) | NI (7.3%) | NI (4.6%) | 256 | NI (8.6%) | NI (10.9%) |
| Bovine liver | NI (10.3%) | 68 | 71 | 523 | 23 | 33.9 |
| α-Galactosidase | ||||||
| Coffee beans | 120 | NI (0%) | NI (0%) | NI (38.4%) | NI (0%) | NI (0%) |
| β-Galactosidase | ||||||
| Bovine liver | NI (5.9%) | 31 | 41 | 361 | 7.4 | 13 |
| α-Mannosidase | ||||||
| Jack bean | NI (37.6%) | NI (0%) | NI (1.3%) | NI (13.3%) | NI (0%) | NI (0.32%) |
| β-Mannosidase | ||||||
| Snail | NI (3.7%) | NI (2.3%) | NI (0%) | NI (14.6%) | NI (0%) | NI (0%) |
| α- l-Fucosidase | ||||||
| Bovine kidney | 205 | 98 | 50 | NI (37.2%) | NI (4.3%) | NI (10.8%) |
| α,α-Trehalase | ||||||
| Porcine kidney | 1000 | NI (3.8%) | NI (0%) | 379 | NI (0%) | NI (0%) |
| Amyloglucosidase | ||||||
| A. niger | — c | NI (0%) | NI (0%) | — | NI (3.8%) | NI (6.8%) |
| α-l-Rhamnosidase | ||||||
| P. decumbens | 91 | NI (11.4%) | NI (15.2%) | NI (24.1%) | NI (3.3%) | NI (4.7%) |
| β-Glucuronidase | ||||||
| E.coli | NI (2.0%) | 42 | NI (28.7%) | NI (5.1%) | 73 | 80 |
| Bovine liver | — | NI (14.9%) | NI (0%) | — | NI (5.2%) | NI (1.7%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.-K.; Kinami, K.; Kato, A.; Li, Y.-X.; Jia, Y.-M.; Fleet, G.W.J.; Yu, C.-Y. Synthesis and Glycosidase Inhibition of Broussonetine M and Its Analogues. Molecules 2019, 24, 3712. https://doi.org/10.3390/molecules24203712
Wu Q-K, Kinami K, Kato A, Li Y-X, Jia Y-M, Fleet GWJ, Yu C-Y. Synthesis and Glycosidase Inhibition of Broussonetine M and Its Analogues. Molecules. 2019; 24(20):3712. https://doi.org/10.3390/molecules24203712
Chicago/Turabian StyleWu, Qing-Kun, Kyoko Kinami, Atsushi Kato, Yi-Xian Li, Yue-Mei Jia, George W. J. Fleet, and Chu-Yi Yu. 2019. "Synthesis and Glycosidase Inhibition of Broussonetine M and Its Analogues" Molecules 24, no. 20: 3712. https://doi.org/10.3390/molecules24203712
APA StyleWu, Q.-K., Kinami, K., Kato, A., Li, Y.-X., Jia, Y.-M., Fleet, G. W. J., & Yu, C.-Y. (2019). Synthesis and Glycosidase Inhibition of Broussonetine M and Its Analogues. Molecules, 24(20), 3712. https://doi.org/10.3390/molecules24203712