Pseudolaric Acid B Induces Growth Inhibition and Caspase-Dependent Apoptosis on Head and Neck Cancer Cell lines through Death Receptor 5
Abstract
:1. Introduction
2. Results
2.1. Effect of PAB on the Viability of HN22 Human HNC Cell Line
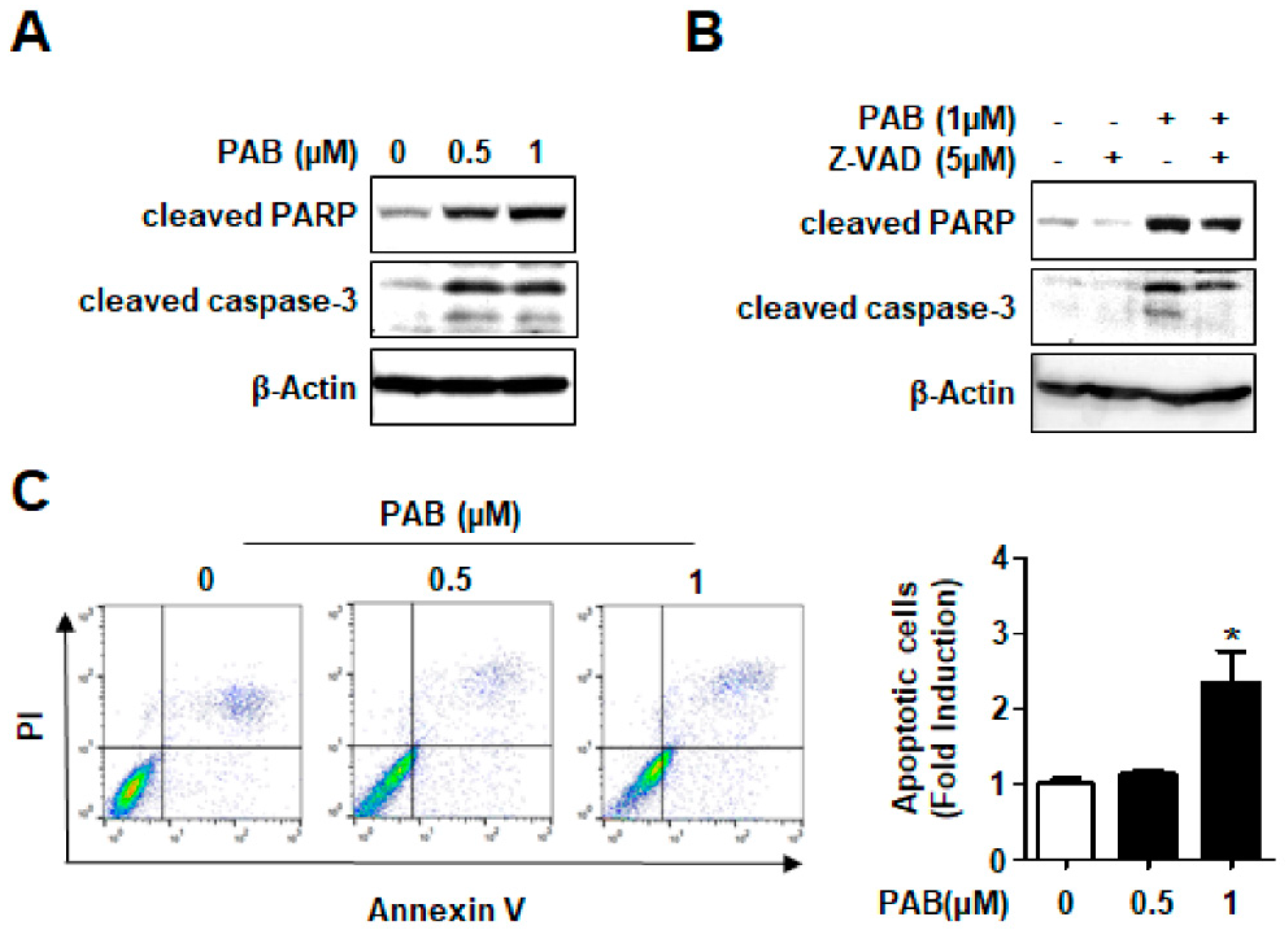
2.2. Effect of PAB on Caspase-Dependent Apoptosis in HN22 Human HNC Cell Line
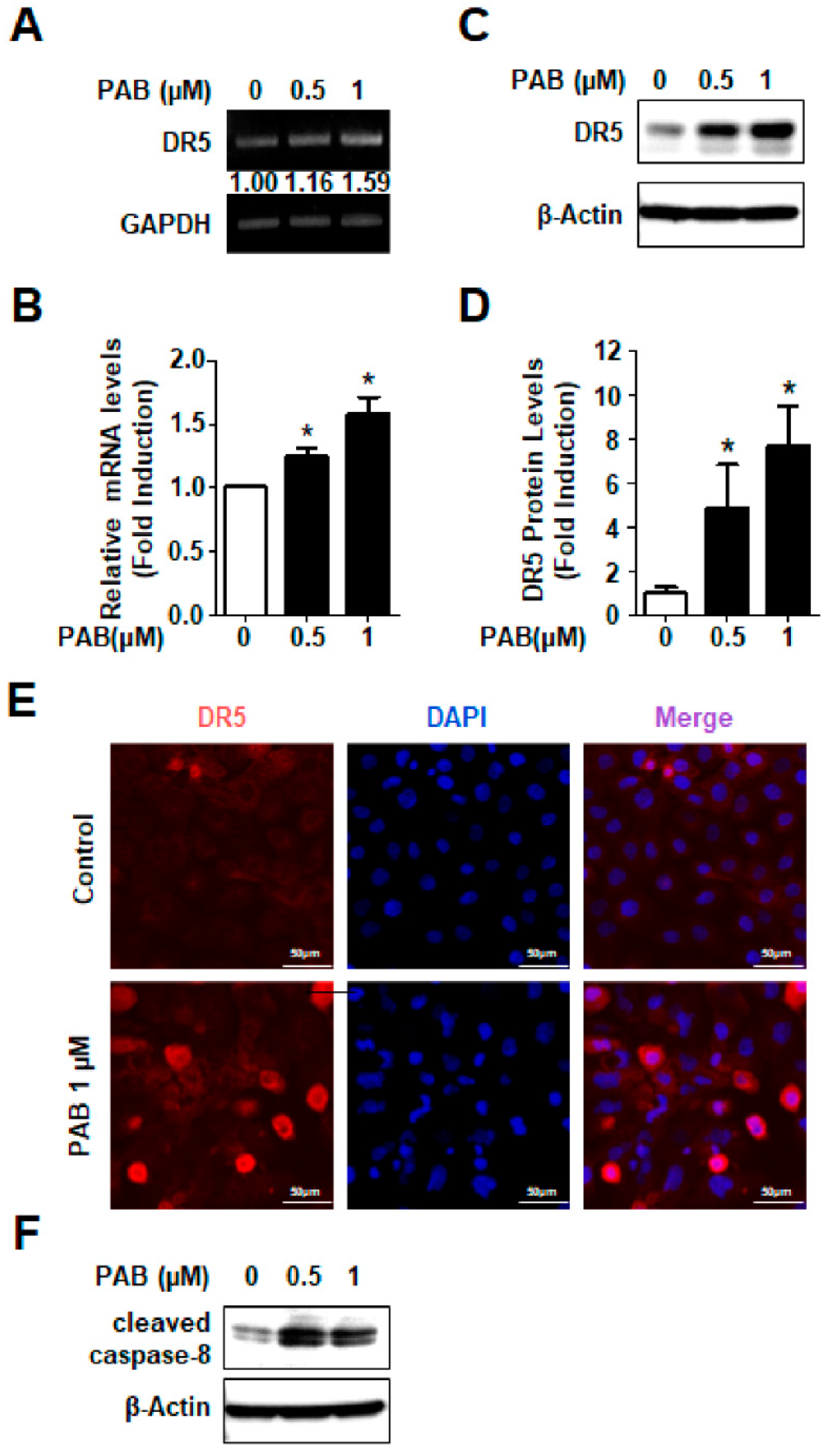
2.3. Involvement of DR5 in PAB-Induced Apoptosis in HN22 Human HNC Cell Line
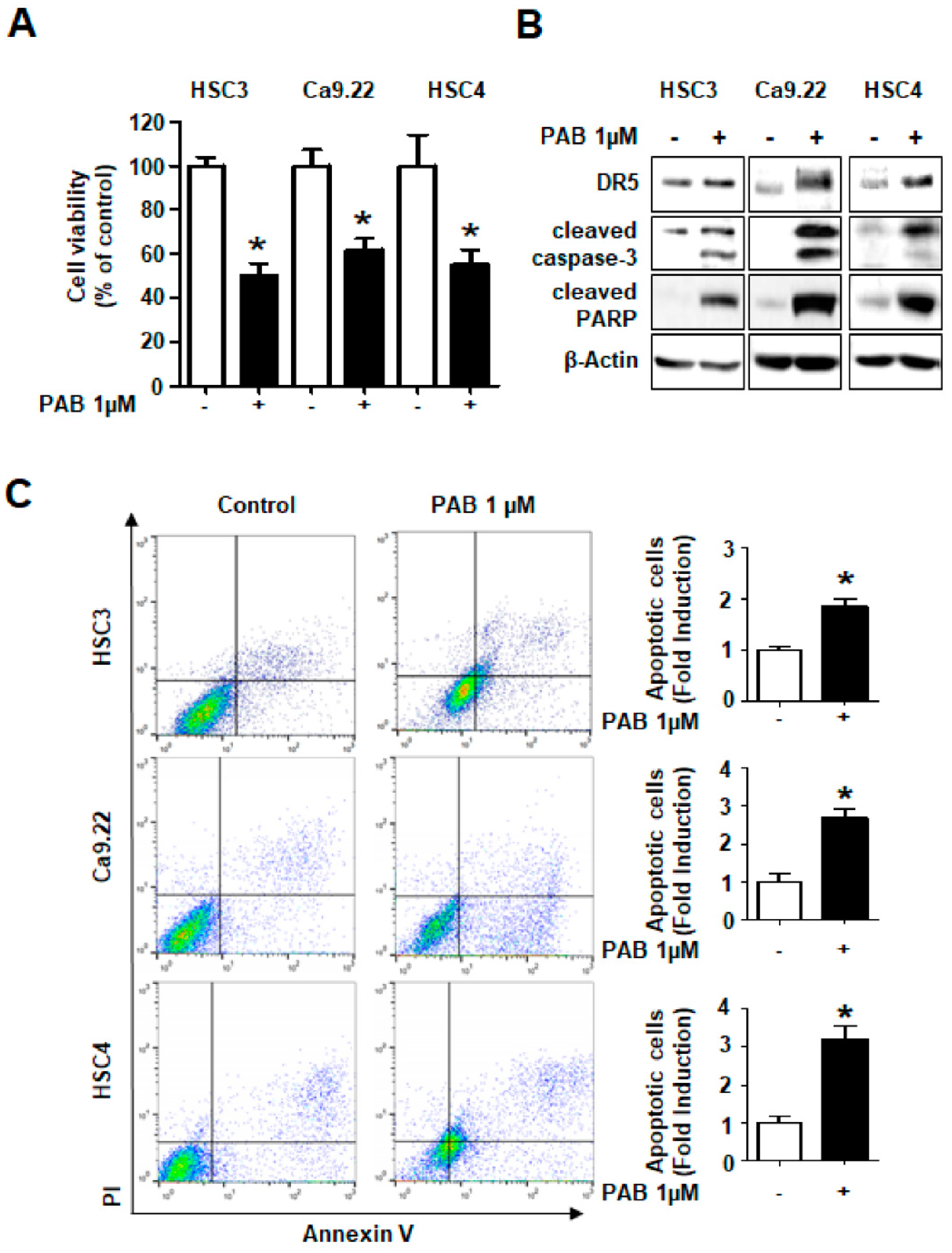
2.4. Growth-Inhibitory and Apoptotic Effects of PAB via DR5 Human HNC Cell Lines
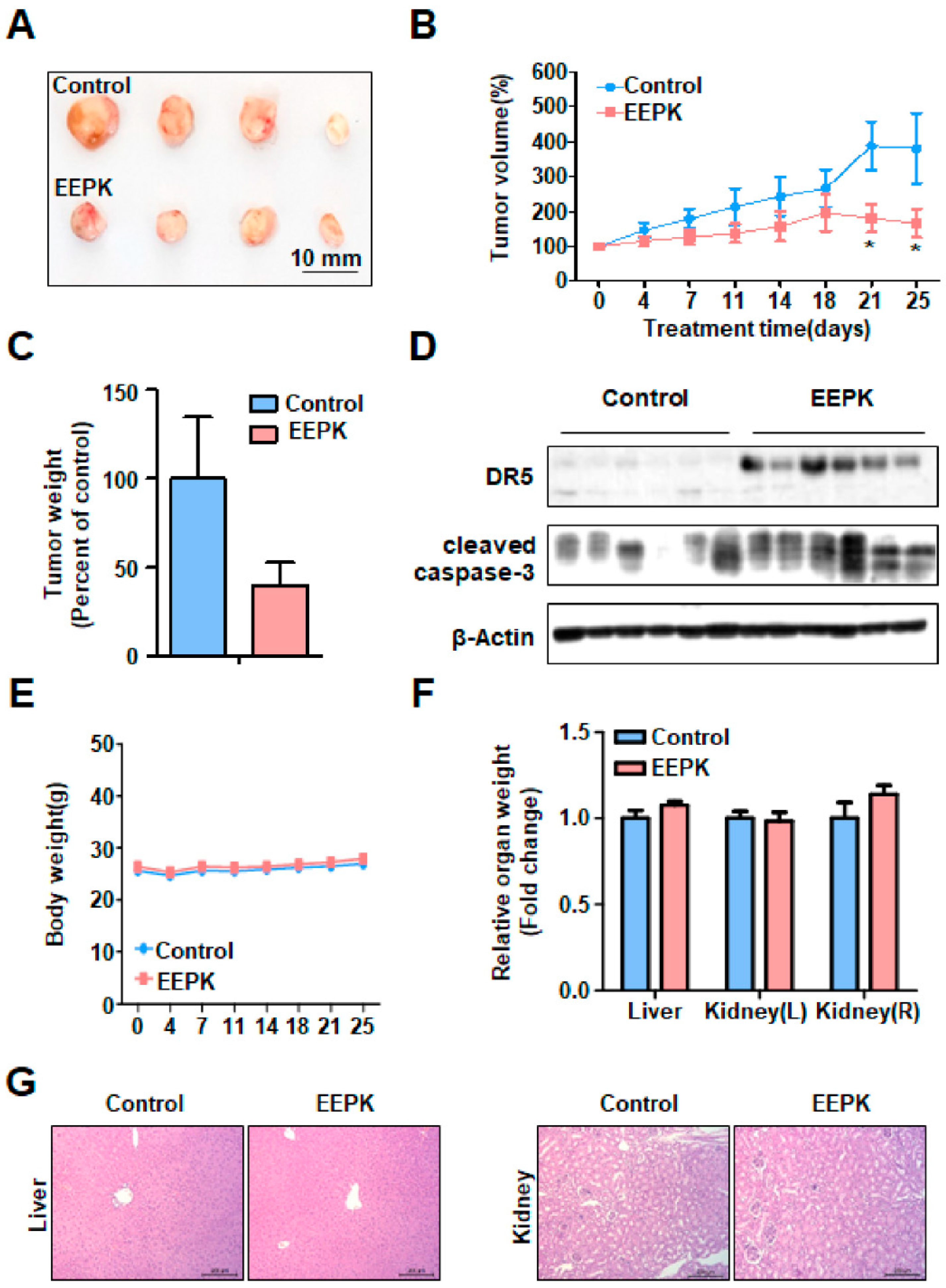
2.5. Anti-Tumorigenic Effect of EEPK in a Tumor Xenograft Model Bearing HN22 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Subsection Chemicals and Antibodies
4.3. Trypan Blue Exclusion Assay
4.4. Live/Dead Assay
4.5. Western Blot Analysis
4.6. Annexin V/PI Double Staining
4.7. Quantitative Real-Time PCR
4.8. Reverse Transcription-Polymerase Chain Reaction
4.9. Immunofluorescence Staining
4.10. Tumor Xenograft Model
4.11. Histopathological Examination of Organs
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.-W.; Zhang, Q.; Guo, Z.-M.; Chen, W.-K.; Liu, W.-W.; Chen, Y.-F.; Li, Q.-L.; Liu, X.-K.; Li, H.; Ou-Yang, D.; et al. Trends in clinical features and survival of oral cavity cancer: Fifty years of experience with 3,362 consecutive cases from a single institution. Cancer Manag. Res. 2018, 10, 4523–4535. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, V.N. Global Scenario of Research in Oral Cancer. J. Maxillofac. Oral Surg. 2019, 18, 354–359. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Trendowski, M. Recent advances in the development of antineoplastic agents derived from natural products. Drugs 2015, 75, 1993–2016. [Google Scholar] [CrossRef]
- Pan, D.J.; Li, Z.L.; Hu, C.Q.; Chen, K.; Chang, J.J.; Lee, K.H. The cytotoxic principles of Pseudolarix kaempferi: Pseudolaric acid-A and -B and related derivatives. Planta Med. 1990, 56, 383–385. [Google Scholar] [CrossRef]
- Chiu, P.; Leung, L.T.; Ko, B.C. Pseudolaric acids: Isolation, bioactivity and synthetic studies. Nat. Prod. Rep. 2010, 7, 1066–1083. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.-C.; Luo, H.; Zhao, L.; Zhang, Z.-D.; Shen, X.-F. Pseudolaric acid B exhibits anti-cancer activity on human hepatocellular carcinoma through inhibition of multiple carcinogenic signaling pathways. Phytomedicine 2018, 59. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Song, W.; Tian, Z.; Yang, L.; Niu, Q.; Zhang, Q.; Xie, M.; Zhou, B.; Xu, Y.; et al. Pseudolaric Acid B Inhibits Proliferation, Invasion and Epithelial-to-Mesenchymal Transition in Human Pancreatic Cancer Cell. Yonsei Med. J. 2018, 59, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Tian, Y.; Feng, W.; Zhao, L.; Zhao, M.; Liu, J.; Wang, Q. Pseudolaric acid B induces endometrial cancer Ishikawa cell apoptosis and inhibits metastasis through AKT-GSK-3beta and ERK1/2 signaling pathways. Anticancer Drugs 2017, 28, 603–612. [Google Scholar] [CrossRef]
- Liu, M.L.; Sun, D.; Li, T.; Chen, H. A Systematic Review of the Immune-Regulating and Anticancer Activities of Pseudolaric Acid B. Front. Pharmacol. 2017, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hong, L. Pseudolaric acid B exerts antitumor activity via suppression of the Akt signaling pathway in HeLa cervical cancer cells. Mol. Med. Rep. 2015, 12, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yue, D.M.; Shu, L.H.; Li, N.J.; Wang, J.H. Pseudolaric acid B induces caspase-dependent cell death in human ovarian cancer cells. Oncol. Rep. 2014, 31, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Yang, Y. Role of pseudolaric acid B in A549 lung cancer cell proliferation and apoptosis. Mol. Med. Rep. 2014, 9, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Yin, S.; Dong, Y.; Guo, X.; Fan, L.; Ye, M.; Hu, H. Pseudolaric acid B induces caspase-dependent apoptosis and autophagic cell death in prostate cancer cells. Phytother. Res. 2013, 27, 885–891. [Google Scholar] [CrossRef]
- Khan, M.; Zheng, B.; Yi, F.; Rasul, A.; Gu, Z.; Li, T.; Gao, H.; Qazi, J.I.; Yang, H.; Ma, T. Pseudolaric Acid B induces caspase-dependent and caspase-independent apoptosis in u87 glioblastoma cells. Evid. Based Complement. Alternat. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Yu, H.-J.; Shin, J.-A.; Choi, E.-S.; Jeon, J.-G.; Cho, N.-P.; Cho, S.-D. The Apoptotic Effect of the Methanol Extract of Polygonum cuspidatum through Up-Regulation Death Receptor 5 and CHOP in HSC-2 Human Oral Cancer Cells. J. Cancer Ther. 2012, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Huong, L.; Shin, J.A.; Choi, E.S.; Cho, N.P.; Kim, H.; Leem, D.H.; Cho, S.-D. β-Phenethyl isothiocyanate induces death receptor 5 to induce apoptosis in human oral cancer cells via p38. Oral Dis. 2012, 18, 513–519. [Google Scholar] [CrossRef]
- Yu, H.J.; Shin, J.; Lee, S.O.; Kwon, K.H.; Cho, S.-D. Extracellular signal-regulated kinase inhibition is required for methanol extract of Smilax china L.-induced apoptosis through death receptor 5 in human oral mucoepidermoid carcinoma cells. Mol. Med. Rep. 2014, 9, 663–668. [Google Scholar] [CrossRef]
- Shin, J.-A.; Shim, J.-H.; Choi, E.-S.; Leem, D.-H.; Kwon, K.H.; Lee, S.-O.; Safe, S.; Cho, N.-P.; Cho, S.-D. Chemopreventive effects of synthetic C-substituted diindolylmethanes originating from cruciferous vegetables in human oral cancer cells. Eur. J. Cancer Prev. 2011, 20, 417–425. [Google Scholar] [CrossRef]
- Katiyar, S. Emerging phytochemicals for the prevention and treatment of head and neck cancer. Molecules 2016, 21, 1610. [Google Scholar] [CrossRef] [PubMed]
- Perdue, R.E., Jr. Procurement of plant materials for antitumor screening. Cancer. Treat. Rep. 1976, 60, 987–998. [Google Scholar] [PubMed]
- Yang, I.H.; Shin, J.A.; Lee, K.E.; Kim, J.; Cho, N.P.; Cho, S.-D. Oridonin induces apoptosis in human oral cancer cells via phosphorylation of histone H2 AX. Eur. J. Oral Sci. 2017, 125, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-J.; Shin, J.-A.; Yang, I.-H.; Won, D.-H.; Ahn, C.H.; Kwon, H.-J.; Lee, J.-S.; Cho, N.-P.; Kim, E.-C.; Yoon, H.-J. Apoptosis induced by caffeic acid phenethyl ester in human oral cancer cell lines: Involvement of Puma and Bax activation. Arch. Oral Biol. 2017, 84, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.-H.; Khadka, S.; Shin, J.-A.; Jung, J.-Y.; Ryu, M.-H.; Yu, H.-J.; Lee, H.N.; Jang, B.; Yang, I.-H.; Won, D.-H. Nitidine chloride acts as an apoptosis inducer in human oral cancer cells and a nude mouse xenograft model via inhibition of STAT3. Oncotarget. 2017, 8, 91306–91315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, V.K.; Chiu, P.; Chung, S.S.; Chow, L.M.; Zhao, Y.Z.; Yang, B.B.; Ko, B.C. Pseudolaric acid B, a novel microtubule-destabilizing agent that circumvents multidrug resistance phenotype and exhibits antitumor activity in vivo. Clin. Cancer Res. 2005, 11, 6002–6011. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.G.; Zhang, X.W.; Geng, M.Y.; Yue, J.M.; Xin, X.L.; Tian, F.; Shen, X.; Tong, L.J.; Li, M.H.; Zhang, C.; et al. Pseudolarix acid B, a new tubulin-binding agent, inhibits angiogenesis by interacting with a novel binding site on tubulin. Mol. Pharmacol. 2006, 69, 1226–1233. [Google Scholar] [CrossRef]
- Yao, G.D.; Yang, J.; Li, Q.; Zhang, Y.; Qi, M.; Fan, S.M.; Hayashi, T.; Tashiro, S.; Onodera, S.; Ikejima, T. Activation of p53 contributes to pseudolaric acid B-induced senescence in human lung cancer cells in vitro. Acta. Pharmacol. Sin. 2016, 37, 919–929. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Wang, M.; Tashiro, S.; Onodera, S.; Ikejima, T. Involvement of JNK-initiated p53 accumulation and phosphorylation of p53 in pseudolaric acid B induced cell death. Exp. Mol. Med. 2006, 38, 428–434. [Google Scholar] [CrossRef] [Green Version]
- Yao, G.; Qi, M.; Ji, X.; Fan, S.; Xu, L.; Hayashi, T.; Tashiro, S.; Onodera, S.; Ikejima, T. ATM-p53 pathway causes G2/M arrest, but represses apoptosis in pseudolaric acid B-treated HeLa cells. Arch. Biochem. Biophys. 2014, 558, 51–60. [Google Scholar] [CrossRef]
- Yu, J.H.; Wang, H.J.; Li, X.R.; Tashiro, S.; Onodera, S.; Ikejima, T. Protein tyrosine kinase, JNK, and ERK involvement in pseudolaric acid B-induced apoptosis of human breast cancer MCF-7 cells. Acta. Pharmacol. Sin. 2008, 29, 1069–1076. [Google Scholar] [CrossRef]
- Sophonnithiprasert, T.; Nilwarangkoon, S.; Nakamura, Y.; Watanapokasin, R. Goniothalamin enhances TRAIL-induced apoptosis in colorectal cancer cells through DR5 upregulation and cFLIP downregulation. Int. J. Oncol. 2015, 47, 2188–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagalingam, A.; Kuppusamy, P.; Singh, S.V.; Sharma, D.; Saxena, N.K. Mechanistic elucidation of the antitumor properties of withaferin a in breast cancer. Cancer Res. 2014, 74, 2617–2629. [Google Scholar] [CrossRef] [PubMed]
- Won, D.H.; Kim, L.H.; Jang, B.; Yang, I.H.; Kwon, H.J.; Jin, B.; Oh, S.H.; Kang, J.H.; Hong, S.D.; Shin, J.A.; et al. In vitro and in vivo anti-cancer activity of silymarin on oral cancer. Tumour Biol. 2018, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.E.; Shin, J.A.; Jeong, J.H.; Jeon, J.G.; Lee, M.H.; Cho, S.D. Anticancer activity of Ashwagandha against human head and neck cancer cell lines. J. Oral Pathol. Med. 2016, 45, 193–201. [Google Scholar] [CrossRef]
- Yu, H.J.; Jung, J.Y.; Jeong, J.H.; Cho, S.D.; Lee, J.S. Induction of apoptosis by parthenolide in human oral cancer cell lines and tumor xenografts. Oral Oncol 2015, 51, 602–609. [Google Scholar] [CrossRef]
- Wang, D.; Xin, Y.; Tian, Y.; Li, W.; Sun, D.; Yang, Y. Pseudolaric acid B inhibits gastric cancer cell metastasis in vitro and in haematogenous dissemination model through PI3K/AKT, ERK1/2 and mitochondria-mediated apoptosis pathways. Exp. Cell Res. 2017, 352, 34–44. [Google Scholar] [CrossRef]
- Zhao, D.; Lin, F.; Wu, X.; Zhao, Q.; Zhao, B.; Lin, P.; Zhang, Y.; Yu, X. Pseudolaric acid B induces apoptosis via proteasome-mediated Bcl-2 degradation in hormone-refractory prostate cancer DU145 cells. Toxicol Vitr. 2012, 26, 595–602. [Google Scholar] [CrossRef]
- Yu, J.H.; Cui, Q.; Jiang, Y.Y.; Yang, W.; Tashiro, S.; Onodera, S.; Ikejima, T. Pseudolaric acid B induces apoptosis, senescence, and mitotic arrest in human breast cancer MCF-7. Acta. Pharmacol. Sin. 2007, 28, 1975–1983. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-J.; Ahn, C.-H.; Yang, I.-H.; Jin, B.; Lee, W.W.; Kim, J.-H.; Ahn, M.-H.; Swarup, N.; Hong, K.-O.; Shin, J.-A.; et al. Pseudolaric Acid B Induces Growth Inhibition and Caspase-Dependent Apoptosis on Head and Neck Cancer Cell lines through Death Receptor 5. Molecules 2019, 24, 3715. https://doi.org/10.3390/molecules24203715
Choi S-J, Ahn C-H, Yang I-H, Jin B, Lee WW, Kim J-H, Ahn M-H, Swarup N, Hong K-O, Shin J-A, et al. Pseudolaric Acid B Induces Growth Inhibition and Caspase-Dependent Apoptosis on Head and Neck Cancer Cell lines through Death Receptor 5. Molecules. 2019; 24(20):3715. https://doi.org/10.3390/molecules24203715
Chicago/Turabian StyleChoi, Su-Jung, Chi-Hyun Ahn, In-Hyoung Yang, Bohwan Jin, Won Woo Lee, Ji-Hoon Kim, Min-Hye Ahn, Neeti Swarup, Kyoung-Ok Hong, Ji-Ae Shin, and et al. 2019. "Pseudolaric Acid B Induces Growth Inhibition and Caspase-Dependent Apoptosis on Head and Neck Cancer Cell lines through Death Receptor 5" Molecules 24, no. 20: 3715. https://doi.org/10.3390/molecules24203715





