Phosphorus-Containing Flame Retardants from Biobased Chemicals and Their Application in Polyesters and Epoxy Resins
Abstract
:1. Introduction
2. Synthesis of Phosphorus-Containing Biobased Flame Retardants
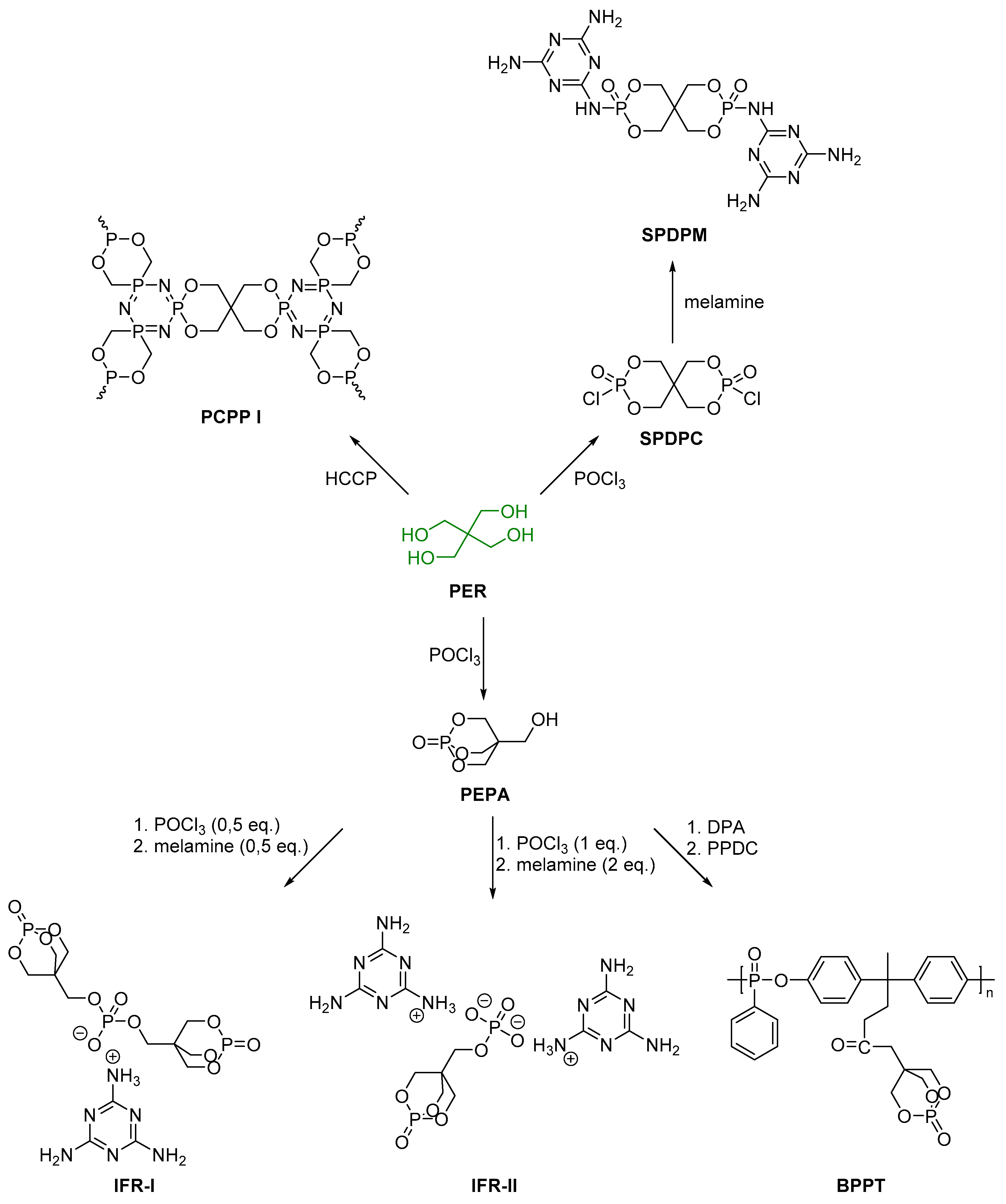
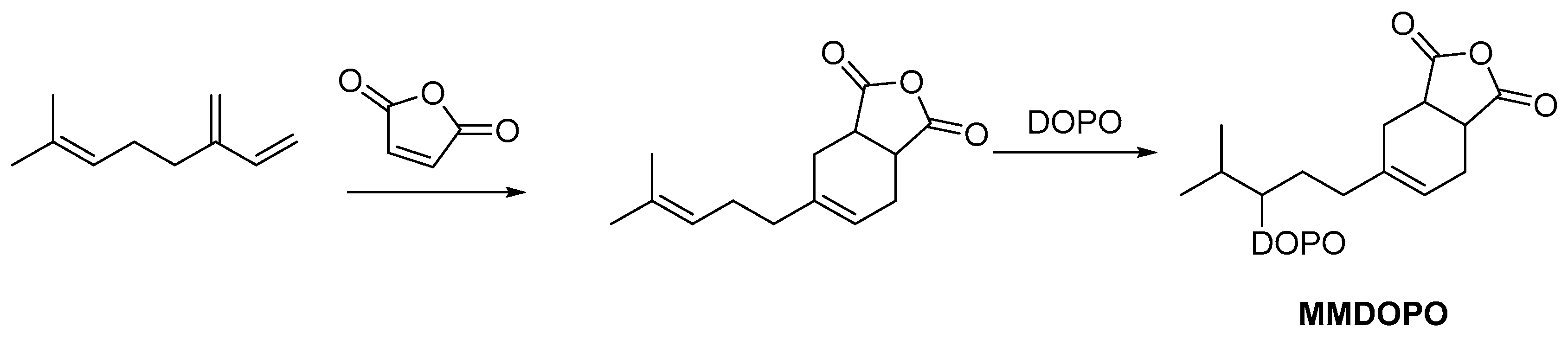
2.1. Biobased Flame Retardants for Poly (Lactic Acid)
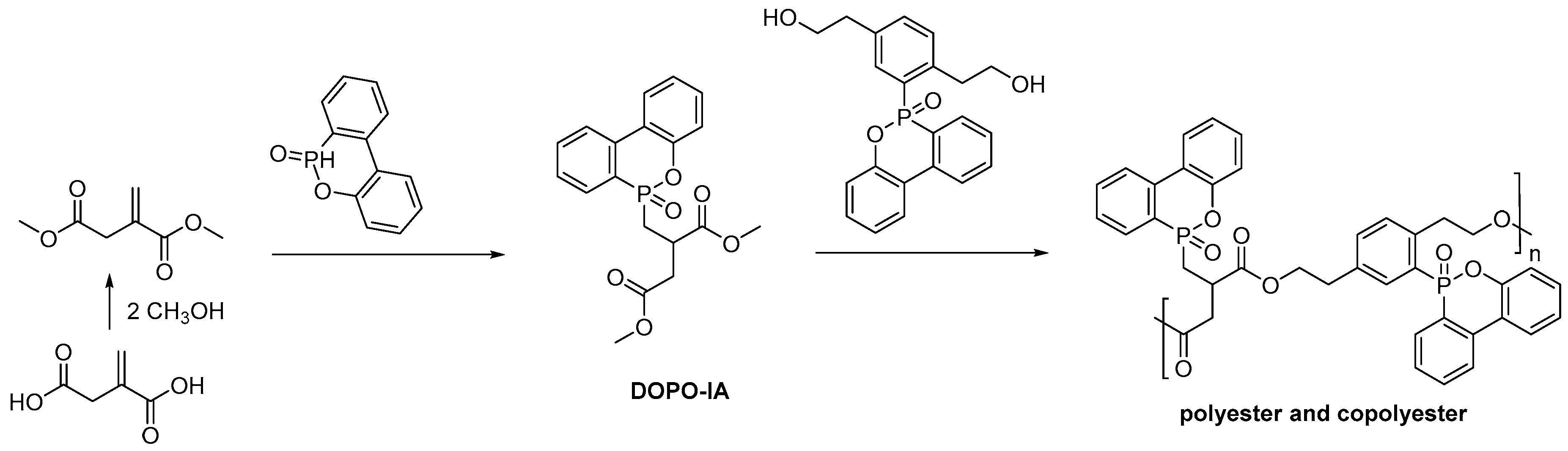
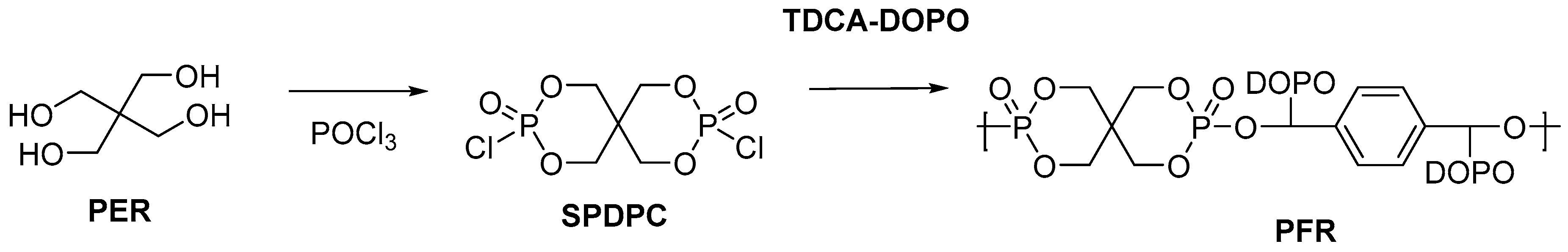
2.2. Biobased Flame Retardants for Poly (Ethylene Therephthalate) and Poly (Butylene Succinate)
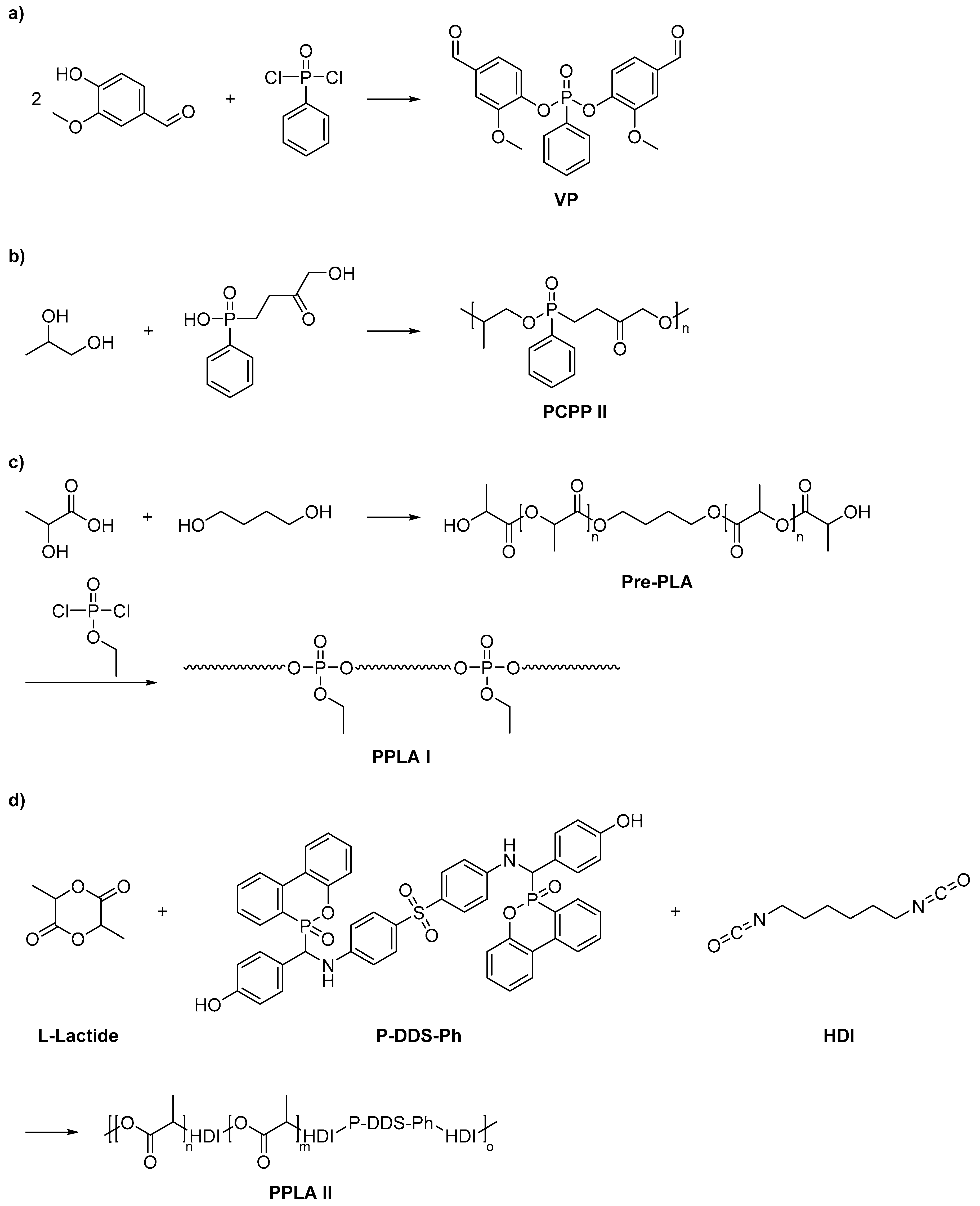
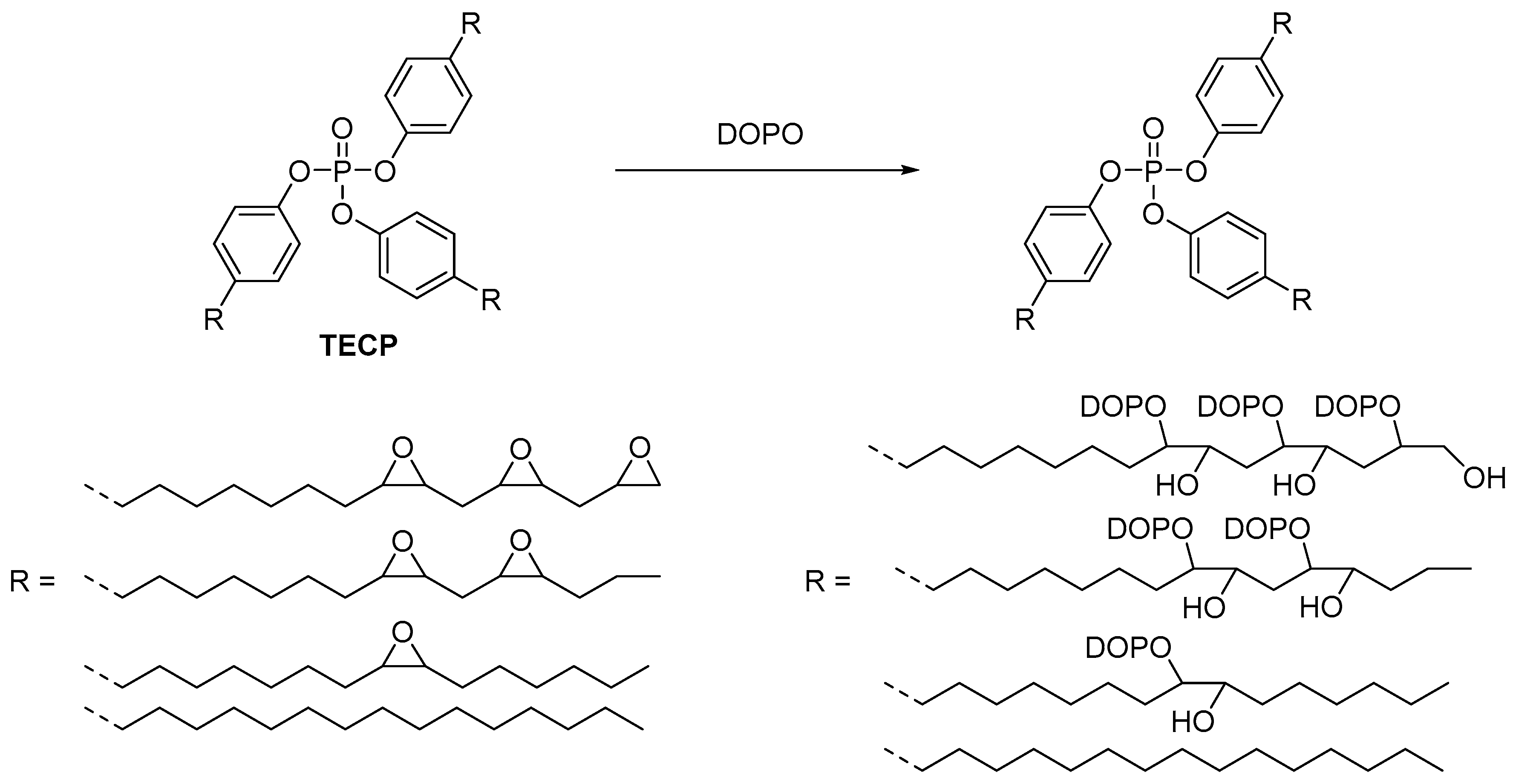
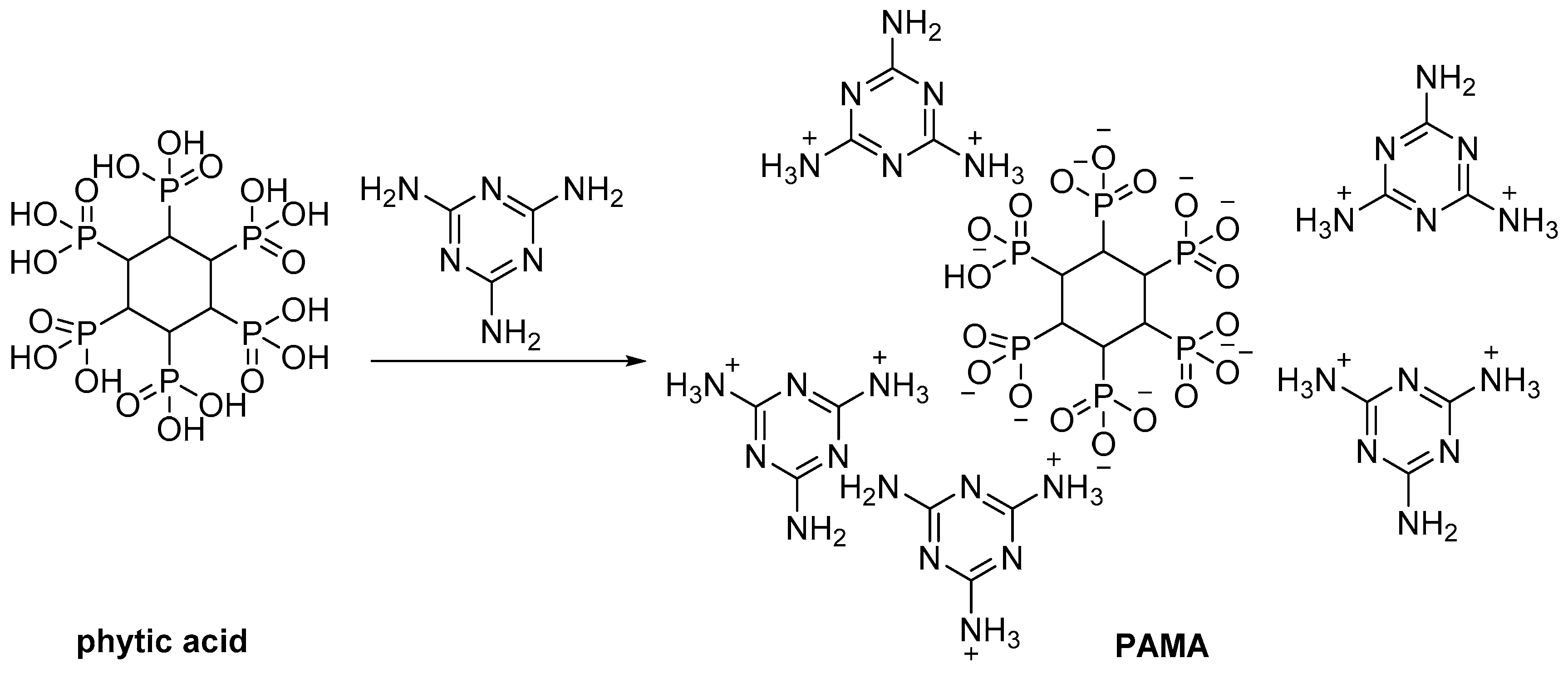
2.3. Biobased Flame Retardants for Epoxy Resin Systems
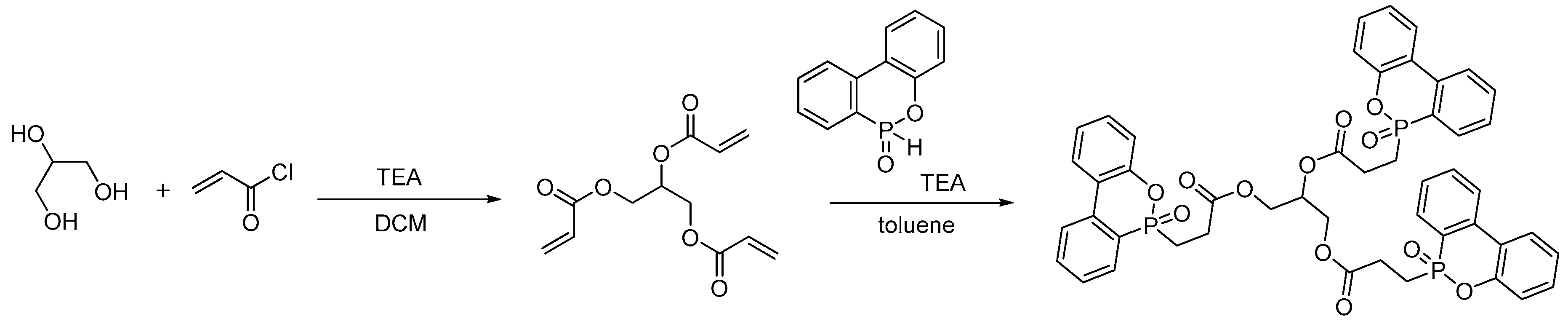
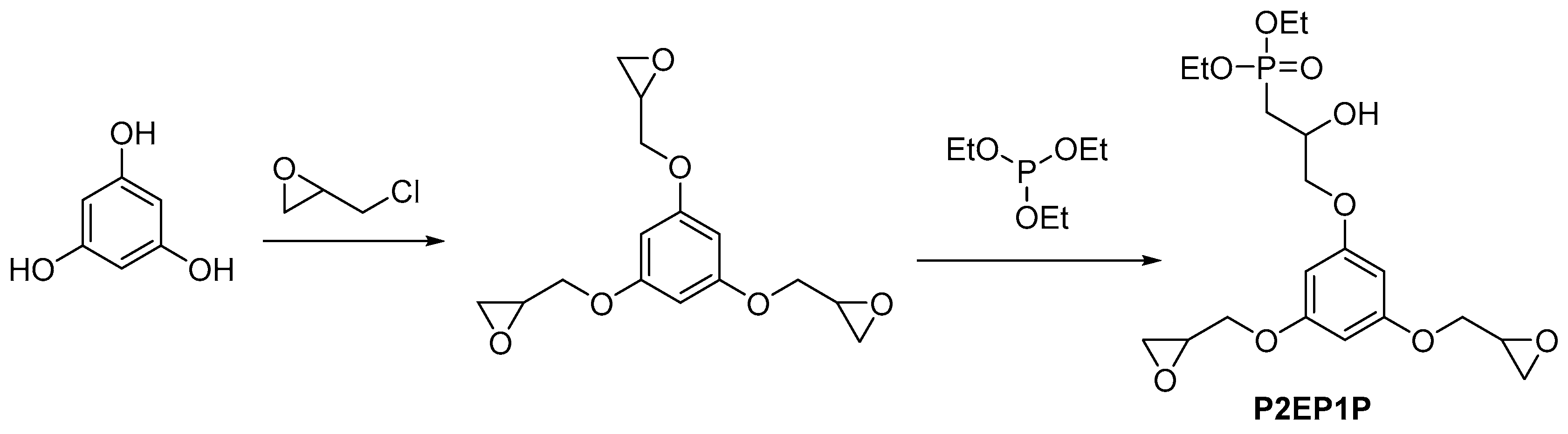
2.3.1. Reactive Flame Retardant Epoxy Monomers
- incorporation of phosphorus-containing groups into an epoxide component or
- incorporation of phosphorus-containing groups into a molecule with a following epoxidation of unsaturated side chains.
2.3.2. Reactive Flame Retardant Curing Agents
2.3.3. Non-Reactive Flame Retardants
3. Biobased Flame Retardants in Polyesters
3.1. Poly (Lactic Acid)
3.2. Poly (Ethylene Therephthalate)
3.3. Poly (Butylene Succinate)
4. Biobased Flame Retardants in Epoxy Resins
5. Outlook
Author Contributions
Conflicts of Interest
References
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Methods for Determining the Biobased Content of Solid, Liquid, and Gaseous Samples Using Radiocarbon Analysis; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Verma, D.; Fortunati, E. Biopolymer Processing and its Composites: An Introduction. In Biomass, Biopolymer-Based Materials, and Bioenergy; Woodhead Publishing: Sawston, Cambridge, UK, 2019; pp. 3–23. [Google Scholar]
- Rudin, A.; Choi, P. The Elements of Polymer Science and Engineering; Academic Press: Cambridge, MA, USA, 2012; ISBN 0123821797. [Google Scholar]
- Emblem, A. Plastics properties for packaging materials. Packag. Technol. 2012, 13, 287–309. [Google Scholar] [CrossRef]
- Mishnaevsky, L.; Branner, K.; Petersen, H.N.; Beauson, J.; McGugan, M.; Sørensen, B.F. Materials for Wind Turbine Blades: An Overview. Materials 2017, 10, 1285. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Keller, J.H.; Altstaedt, V. Full-field shear analyses of sandwich core materials using Digital Image Correlation (DIC). Compos. Part B Eng. 2015, 70, 156–166. [Google Scholar] [CrossRef]
- Müller, R.-J.; Schrader, H.; Profe, J.; Dresler, K.; Deckwer, W.-D. Enzymatic Degradation of Poly(ethylene terephthalate): Rapid Hydrolyse using a Hydrolase fromT. fusca. Macromol. Rapid Commun. 2005, 26, 1400–1405. [Google Scholar] [CrossRef]
- Endres, H.J.; Siebert-Raths, A.; Behnsen, H.; Schulz, C. Biopolymers Facts and Statistics; IfBB – Institut für Biokunststoffe und Bioverbundwerkstoffe: Hannover, Germany, 2016; pp. 2363–8559. [Google Scholar]
- Robertson, G.L.; Sand, C.K. Bio-Derived Polymers for Food Packaging; Institute of Food Technologists: Chicago, IL, USA, 2018. [Google Scholar]
- Kriegel, R.M.; Huang, X.; Schultheis, M.W. Bio-Based Polyethylene Terephthalate Polymer and Method of Making Same. US2009246430A1, 1 October 2009. [Google Scholar]
- Harmsen, P.F.H.; Hackmann, M.M.; Bos, H.L. Green building blocks for bio-based plastics. Biofuels Bioprod. Biorefining 2014, 8, 306–324. [Google Scholar] [CrossRef]
- Pellis, A.; Acero, E.H.; Ferrario, V.; Ribitsch, D.; Guebitz, G.M.; Gardossi, L. The Closure of the Cycle: Enzymatic Synthesis and Functionalization of Bio-Based Polyesters. Trends Biotechnol. 2016, 34, 316–328. [Google Scholar] [CrossRef]
- Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Fierro, V.; Celzard, A. PLA with Intumescent System Containing Lignin and Ammonium Polyphosphate for Flame Retardant Textile. Polymers 2016, 8, 331. [Google Scholar] [CrossRef]
- Jensen, J.; Skelton, K. Wind turbine blade recycling: Experiences, challenges and possibilities in a circular economy. Renew. Sustain. Energy Rev. 2018, 97, 165–176. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Kiekens, P. Recent Advances in the Design of Water Based-Flame Retardant Coatings for Polyester and Polyester-Cotton Blends. Polymers 2016, 8, 357. [Google Scholar] [CrossRef]
- Chen, L.; Pelton, R.E.; Smith, T.M. Comparative life cycle assessment of fossil and bio-based polyethylene terephthalate (PET) bottles. J. Clean. Prod. 2016, 137, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhou, S.; Guo, W.W.; Wang, P.L.; Xing, W.; Song, L.; Hu, Y. Renewable Cardanol-Based Phosphate as a Flame Retardant Toughening Agent for Epoxy Resins. ACS Sustain. Chem. Eng. 2017, 5, 3409–3416. [Google Scholar] [CrossRef]
- Schartel, B.; Balabanovich, A.I.; Braun, U.; Knoll, U.; Artner, J.; Ciesielski, M.; Doring, M.; Pérez, R.; Sandler, J.K.W.; Altstädt, V.; et al. Pyrolysis of epoxy resins and fire behavior of epoxy resin composites flame-retarded with 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide additives. J. Appl. Polym. Sci. 2007, 104, 2260–2269. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wang, D.-Y. Renewable Cardanol-Based Surfactant Modified Layered Double Hydroxide as a Flame Retardant for Epoxy Resin. ACS Sustain. Chem. Eng. 2015, 3, 3281–3290. [Google Scholar] [CrossRef]
- Greiner, L.; Kukla, P.; Eibl, S.; Döring, M. Phosphorus Containing Polyacrylamides as Flame Retardants for Epoxy-Based Composites in Aviation. Polymers 2019, 11, 284. [Google Scholar] [CrossRef]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Bell, B.M.; Briggs, J.R.; Campbell, R.M.; Chambers, S.M.; Gaarenstroom, P.D.; Hippler, J.G.; Hook, B.D.; Kearns, K.; Kenney, J.M.; Kruper, W.J.; et al. Glycerin as a Renewable Feedstock for Epichlorohydrin Production. The GTE Process. Clean Soil Air Water 2008, 36, 657–661. [Google Scholar] [CrossRef]
- Rad, E.R.; Vahabi, H.; de Anda, A.R.; Saeb, M.R.; Thomas, S. Bio-epoxy resins with inherent flame retardancy. Prog. Org. Coat. 2019, 135, 608–612. [Google Scholar] [CrossRef]
- Perez, R.M.; Sandler, J.K.W.; Altstädt, V.; Hoffmann, T.; Pospiech, D.; Ciesielski, M.; Doring, M. Effect of DOP-based compounds on fire retardancy, thermal stability, and mechanical properties of DGEBA cured with 4, 4′-DDS. J. Mater. Sci. 2006, 41, 341–353. [Google Scholar] [CrossRef]
- Qian, L.-J.; Ye, L.-J.; Xu, G.-Z.; Liu, J.; Guo, J.-Q. The non-halogen flame retardant epoxy resin based on a novel compound with phosphaphenanthrene and cyclotriphosphazene double functional groups. Polym. Degrad. Stab. 2011, 96, 1118–1124. [Google Scholar] [CrossRef]
- Gao, L.-P.; Wang, D.-Y.; Wang, Y.-Z.; Wang, J.-S.; Yang, B. A flame-retardant epoxy resin based on a reactive phosphorus-containing monomer of DODPP and its thermal and flame-retardant properties. Polym. Degrad. Stab. 2008, 93, 1308–1315. [Google Scholar] [CrossRef]
- Guo, J.; Song, H.; Liu, H.; Luo, C.; Ren, Y.; Ding, T.; Khan, M.A.; Young, D.P.; Liu, X.; Zhang, X.; et al. Polypyrrole-interface-functionalized nano-magnetite epoxy nanocomposites as electromagnetic wave absorbers with enhanced flame retardancy. J. Mater. Chem. C 2017, 5, 5334–5344. [Google Scholar] [CrossRef]
- Hussain, M.; Varley, R.J.; Mathys, Z.; Cheng, Y.B.; Simon, G.P. Effect of organo-phosphorus and nano-clay materials on the thermal and fire performance of epoxy resins. J. Appl. Polym. Sci. 2004, 91, 1233–1253. [Google Scholar] [CrossRef]
- Zhang, X.; He, Q.; Gu, H.; Colorado, H.A.; Wei, S.; Guo, Z. Flame-Retardant Electrical Conductive Nanopolymers Based on Bisphenol F Epoxy Resin Reinforced with Nano Polyanilines. ACS Appl. Mater. Interfaces 2013, 5, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Kalali, E.N.; Wang, X. Functionalized layered double hydroxide-based epoxy nanocomposites with improved flame retardancy and mechanical properties. J. Mater. Chem. A 2015, 3, 6819–6826. [Google Scholar] [CrossRef] [Green Version]
- Polen, T.; Spelberg, M.; Bott, M. Toward biotechnological production of adipic acid and precursors from biorenewables. J. Biotechnol. 2013, 167, 75–84. [Google Scholar] [CrossRef]
- Raj, K.; Partow, S.; Correia, K.; Khusnutdinova, A.N.; Yakunin, A.F.; Mahadevan, R. Biocatalytic production of adipic acid from glucose using engineered Saccharomyces cerevisiae. Metab. Eng. Commun. 2018, 6, 28–32. [Google Scholar] [CrossRef]
- Adom, F.; Dunn, J.B.; Han, J.; Sather, N. Life-Cycle Fossil Energy Consumption and Greenhouse Gas Emissions of Bioderived Chemicals and Their Conventional Counterparts. Environ. Sci. Technol. 2014, 48, 14624–14631. [Google Scholar] [CrossRef]
- Yim, H.; Haselbeck, R.; Niu, W.; Pujol-Baxley, C.; Burgard, A.; Boldt, J.; Khandurina, J.; Trawick, J.D.; Osterhout, R.E.; Stephen, R.; et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat. Methods 2011, 7, 445–452. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wu, C.S.; Hsu, K.Y.; Chang, T.C. Flame-retardant epoxy resins fromo-cresol novolac epoxy cured with a phosphorus-containing aralkyl novolac. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2329–2339. [Google Scholar] [CrossRef]
- Buck, K.T.; Boeing, A.J.; Dolfini, J.E. Method of Producing Benzaldehyde. U.S. Patent US4673766, 16 June 1987. [Google Scholar]
- Van Haveren, J.; Scott, E.L.; Sanders, J. Bulk chemicals from biomass. Biofuels Bioprod. Biorefining 2008, 2, 41–57. [Google Scholar] [CrossRef]
- Barreto, A.; Esmeraldo, M.; Rosa, D.; Fechine, P.; Mazzetto, S. Cardanol biocomposites reinforced with jute fiber: Microstructure, biodegradability, and mechanical properties. Polym. Compos. 2010, 31, 1928–1937. [Google Scholar] [CrossRef]
- Miao, J.T.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Biobased Heat Resistant Epoxy Resin with Extremely High Biomass Content from 2,5-Furandicarboxylic Acid and Eugenol. ACS Sustain. Chem. Eng. 2017, 5, 7003–7011. [Google Scholar] [CrossRef]
- Ecochard, Y.; DeCostanzi, M.; Negrell, C.; Sonnier, R.; Caillol, S. Cardanol and Eugenol Based Flame Retardant Epoxy Monomers for Thermostable Networks. Molecules 2019, 24, 1818. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.J.; Al-Bayati, F.A. Isolation and identification of antibacterial compounds from Thymus kotschyanus aerial parts and Dianthus caryophyllus flower buds. Phytomedicine 2009, 16, 632–637. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Gruter, G.J.M.; Coelho, J.F.J.; Silvestre, A.J.D.; Matos, M.; Freire, C.S.R. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Sadler, J.M.; Toulan, F.R.; Nguyen, A.P.T.; Kayea III, R.V.; Ziaee, S.; Palmese, G.R.; La Scala, J.J. Isosorbide as the structural component of bio-based unsaturated polyesters for use as thermosetting resins. Carbohydr. Polym. 2014, 100, 97–106. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Jiang, Y.; Fan, L.; Feng, J.; Zhu, J. Synthesis and properties of phosphorus-containing bio-based epoxy resin from itaconic acid. Sci. China Chem. 2014, 57, 379–388. [Google Scholar] [CrossRef]
- Willke, T.; Vorlop, K.-D. Biotechnological production of itaconic acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295. [Google Scholar] [CrossRef]
- Ghaffar, T.; Irshad, M.; Anwar, Z.; Aqil, T.; Zulifqar, Z.; Tariq, A.; Kamran, M.; Ehsan, N.; Mehmood, S. Recent trends in lactic acid biotechnology: A brief review on production to purification. J. Radiat. Res. Appl. Sci. 2014, 7, 222–229. [Google Scholar] [CrossRef]
- Wazarkar, K.; Kathalewar, M.; Sabnis, A. Reactive Modification of Thermoplastic and Thermoset Polymers using Flame Retardants: An Overview. Polym. Plast. Technol. Eng. 2016, 55, 71–91. [Google Scholar] [CrossRef]
- Van Es, D.S.; Van der Klis, F.; Van Haveren, J. Succinic Acid from Biomass. U.S. Patent WO 2012044168, 5 May 2012. [Google Scholar]
- Girisuta, B. Levulinic Acid from Lignocellulosic Biomass. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2007. [Google Scholar]
- Prieur, B.; Meub, M.; Wittemann, M.; Klein, R.; Bellayer, S.; Fontaine, G.; Bourbigot, S. Phosphorylation of lignin: Characterization and investigation of the thermal decomposition. RSC Adv. 2017, 7, 16866–16877. [Google Scholar] [CrossRef]
- Prieur, B.; Meub, M.; Wittemann, M.; Klein, R.; Bellayer, S.; Fontaine, G.; Bourbigot, S. Phosphorylation of lignin to flame retard acrylonitrile butadiene styrene (ABS). Polym. Degrad. Stab. 2016, 127, 32–43. [Google Scholar] [CrossRef]
- Ferry, L.; Dorez, G.; Taguet, A.; Otazaghine, B.; Lopez-Cuesta, J. Chemical modification of lignin by phosphorus molecules to improve the fire behavior of polybutylene succinate. Polym. Degrad. Stab. 2015, 113, 135–143. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin, a key-intermediate of biobased polymers. Eur. Polym. J. 2015, 68, 488–502. [Google Scholar] [CrossRef]
- Laskar, D.D.; Yang, B.; Wang, H.; Lee, J. Pathways for biomass-derived lignin to hydrocarbon fuels. Biofuels, Bioprod. Biorefining 2013, 7, 602–626. [Google Scholar] [CrossRef]
- Taing, O.; Taing, K. Production of malic and succinic acids by sugar-tolerant yeast Zygosaccharomyces rouxii. Eur. Food Res. Technol. 2007, 224, 343–347. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.; Xia, J.; Mao, W.; Li, S. Study on synthesis of novel phosphorus-containing flame retardant epoxy curing agents from renewable resources and the comprehensive properties of their combined cured products. Prog. Org. Coatings 2017, 110, 195–203. [Google Scholar] [CrossRef]
- Kim, E.-M.; Eom, J.-H.; Um, Y.; Kim, Y.; Woo, H.M. Microbial Synthesis of Myrcene by Metabolically Engineered Escherichia coli. J. Agric. Food Chem. 2015, 63, 4606–4612. [Google Scholar] [CrossRef]
- Behr, A.; Johnen, L. Myrcene as a Natural Base Chemical in Sustainable Chemistry: A Critical Review. ChemSusChem 2009, 12, 1072–1095. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Development of novel phosphorus-containing epoxy resins from renewable resources. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 6717–6727. [Google Scholar] [CrossRef]
- Ran, N.; Knop, D.R.; Draths, K.M.; Frost, J.W. Benzene-Free Synthesis of Hydroquinone. J. Am. Chem. Soc. 2001, 123, 10927–10934. [Google Scholar] [CrossRef] [PubMed]
- Ménard, R.; Fache, M.; Negrell, C.; Ferry, L.; Sonnier, R.; David, G. From a bio-based phosphorus-containing epoxy monomer to fully bio-based flame-retardant thermosets. RSC Adv. 2015, 5, 70856–70867. [Google Scholar] [CrossRef]
- Glombitza, K.-W. Highly Hydroxylated Phenols of the Phaeophyceae. In Marine Natural Products Chemistry; Springer Science: Boston, MA, USA, 1977; pp. 191–204. [Google Scholar]
- Zhang, T.; Yan, H.; Shen, L.; Fang, Z.; Zhang, X.; Wang, J.; Zhang, B. Chitosan/Phytic Acid Polyelectrolyte Complex: A Green and Renewable Intumescent Flame Retardant System for Ethylene–Vinyl Acetate Copolymer. Ind. Eng. Chem. Res. 2014, 53, 19199–19207. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Guan, J.-P.; Tang, R.-C.; Liu, K.-Q. Phytic acid as a bio-based phosphorus flame retardant for poly(lactic acid) nonwoven fabric. J. Clean. Prod. 2016, 124, 114–119. [Google Scholar] [CrossRef]
- Lolas, G.M.; Markakis, P. Phytic acid and other phosphorus compounds of beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 1975, 23, 13–15. [Google Scholar] [CrossRef]
- Kraus, G.A. Synthetic Methods for the Preparation of 1,3-Propanediol. CLEAN Soil Air Water 2008, 36, 648–651. [Google Scholar] [CrossRef]
- Ogunniyi, D. Castor oil: A vital industrial raw material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef]
- Wang, X.L.; Chen, L.; Wu, J.N.; Fu, T.; Wang, Y.Z. Flame-Retardant Pressure-Sensitive Adhesives Derived from Epoxidized Soybean Oil and Phosphorus-Containing Dicarboxylic Acids. ACS Sustain. Chem. Eng. 2017, 5, 3353–3361. [Google Scholar] [CrossRef]
- Barrow, K.; Collins, J.; Leight, D.; Rogers, P.; Warr, R. Sorbitol production by Zymomonas mobilis. Appl. Microbiol. Biotechnol. 1984, 20, 225–232. [Google Scholar] [CrossRef]
- Ladero, V.; Ramos, A.; Wiersma, A.; Goffin, P.; Schanck, A.; Kleerebezem, M.; Hugenholtz, J.; Smid, E.J.; Hols, P. High-Level Production of the Low-Calorie Sugar Sorbitol by Lactobacillus plantarum through Metabolic Engineering. Appl. Environ. Microbiol. 2007, 73, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, J.M.; Domine, M.E.; Parvulescu, V.; Petru, F. Sustainability metrics for succinic acid production: A comparison between biomass-based and petrochemical routes. Catal. Today 2015, 239, 17–24. [Google Scholar] [CrossRef]
- Dhamaniya, S.; Jacob, J. Synthesis and characterization of polyesters based on tartaric acid derivatives. Polymer 2010, 51, 5392–5399. [Google Scholar] [CrossRef]
- Howell, B.A.; Carter, K.E.; Dangalle, H. Flame Retardants Based on Tartaric Acid: A Renewable By-Product of the Wine Industry. In ACS Symposium Series; American Chemical Society (ACS): Washington, DC, USA, 2011; Volume 1063, pp. 133–152. [Google Scholar]
- Collias, D.I.; Harris, A.M.; Nagpal, V.; Cottrell, I.W.; Schultheis, M.W. Biobased Terephthalic Acid Technologies: A Literature Review. Ind. Biotechnol. 2014, 10, 91–105. [Google Scholar] [CrossRef]
- Tachibana, Y.; Kimura, S.; Kasuya, K.-I. Synthesis and Verification of Biobased Terephthalic Acid from Furfural. Sci. Rep. 2015, 5, 8249. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.D.; Grande, C.A.; Rodrigues, A.E. Vanillin production from lignin oxidation in a batch reactor. Chem. Eng. Res. Des. 2010, 88, 1024–1032. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Wiredu, B.; Razzaq, A. Vanillin based polymers: I. An electrochemical route to polyvanillin. Green Chem. 2012, 14, 2395. [Google Scholar] [CrossRef]
- Fourcade, D.; Ritter, B.S.; Walter, P.; Schönfeld, R.; Mülhaupt, R. Renewable resource-based epoxy resins derived from multifunctional poly(4-hydroxybenzoates). Green Chem. 2013, 15, 910. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Trossarelli, L. Study of the mechanism of intumescence in fire retardant polymers: Part I—Thermal degradation of ammonium polyphosphate-pentaerythritol mixtures. Polym. Degrad. Stab. 1984, 6, 243–252. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R. Carbonization mechanisms resulting from intumescence association with the ammonium polyphosphate-pentaerythritol fire retardant system. Carbon 1993, 31, 1219–1230. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, P.; Hu, Y.; Hu, K. Thermal degradation study of intumescent flame retardants by TG and FTIR: Melamine phosphate and its mixture with pentaerythritol. J. Anal. Appl. Pyrolysis 2009, 86, 207–214. [Google Scholar] [CrossRef]
- Tao, K.; Li, J.; Xu, L.; Zhao, X.; Xue, L.; Fan, X.; Yan, Q. A novel phosphazene cyclomatrix network polymer: Design, synthesis and application in flame retardant polylactide. Polym. Degrad. Stab. 2011, 96, 1248–1254. [Google Scholar] [CrossRef]
- Zhan, J.; Song, L.; Nie, S.; Hu, Y. Combustion properties and thermal degradation behavior of polylactide with an effective intumescent flame retardant. Polym. Degrad. Stab. 2009, 94, 291–296. [Google Scholar] [CrossRef]
- Xuan, S.; Wang, X.; Song, L.; Xing, W.; Lu, H.; Hu, Y. Study on flame-retardancy and thermal degradation behaviors of intumescent flame-retardant polylactide systems. Polym. Int. 2011, 60, 1541–1547. [Google Scholar] [CrossRef]
- Tang, X.; Jing, J.; Zhang, Y.; Fang, Z. Synthesis of a highly efficient phosphorus-containing flame retardant utilizing plant-derived diphenolic acids and its application in polylactic acid. RSC Adv. 2016, 6, 49019–49027. [Google Scholar]
- Bozell, J.J.; Moens, L.; Elliott, D.; Wang, Y.; Neuenscwander, G.; Fitzpatrick, S.; Bilski, R.; Jarnefeld, J. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, Z.; Wang, X.; Pan, Y.T.; Kuehnert, I.; Gehde, M.; Wang, D.-Y.; Leuteritz, A. Renewable vanillin based flame retardant for poly(lactic acid): A way to enhance flame retardancy and toughness simultaneously. RSC Adv. 2018, 8, 42189–42199. [Google Scholar] [CrossRef]
- Lin, H.J.; Liu, S.R.; Han, L.J.; Wang, X.M.; Bian, Y.J.; Dong, L.S. Effect of a phosphorus-containing oligomer on flame-retardant, rheological and mechanical properties of poly (lactic acid). Polym. Degrad. Stab. 2013, 98, 1389–1396. [Google Scholar] [CrossRef]
- Wang, D.Y.; Song, Y.P.; Lin, L.; Wang, X.L.; Wang, Y.Z. A novel phosphorus-containing poly(lactic acid) toward its flame retardation. Polymer 2011, 52, 233–238. [Google Scholar] [CrossRef]
- Yu, S.; Xiang, H.; Zhou, J.; Zhu, M. Enhanced flame-retardant performance of poly (lactic acid)(PLA) composite by using intrinsically phosphorus-containing PLA. Prog. Nat. Sci. 2018, 28, 590–597. [Google Scholar] [CrossRef]
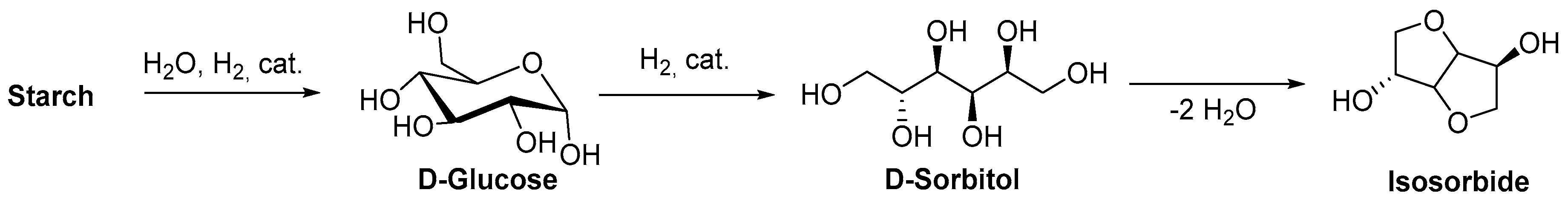
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.-P. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Fletcher, H.G.; Goepp, R.M. Hexitol Anhydrides.11,4,3,6-Dianhydro-L-iditol and the Structures of Isomannide and Isosorbide. J. Am. Chem. Soc. 1946, 68, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Flèche, G.; Huchette, M. Isosorbide. Preparation, Properties and Chemistry. Starch Stärke 1986, 38, 26–30. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Synthesis and characterization of isosorbide bis -phosphorus esters. Heteroat. Chem. 2017, 28, e21369. [Google Scholar] [CrossRef]
- Mauldin, T.C.; Zammarano, M.; Gilman, J.W.; Shields, J.R.; Boday, D.J. Synthesis and characterization of isosorbide-based polyphosphonates as biobased flame-retardants. Polym. Chem. 2014, 5, 5139. [Google Scholar] [CrossRef]
- Hu, C.; Bourbigot, S.; Delaunay, T.; Collinet, M.; Marcille, S.; Fontaine, G. Synthesis of isosorbide based flame retardants: Application for polybutylene succinate. Polym. Degrad. Stab. 2019, 164, 9–17. [Google Scholar] [CrossRef]
- Daniel, Y.G.; Howell, B.A. Flame retardant properties of isosorbide bis -phosphorus esters. Polym. Degrad. Stab. 2017, 140, 25–31. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, X.; Tai, Q.; Huang, H.; Hu, Y. Modification of lignin and its application as char agent in intumescent flame-retardant poly(lactic acid). Polym. Eng. Sci. 2012, 12, 2620–2626. [Google Scholar] [CrossRef]
- De Carvalho, J.C.; Magalhaes, A.I.; Soccol, C.R. Biobased itaconic acid market and research trends—Is it really a promising chemical. Chim. Oggi Chem. Today 2018, 36, 56–58. [Google Scholar]
- Pospiech, D.; Korwitz, A.; Komber, H.; Jehnichen, D.; Häußler, L.; Scheibner, H.; Liebmann, M.; Jähnichen, K.; Voit, B. Biobased Aliphatic Polyesters with DOPO Substituents for Enhanced Flame Retardancy. Macromol. Chem. Phys. 2015, 216, 1447–1461. [Google Scholar] [CrossRef]
- Sonnier, R.; Taguet, A.; Ferry, L.; Lopez-Cuesta, J.M. Towards Bio-based Flame Retardant Polymers; Springer: New York, NY, USA, 2018. [Google Scholar]
- Xie, M.; Zhang, S.; Ding, Y.; Wang, F.; Liu, P.; Tang, H.; Wang, Y.; Yang, M. Synthesis of a heat-resistant DOPO derivative and its application as flame-retardant in engineering plastics. J. Appl. Polym. Sci. 2017, 134, 134. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gooneie, A.; Simonetti, P.; Nazir, R.; Kaiser, J.-P.; Rippl, A.; Hirsch, C.; Lehner, S.; Rupper, P.; Hufenus, R.; et al. Comprehensive study on flame retardant polyesters from phosphorus additives. Polym. Degrad. Stab. 2018, 155, 22–34. [Google Scholar] [CrossRef]
- Carosio, F.; Cuttica, F.; Di Blasio, A.; Alongi, J.; Malucelli, G. Layer by layer assembly of flame retardant thin films on closed cell PET foams: Efficiency of ammonium polyphosphate versus DNA. Polym. Degrad. Stab. 2015, 113, 189–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Wang, B.; Liew, K.M.; Song, L.; Wang, C.; Hu, Y. Highly Effective P–P Synergy of a Novel DOPO-Based Flame Retardant for Epoxy Resin. Ind. Eng. Chem. Res. 2017, 56, 1245–1255. [Google Scholar] [CrossRef]
- Alongi, J.; Cuttica, F.; Carosio, F. DNA coatings from byproducts: A panacea for the flame retardancy of EVA, PP, ABS, PET, and PA6? ACS Sustain. Chem. Eng. 2016, 4, 3544–3551. [Google Scholar] [CrossRef]
- Alongi, J.; Milnes, J.; Malucelli, G.; Bourbigot, S.; Kandola, B. Thermal degradation of DNA-treated cotton fabrics under different heating conditions. J. Anal. Appl. Pyrolysis 2014, 108, 212–221. [Google Scholar] [CrossRef]
- Carosio, F.; Di Blasio, A.; Alongi, J.; Malucelli, G. Green DNA-based flame retardant coatings assembled through Layer by Layer. Polymer 2013, 54, 5148–5153. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Di Blasio, A.; Cuttica, F.; Carosio, F.; Bosco, F.; Malucelli, G. Intrinsic intumescent-like flame retardant properties of DNA-treated cotton fabrics. Carbohydr. Polym. 2013, 96, 296–304. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Di Blasio, A.; Carosio, F.; Bosco, F.; Malucelli, G. DNA: A novel, green, natural flame retardant and suppressant for cotton. J. Mater. Chem. A 2013, 1, 4779. [Google Scholar] [CrossRef]
- Malucelli, G. 9 Textile Finishing with Biomacromolecules: A low Environmental Impact Approach in Flame Retardancy. In The Impact and Prospects of Green Chemistry for Textile Technology; Shahid ul, I., Butola, B.S., Eds.; Woodhead Publishing: Sawston/Cambridge, UK, 2019; pp. 251–279. ISBN 978-0-08-102491-1. [Google Scholar]
- Carosio, F.; Di Blasio, A.; Cuttica, F.; Alongi, J.; Malucelli, G. Flame Retardancy of Polyester and Polyester–Cotton Blends Treated with Caseins. Ind. Eng. Chem. Res. 2014, 53, 3917–3923. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Bosco, F.; Carosio, F.; Di Blasio, A.; Cuttica, F.; Antonucci, V.; Giordano, M.; Malucelli, G. Caseins and hydrophobins as novel green flame retardants for cotton fabrics. Polym. Degrad. Stab. 2014, 99, 111–117. [Google Scholar] [CrossRef]
- Richter, C.A.; Birnbaum, L.S.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; Saal, F.S.V. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007, 24, 199–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vom Saal, F.S.; Akingbemi, B.T.; Belcher, S.M.; Birnbaum, L.S.; Crain, D.A.; Eriksen, M.; Farabollini, F.; Guillette, L.J.; Hauser, R.; Heindel, J.J.; et al. Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 2007, 24, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, A.W.; Beach, E.C.; Stetzik, L.A.; Perry, A.; D’Addezio, A.S.; Cushing, B.S.; Patisaul, H.B. A Novel Model for Neuroendocrine Toxicology: Neurobehavioral Effects of BPA Exposure in a Prosocial Species, the Prairie Vole (Microtus ochrogaster). Endocrinology 2014, 155, 3867–3881. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.P. Biobased thermosetting epoxy: Present and future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef]
- Wan, J.; Gan, B.; Molina-Aldareguia, J.; Li, C.; Wang, X. A novel biobased epoxy resin with high mechanical stiffness and low flammability: Synthesis, characterization and properties. J. Mater. Chem. A 2015, 3, 21907–21921. [Google Scholar] [CrossRef]
- Dai, J.; Peng, Y.; Teng, N.; Liu, Y.; Liu, C.; Shen, X.; Mahmud, S.; Zhu, J.; Liu, X. High-Performing and Fire-Resistant Biobased Epoxy Resin from Renewable Sources. ACS Sustain. Chem. Eng. 2018, 6, 7589–7599. [Google Scholar] [CrossRef]
- Liggins, J.; Bluck, L.J.; Runswick, S.; Atkinson, C.; Coward, W.; Bingham, S.A. Daidzein and genistein content of fruits and nuts. J. Nutr. Biochem. 2000, 11, 326–331. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Jiang, Y.; Tang, Z.; Zhang, C.; Zhu, J. Bio-based epoxy resin from itaconic acid and its thermosets cured with anhydride and comonomers. Green Chem. 2012, 15, 245–254. [Google Scholar] [CrossRef]
- Szolnoki, B.; Toldy, A.; Konrad, P.; Szebényi, G.; Marosi, G. Comparison of additive and reactive phosphorus-based flame retardants in epoxy resins. Period. Polytech. Chem. Eng. 2013, 57, 85. [Google Scholar] [CrossRef] [Green Version]
- Tsujimoto, T.; Uyama, H.; Kobayashi, S. Green Nanocomposites from Renewable Resources: Biodegradable Plant Oil-Silica Hybrid Coatings. Macromol. Rapid Commun. 2003, 24, 711–714. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Synthesis and properties of thermosetting polymers from a phosphorous-containing fatty acid derivative. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5630–5644. [Google Scholar] [CrossRef]
- Koeduka, T.; Fridman, E.; Gang, D.R.; Vassão, D.G.; Jackson, B.L.; Kish, C.M.; Orlova, I.; Spassova, S.M.; Lewis, N.G.; Noel, J.P.; et al. Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proc. Natl. Acad. Sci. USA 2006, 103, 10128–10133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calo, E.; Maffezzoli, A.; Mele, G.; Martina, F.; Mazzetto, S.E.; Tarzia, A.; Stifani, C. Synthesis of a novel cardanol-based benzoxazine monomer and environmentally sustainable production of polymers and bio-composites. Green Chem. 2007, 9, 754. [Google Scholar] [CrossRef]
- Mallikappa, D.; Reddy, R.P.; Murthy, C. Performance and emission characteristics of double cylinder CI engine operated with cardanol bio fuel blends. Renew. Energy 2012, 38, 150–154. [Google Scholar] [CrossRef]
- Menon, A.R.R.; Pillai, C.K.S.; Nando, G.B. Chemical crosslink densit and network structure of natural rubber vulcanizates modified with phosphorylated cardnol prepolymer. J. Appl. Polym. Sci. 1994, 51, 2157–2164. [Google Scholar] [CrossRef]
- Antony, R.; Pillai, C.K.S.; Scariah, K.J. GPC studies on the cationic polymerization of cardanol initiated by borontrifluoridediethyletherate. J. Appl. Polym. Sci. 1990, 41, 1765–1775. [Google Scholar] [CrossRef]
- John, G.; Masuda, M.; Okada, Y.; Yase, K.; Shimizu, T. Nanotube Formation from Renewable Resources via Coiled Nanofibers. Adv. Mater. 2001, 13, 715–718. [Google Scholar] [CrossRef]
- Saladino, R.; Neri, V.; Mincione, E.; Marini, S.; Coletta, M.; Fiorucci, C.; Filippone, P. A new and efficient synthesis of ortho- and para-benzoquinones of cardanol derivatives by the catalytic system MeReO3–H2O2. J. Chem. Soc. Perkin Trans. 2000, 1, 581–586. [Google Scholar] [CrossRef]
- Toldy, A.; Szolnoki, B.; Csontos, I.; Marosi, G. Green synthesis and characterization of phosphorus flame retardant crosslinking agents for epoxy resins. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Abell, J.P.; Yamamoto, H. Catalytic Enantioselective Pudovik Reaction of Aldehydes and Aldimines with Tethered Bis(8-quinolinato) (TBOx) Aluminum Complex. J. Am. Chem. Soc. 2008, 130, 10521–10523. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zeng, B.; Gao, H.; Xu, Y.; Luo, W.; Liu, X.; Dai, L. Improving thermal and flame-retardant properties of epoxy resins by a novel reactive phosphorous-containing curing agent. Polym. Eng. Sci. 2014, 54, 1192–1200. [Google Scholar] [CrossRef]
- Lin, C.H.; Chou, Y.C.; Shiao, W.F.; Wang, M.W. High temperature, flame-retardant, and transparent epoxy thermosets prepared from an acetovanillone-based hydroxyl poly(ether sulfone) and commercial epoxy resins. Polymer 2016, 97, 300–308. [Google Scholar] [CrossRef]
- Lin, C.M.; Chen, C.H.; Lin, C.H.; Juang, T.Y. High-performance bio-based benzoxazines derived from phosphinated biphenols and furfurylamine. Eur. Polym. J. 2018, 108, 48–56. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Yang, H.; Xing, W.; Lu, H. Synthesis and characterization of a DOPO-substitued organophosphorus oligomer and its application in flame retardant epoxy resins. Prog. Org. Coatings 2011, 71, 72–82. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Xia, J.; Song, J.; Huang, K.; Li, M. Novel renewable resource-based UV-curable copolymers derived from myrcene and tung oil: Preparation, characterization and properties. Ind. Crops Prod. 2015, 63, 17–25. [Google Scholar] [CrossRef]
- Chen, H.B.; Dong, X.; Schiraldi, D.A.; Chen, L.; Wang, D.Y.; Wang, Y.Z. Phosphorus-containing poly(trimethylene terephthalate) derived from 2-(6-oxido-6H-dibenz<c,e><1,2>oxaphosphorin-6-yl)-1,4-hydroxyethoxy phenylene: Synthesis, thermal degradation, combustion and pyrolysis behavior. J. Anal. Appl. Pyrolysis 2013, 99, 40–48. [Google Scholar] [CrossRef]
- Li, T.; Shi, Z.; He, X.; Jiang, P.; Lu, X.; Zhang, R.; Wang, X. Aging-Resistant Functionalized LDH-SAS/Nitrile-Butadiene Rubber Composites: Preparation and Study of Aging Kinetics/Anti-Aging Mechanism. Materials 2018, 11, 836. [Google Scholar] [CrossRef]
- Schmidt, C.; Ciesielski, M.; Greiner, L.; Döring, M. Novel organophosphorus flame retardants and their synergistic application in novolac epoxy resin. Polym. Degrad. Stab. 2018, 158, 190–201. [Google Scholar] [CrossRef]
- Wang, P.J.; Liao, D.J.; Hu, X.P.; Pan, N.; Li, W.X.; Wang, D.Y.; Yao, Y. Facile fabrication of biobased P N C-containing nano-layered hybrid: Preparation, growth mechanism and its efficient fire retardancy in epoxy. Polym. Degrad. Stab. 2019, 159, 153–162. [Google Scholar] [CrossRef]
- Graf, E. Antioxidant functions of phytic acid. Free. Radic. Biol. Med. 1990, 8, 61–69. [Google Scholar] [CrossRef]
- Zhao, X.; Xiao, D.; Alonso, J.P.; Wang, D.Y. Inclusion complex between beta-cyclodextrin and phenylphosphonicdiamide as novel bio-based flame retardant to epoxy: Inclusion behavior, characterization and flammability. Mater. Des. 2017, 114, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Yang, L.; Martin, F.H.; Zhang, X.Q.; Wang, R.; Wang, D.Y. Influence of phenylphosphonate based flame retardant on epoxy/glass fiber reinforced composites (GRE): Flammability, mechanical and thermal stability properties. Compos. Part B Eng. 2017, 110, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Vanillin, a promising biobased building-block for monomer synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- Jin, S.; Qian, L.; Qiu, Y.; Chen, Y.; Xin, F. High-efficiency flame retardant behavior of bi-DOPO compound with hydroxyl group on epoxy resin. Polym. Degrad. Stab. 2019, 166, 344–352. [Google Scholar] [CrossRef]
- Lehr, V.; Sarlea, M.; Ott, L.; Vogel, H. Catalytic dehydration of biomass-derived polyols in sub- and supercritical water. Catal. Today 2007, 121, 121–129. [Google Scholar] [CrossRef]
- Howell, B.A.; Sun, W. Biobased flame retardants from tartaric acid and derivatives. Polym. Degrad. Stab. 2018, 157, 199–211. [Google Scholar] [CrossRef]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of Poly(Lactic Acid): A Review. J. Macromol. Sci. Part C 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Domenek, S.; Courgneau, C.; Ducruet, V. Characteristics and Applications of Poly(lactide). Biopolymers 2011, 183–223. [Google Scholar]
- Obuchi, S.; Ogawa, S. Packaging and Other Commercial Applications. Poly (Lactic Acid) 2010, 457–467. [Google Scholar]
- Hamad, K. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Bourbigot, S.; Fontaine, G. Flame retardancy of polylactide: An overview. Polym. Chem. 2010, 1, 1413. [Google Scholar] [CrossRef]
- Chow, W.; Teoh, E.; Karger-Kocsis, J. Flame retarded poly(lactic acid): A review. eXPRESS Polym. Lett. 2018, 12, 396–417. [Google Scholar] [CrossRef]
- Tawiah, B.; Yu, B.; Fei, B. Advances in Flame Retardant Poly(Lactic Acid). Polymers 2018, 10, 876. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, Y.; Tang, X.; Li, X.; Peng, M.; Fang, Z. Combination of a bio-based polyphosphonate and modified graphene oxide toward superior flame retardant polylactic acid. RSC Adv. 2018, 8, 4304–4313. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Han, L.; Dong, L. Thermal degradation behavior and gas phase flame-retardant mechanism of polylactide/PCPP blends. J. Appl. Polym. Sci. 2014, 131, 131. [Google Scholar] [CrossRef]
- Réti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability properties of intumescent PLA including starch and lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Aguedo, M.; Richel, A.; Brohez, S.; Delvosalle, C.; Dubois, P. Phosphorus and nitrogen derivatization as efficient route for improvement of lignin flame retardant action in PLA. Eur. Polym. J. 2016, 84, 652–667. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Delvosalle, C.; Dubois, P. Phytic acid–lignin combination: A simple and efficient route for enhancing thermal and flame retardant properties of polylactide. Eur. Polym. J. 2017, 94, 270–285. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Dumazert, L.; Lopez-Cuesta, J.-M.; Brohez, S.; Delvosalle, C.; Dubois, P. Metallic phytates as efficient bio-based phosphorous flame retardant additives for poly(lactic acid). Polym. Degrad. Stab. 2015, 119, 217–227. [Google Scholar] [CrossRef]
- Laoutid, F.; Vahabi, H.; Shabanian, M.; Aryanasab, F.; Zarrintaj, P.; Saeb, M.R. A new direction in design of bio-based flame retardants for poly(lactic acid). Fire Mater. 2018, 42, 914–924. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Khelifa, F.; Rose, G.; Brohez, S.; Delvosalle, C.; Dubois, P. Cellulose/phosphorus combinations for sustainable fire retarded polylactide. Eur. Polym. J. 2016, 74, 218–228. [Google Scholar] [CrossRef]
- Fox, D.M.; Lee, J.; Citro, C.J.; Novy, M. Flame retarded poly(lactic acid) using POSS-modified cellulose. 1. Thermal and combustion properties of intumescing composites. Polym. Degrad. Stab. 2013, 98, 590–596. [Google Scholar] [CrossRef]
- Fox, D.M.; Novy, M.; Brown, K.; Zammarano, M.; Harris, R.H.; Murariu, M.; McCarthy, E.D.; Seppala, J.E.; Gilman, J.W. Flame retarded poly(lactic acid) using POSS-modified cellulose. 2. Effects of intumescing flame retardant formulations on polymer degradation and composite physical properties. Polym. Degrad. Stab. 2014, 106, 54–62. [Google Scholar] [CrossRef]
- Vahabi, H.; Shabanian, M.; Aryanasab, F.; Mangin, R.; Laoutid, F.; Saeb, M.R. Inclusion of modified lignocellulose and nano-hydroxyapatite in development of new bio-based adjuvant flame retardant for poly(lactic acid). Thermochim. Acta 2018, 666, 51–59. [Google Scholar] [CrossRef]
- Chen, C.; Gu, X.; Jin, X.; Sun, J.; Zhang, S. The effect of chitosan on the flammability and thermal stability of polylactic acid/ammonium polyphosphate biocomposites. Carbohydr. Polym. 2017, 157, 1586–1593. [Google Scholar] [CrossRef]
- Feng, J.X.; Su, S.P.; Feng, J.; Su, S.; Zhu, J. An intumescent flame retardant system using β-cyclodextrin as a carbon source in polylactic acid (PLA). Polym. Adv. Technol. 2011, 22, 1115–1122. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Wang, B.; Wen, P.; Song, L.; Hu, Y.; Zhang, P. Comparative Study on the Effect of Beta-Cyclodextrin and Polypseudorotaxane As Carbon Sources on the Thermal Stability and Flame Retardance of Polylactic Acid. Ind. Eng. Chem. Res. 2013, 52, 3287–3294. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xuan, S.; Xing, W.; Bai, Z.; Lu, H. Flame Retardancy and Thermal Degradation of Intumescent Flame Retardant Poly(lactic acid)/Starch Biocomposites. Ind. Eng. Chem. Res. 2011, 50, 713–720. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, X.; Gu, X.; Chen, C.; Li, H.; Zhang, Z.; Sun, J. The preparation of fully bio-based flame retardant poly(lactic acid) composites containing casein. J. Appl. Polym. Sci. 2018, 135, 46599. [Google Scholar] [CrossRef]
- Zhang, N.; Shen, J.; Pasquinelli, M.A.; Hinks, D.; Tonelli, A.E. Formation and characterization of an inclusion complex of triphenyl phosphate and β-cyclodextrin and its use as a flame retardant for polyethylene terephthalate. Polym. Degrad. Stab. 2015, 120, 244–250. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, X.; Tao, X. Intumescent flame retardant and anti-dripping of PET fabrics through layer-by-layer assembly of chitosan and ammonium polyphosphate. Prog. Org. Coatings 2019, 134, 162–168. [Google Scholar] [CrossRef]
- Stelzig, T.; Bommer, L.; Gaan, S.; Buczko, A. DOPO-Based Hybrid Flame Retardants. U.S. Patents 20170081590A1, 23 March 2017. [Google Scholar]
- Seiji, E.; Takao, K.; Akitada, O.; Tatsuhiko, S.; Tadashi, I. Neue phosphor-enthaltende verbindungen. U.S. Patent C07F9/657172, 13 October 1976. [Google Scholar]
- Wermter, H.; Futterer, T.; Fünderich, S. Flame Retardant Composition for Thermoplastic Molding Compounds. U.S. Patents 20120322923A1, 20 December 2012. [Google Scholar]
- LeComte, H.A.; Liggat, J.J. Commercial fire-retarded PET formulations—Relationship between thermal degradation behaviour and fire-retardant action. Polym. Degrad. Stab. 2008, 93, 498–506. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Correlo, V.M.; Sol, P.C.; Costa-Pinto, A.R.; Malafaya, P.B.; Salgado, A.J.; Bhattacharya, M.; Charbord, P.; Neves, N.M.; Reis, R.L. Assessment of the suitability of chitosan/polybutylene succinate scaffolds seeded with mouse mesenchymal progenitor cells for a cartilage tissue engineering approach. Tissue Eng. Part A 2008, 14, 1651–1661. [Google Scholar] [CrossRef]
- Petchwattana, N.; Covavisaruch, S.; Wibooranawong, S.; Naknaen, P. Antimicrobial food packaging prepared from poly(butylene succinate) and zinc oxide. Measurement 2016, 93, 442–448. [Google Scholar] [CrossRef]
- Fujimaki, T. Processability and properties of aliphatic polyesters, ‘BIONOLLE’, synthesized by polycondensation reaction. Polym. Degrad. Stab. 1998, 59, 209–214. [Google Scholar] [CrossRef]
- Miao, J.-T.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Biobased epoxy resin derived from eugenol with excellent integrated performance and high renewable carbon content. Polym. Int. 2018, 67, 1194–1202. [Google Scholar] [CrossRef]
- Weibin, C.; Jianqing, Z.; Xuehao, L. Analysis of Standard Situation of PSA Tapes in China and Abroad. Sci. J. Chem. 2018, 6, 24. [Google Scholar] [CrossRef]
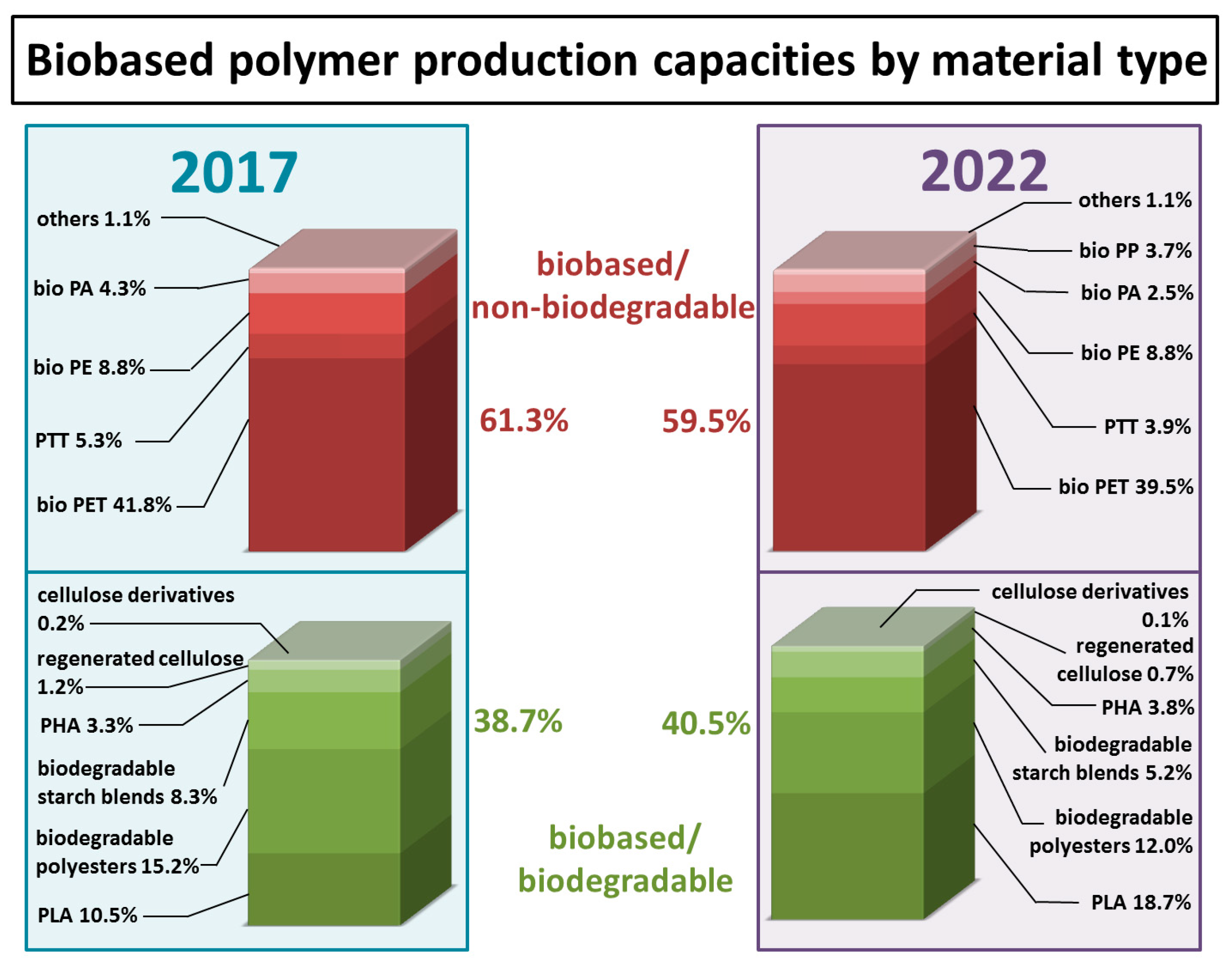



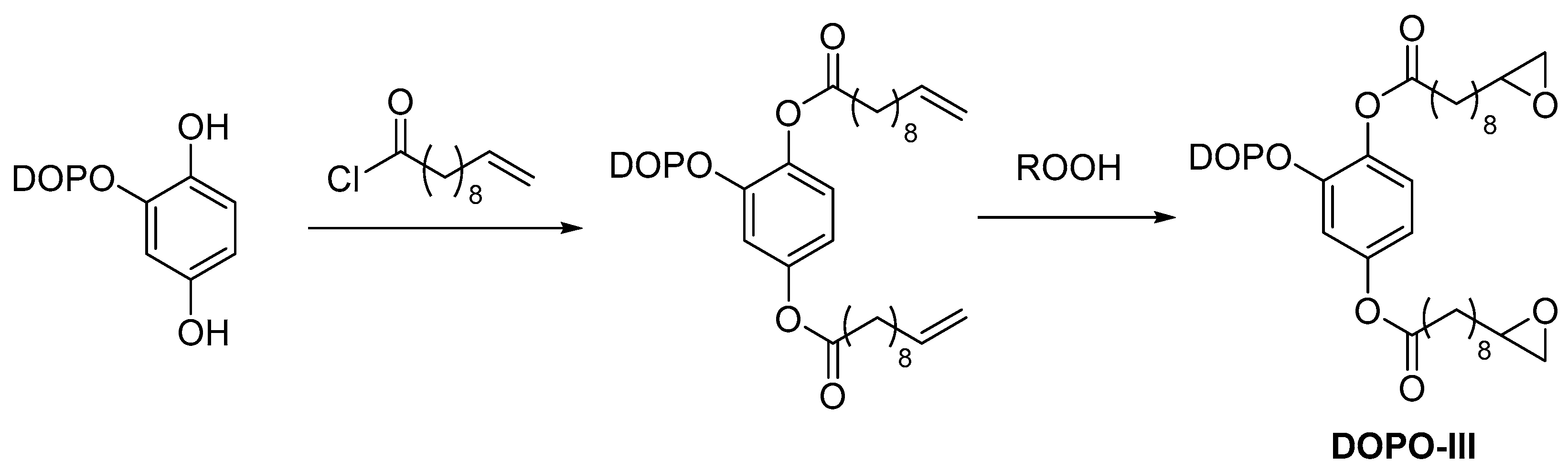
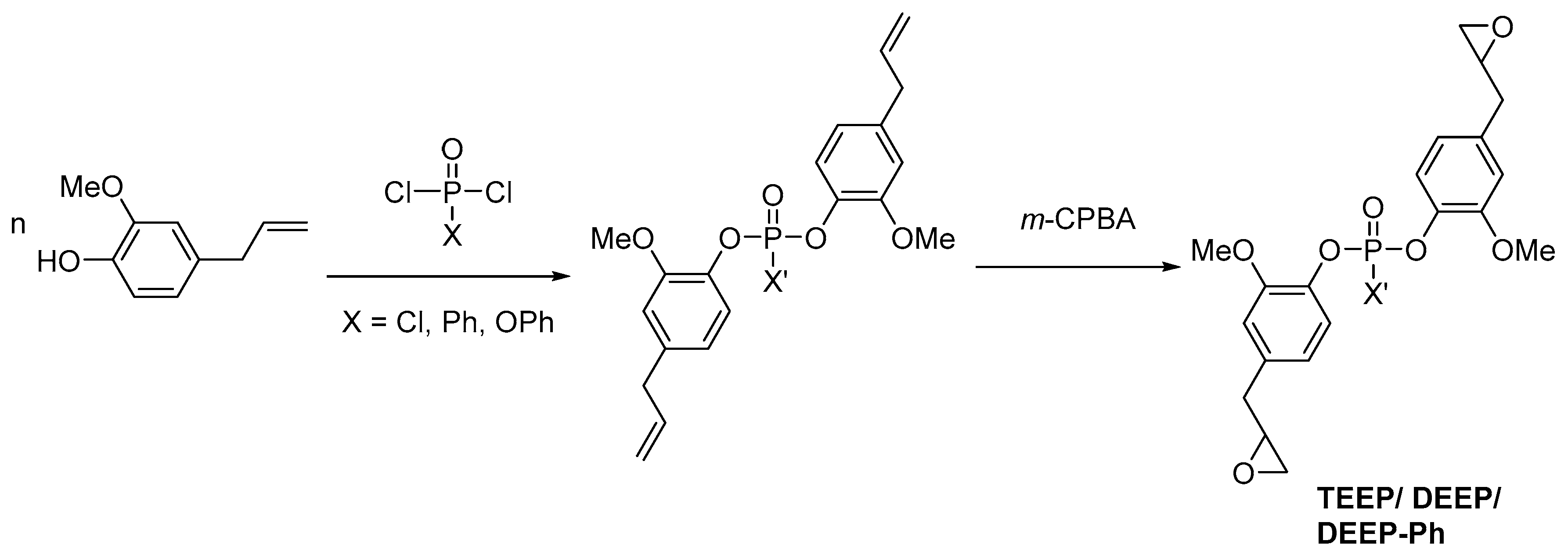

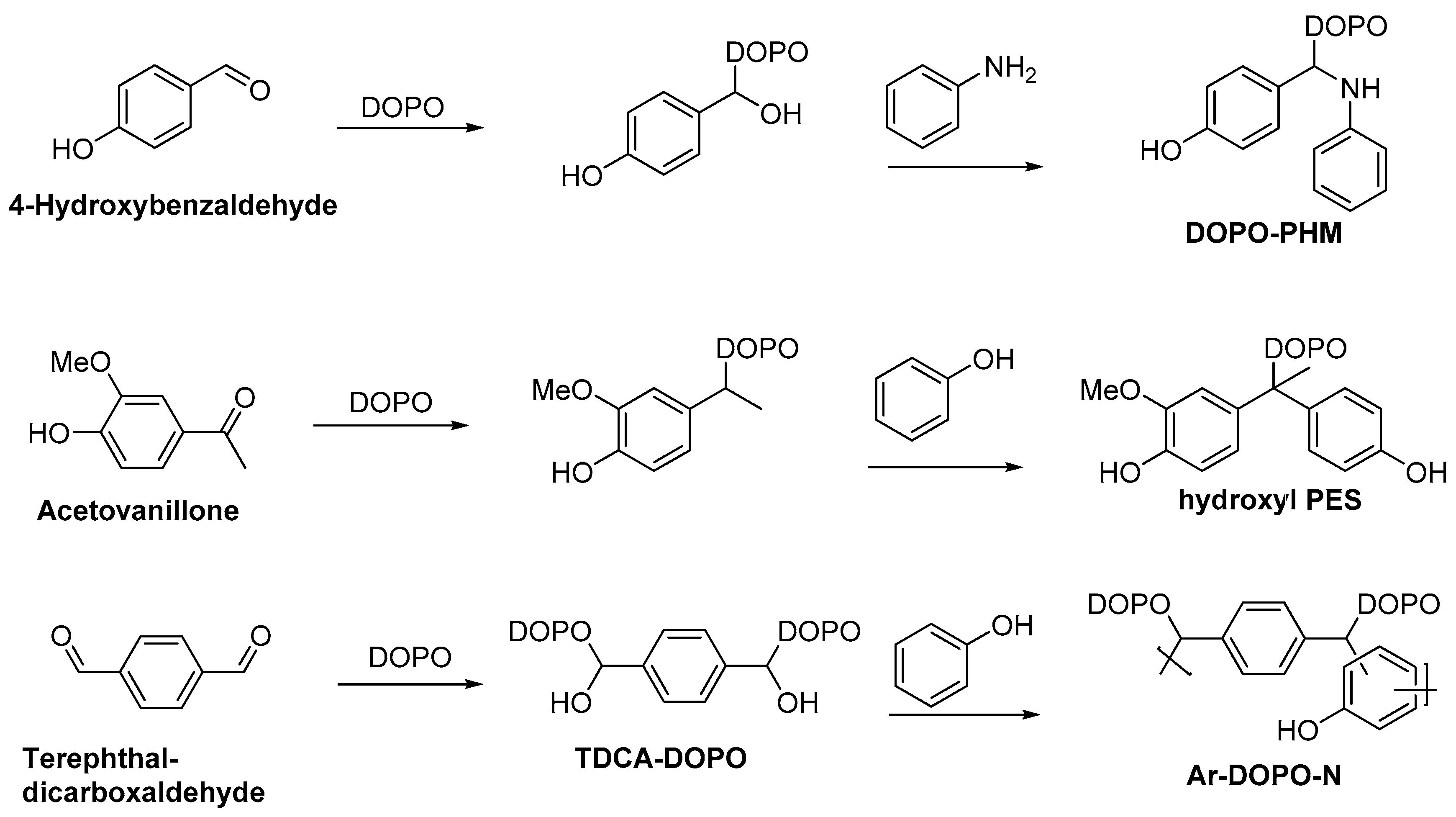
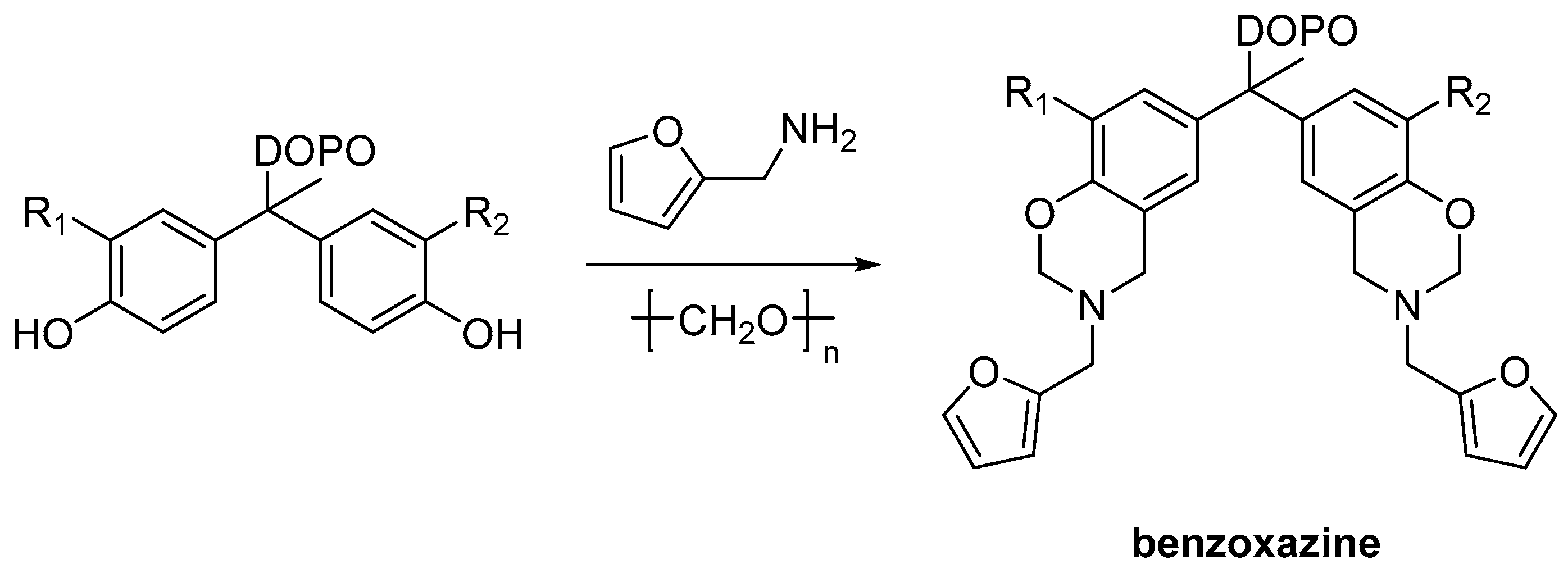



| Monomer/Chemical Building Block | Biotechnical Process | Application | Reference |
|---|---|---|---|
| Adipic acid | Fermentation of glucose + hydrogenation of cis,cis-muconic acid or glucaric acid | Resins, polyesteramines, polyesterurethanes | [13,32,33,34] |
| 1,4-butanediol | Fermentation of sugars, hydrogenation of succinic acid | PBAT, PBS, PBT | [13,34,35] |
| Benzaldehyde | Steam distillation | Resins, flame retardants | [36,37] |
| Benzene, toluene, xylene (BTX) | Pyrolysis of lignocellulose residues | Building block for PET | [12,38] |
| Cardanol | Extraction from cashew nuts via hot oil or roasting process, distillation | Resins | [18,20,39] |
| Ethylene glycol | Ethanol dehydration, hydrogenolysis of xylitol, sorbitol or glycerol | PET, PEF | [13,38] |
| Eugenol | Extraction from Dianthus | Resins | [40,41,42] |
| 2,5-Furandicarboxylic acid | Fermentation, dehydration of fructose/glucose, oxidation | PEF, PBF, polyesterurethanes | [13,43] |
| Isobutanol | Yeast fermentation of sugars | PET (production of terephthalic) | [12,13] |
| Isosorbide | Sorbitol dehydration | Thermosetting resins | [13,44] |
| Itaconic acid | Fermentation of carbohydrates | Photocurable precursors, plasticizers, resins, flame retardants | [13,45,46] |
| Lactic acid | Fermentation | PLA | [13,47,48] |
| Levullinic acid | Acid hydrolysis of lignocellulose | PBS | [13,49,50] |
| Lignin | Plant biomass | Resins, PET, Flame retardant agents | [51,52,53,54,55] |
| Malic acid | fermentation | Functionalized chiral polyesters | [13,56] |
| Myrcene | Extraction from essential oils, pyrolysis of β-pinene | Resins, Polyesters, Polymyrcene | [57,58,59] |
| p-Benzoquinone | Microbial catalysis of glucose, oxidation | Resins | [60,61] |
| Phloroglucinol | Extraction of seaweeds | Resins | [62,63] |
| Phytic acid | Extraction from beans | Flame retardant | [64,65,66] |
| 1,3-Propanediol | Fermentation | PTT, fibers, elastomers, polyesterurethanes | [12,13,67] |
| Sebacic acid | Caustic fusion from castor oil | Polyesters, Resins | [68,69] |
| Sorbitol | Fermentation and hydrogenation | Functional polyesters, coatings | [13,70,71] |
| Succinic acid | Fermentation of sugars | Textiles, coatings, PBS, PBT | [13,49,72] |
| Tartaric acid | Precipitation in wine production | Polyester, flame retardant | [73,74] |
| Terephthalic acid | Isobutylene oxidation, fermentation | PET, coatings | [12,13,75,76] |
| Vanillin | Oxidation of lignin | Resins, polyvanillin | [77,78] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sag, J.; Goedderz, D.; Kukla, P.; Greiner, L.; Schönberger, F.; Döring, M. Phosphorus-Containing Flame Retardants from Biobased Chemicals and Their Application in Polyesters and Epoxy Resins. Molecules 2019, 24, 3746. https://doi.org/10.3390/molecules24203746
Sag J, Goedderz D, Kukla P, Greiner L, Schönberger F, Döring M. Phosphorus-Containing Flame Retardants from Biobased Chemicals and Their Application in Polyesters and Epoxy Resins. Molecules. 2019; 24(20):3746. https://doi.org/10.3390/molecules24203746
Chicago/Turabian StyleSag, Jacob, Daniela Goedderz, Philipp Kukla, Lara Greiner, Frank Schönberger, and Manfred Döring. 2019. "Phosphorus-Containing Flame Retardants from Biobased Chemicals and Their Application in Polyesters and Epoxy Resins" Molecules 24, no. 20: 3746. https://doi.org/10.3390/molecules24203746
APA StyleSag, J., Goedderz, D., Kukla, P., Greiner, L., Schönberger, F., & Döring, M. (2019). Phosphorus-Containing Flame Retardants from Biobased Chemicals and Their Application in Polyesters and Epoxy Resins. Molecules, 24(20), 3746. https://doi.org/10.3390/molecules24203746






