Synthesis of 1,1′-Bis(1-Methyl/Chloro-2,3,4,5-Tetraphenyl-1-Silacyclopentadienyl) [Ph4C4Si(Me/Cl)-(Me/Cl)SiC4Ph4] from Silole Anion [MeSiC4Ph4]−•[Li+ or Na+] and Silole Dianion [SiC4Ph4]2−•2[Li+]; Oxidative Coupling of Silole Anion [MeSiC4Ph4]−•[Li+ or Na+] by Ferrous Chloride (FeCl2) and Oxidative Coupling and Chlorination of Silole Dianion [SiC4Ph4]2−•2[Li+] by Cupric Chloride (CuCl2)
Abstract
:1. Introduction
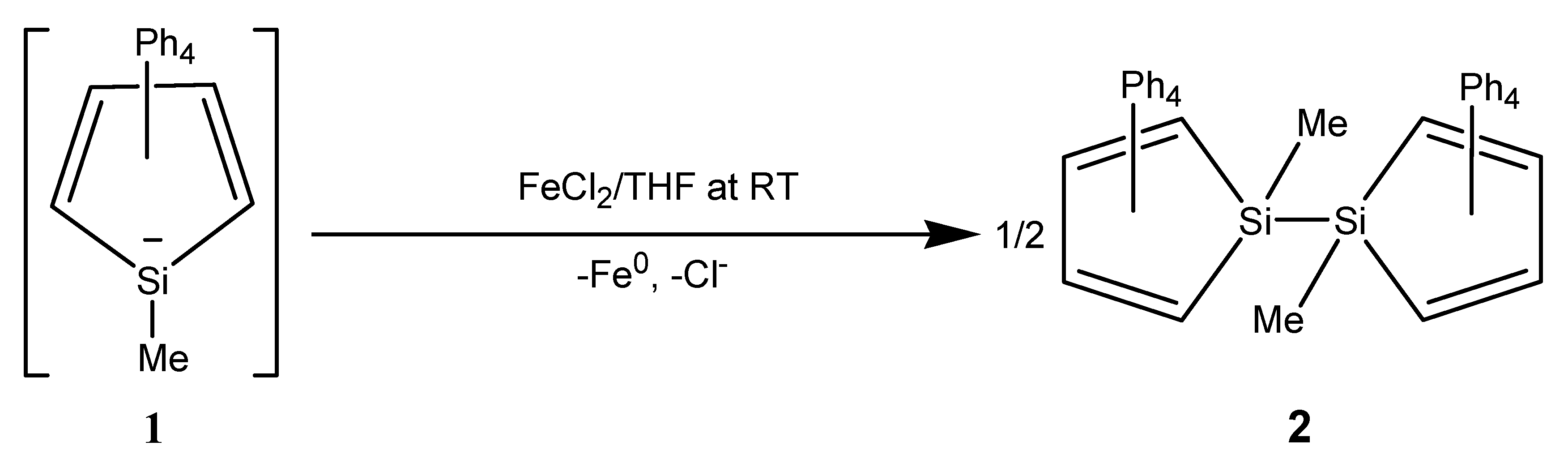
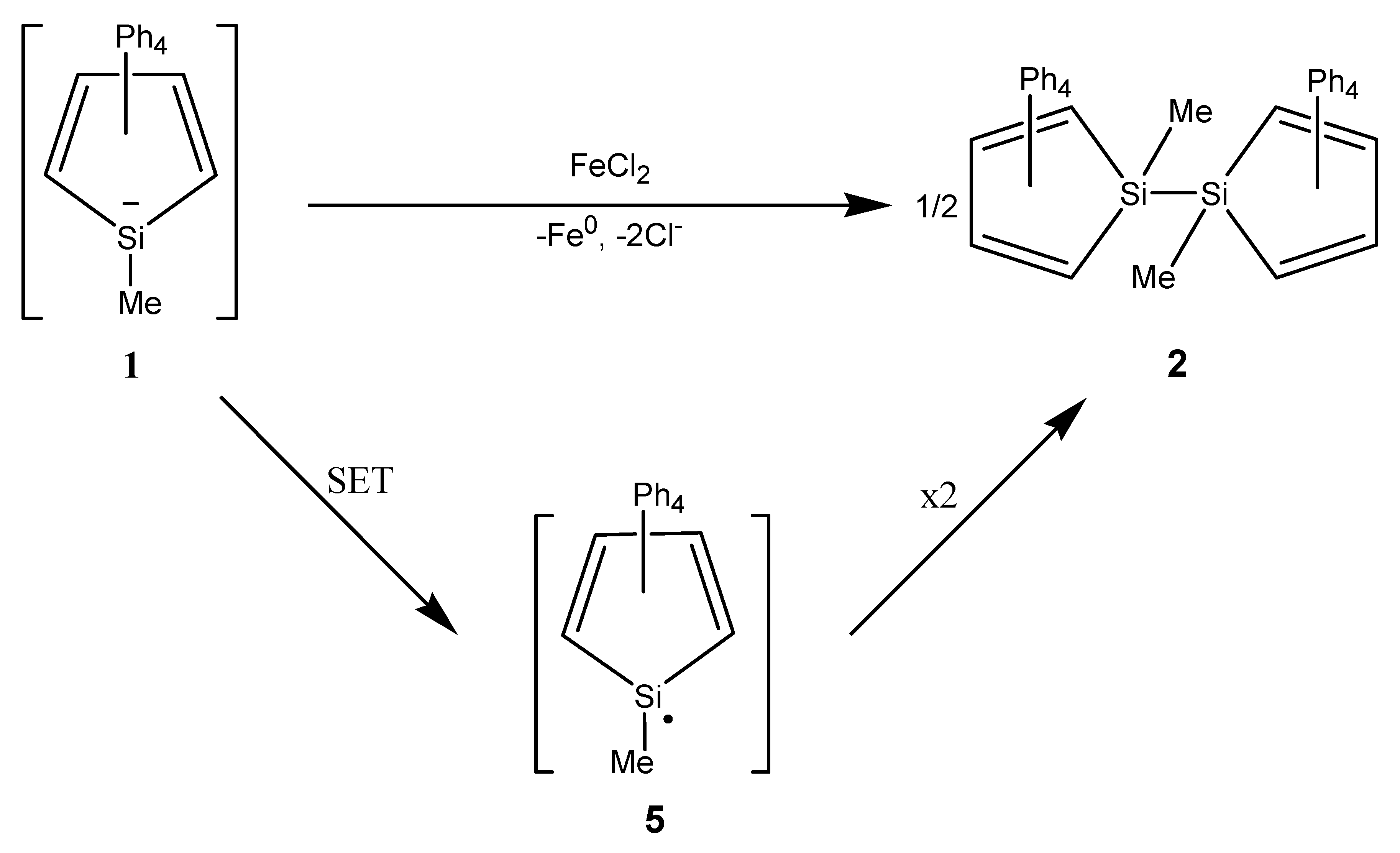
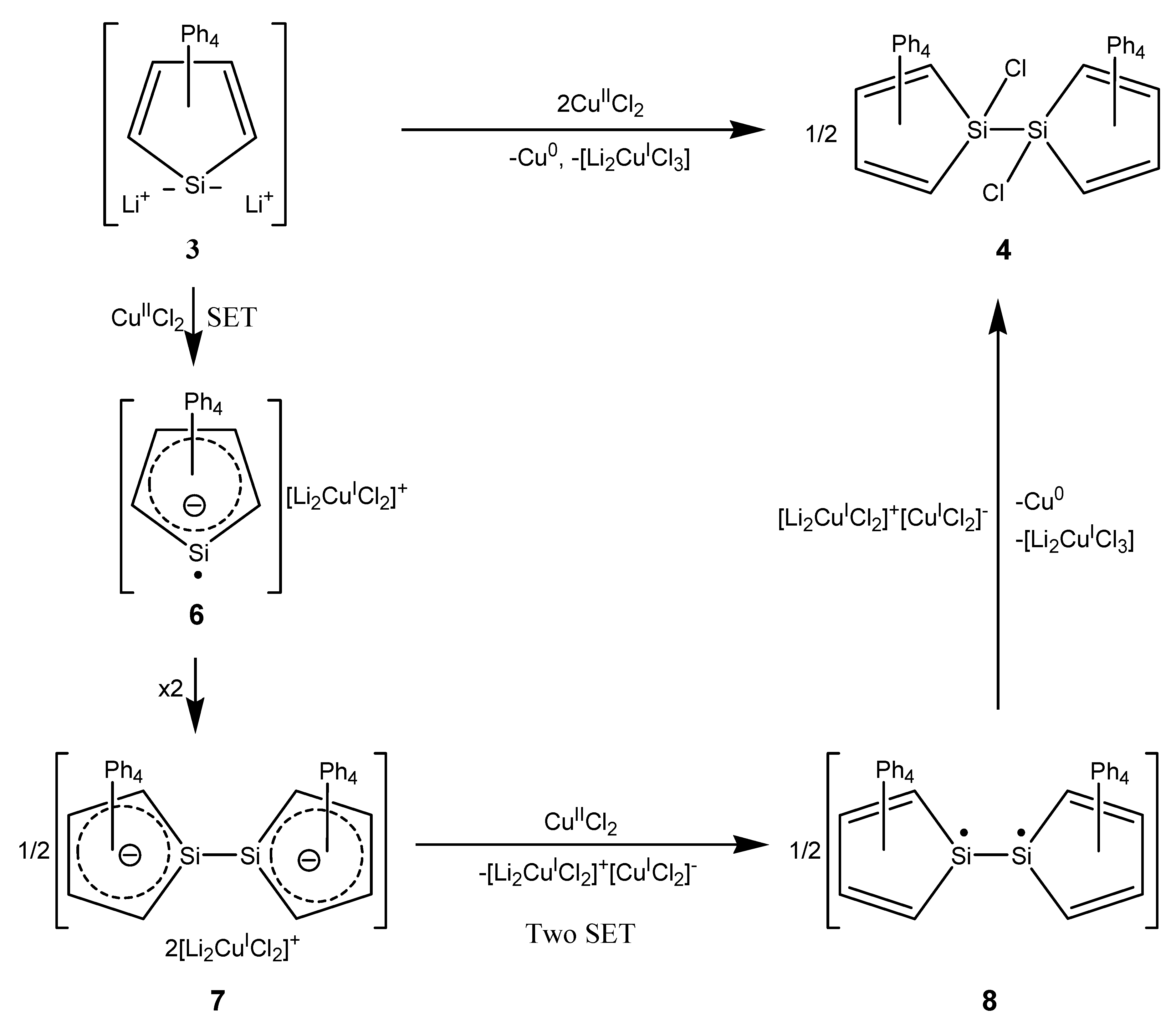
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Funding
Conflicts of Interest
References
- Lee, V.Y.; Sekiguchi, A.; Ichinohe, M.; Fukaya, N. Stable aromatic compounds containing heavier group 14 elements. J. Organomet. Chem. 2000, 611, 228–235. [Google Scholar] [CrossRef]
- Hissler, M.; Dyer, P.W.; Réau, R. Linear organic π-conjugated systems featuring the heavy group 14 and 15 elements. Co-ord. Chem. Rev. 2003, 244, 1–44. [Google Scholar] [CrossRef]
- Grützmacher, H. Five-membered aromatic and antianromatic rings with gallium, germanium, and bismuth centers: A short march through the periodic table. Angew. Chem. Int. Ed. 1995, 34, 295–298. [Google Scholar] [CrossRef]
- Lee, V.Y.; Sekiguchi, A. Aromaticity of group 14 organometallics: Experimental aspects. Angew. Chem. Int. Ed. 2007, 38, 6596–6620. [Google Scholar] [CrossRef]
- Saito, M.; Yoshioka, M. The anions and dianions of group 14 metalloles. Co-ord. Chem. Rev. 2005, 249, 765–780. [Google Scholar] [CrossRef]
- Saito, M. Challenge to expand the concept of aromaticity to tin- and lead-containing carbocyclic compounds: Synthesis, structures and reactions of dilithiostannoles and dilithioplumbole. Co-ord. Chem. Rev. 2012, 256, 627–636. [Google Scholar] [CrossRef]
- Joo, W.-C.; Hong, J.-H.; Choi, S.-B.; Son, H.-E.; Kim, C.H. Synthesis and reactivity of 1,1-disodio-2,3,4,5-tetraphenyl-1-silacyclopentadiene. J. Organomet. Chem. 1990, 391, 27–36. [Google Scholar] [CrossRef]
- Hong, J.-H.; Boudjouk, P. Synthesis and characterization of a delocalized germanium-containing dianion: Dilithio-2,3,4,5-tetraphenyl-germole. Bull. Soc. Chim. Fr. 1995, 132, 495–498. [Google Scholar] [CrossRef]
- Hong, J.H.; Boudjouk, P. A stable aromatic species containing silicon. Synthesis and characterization of the 1-tert-butyl-2,3,4,5-tetraphenyl-1-silacyclopentadienide anion. J. Am. Chem. Soc. 1993, 115, 5883–5884. [Google Scholar] [CrossRef]
- Hong, J.-H.; Boudjouk, P.; Castellino, S. Synthesis and characterization of two aromatic silicon-containing dianions: The 2,3,4,5-tetraphenylsilole dianion and the 1,1′-disila-2,2′,3,3′,4,4′,5,5′-octaphenylfulvalene dianion. Organometallics 1994, 13, 3387–3389. [Google Scholar] [CrossRef]
- West, R.; Sohn, H.; Bankwitz, U.; Calabrese, J.; Apeloig, T.; Müller, T. Dilithium derivative of tetraphenylsilole: An η1-η5 dilithium structure. J. Am. Chem. Soc. 1995, 117, 11608–11609. [Google Scholar] [CrossRef]
- Freeman, W.P.; Tilley, T.D.; Yap, G.P.A.; Rheingold, A.L. Siloyl anions and silole dianions: Structure of [K(18-crown-6)+]2[C4Me4Si2−]. Angew. Chem. Int. Ed. 1996, 35, 882–884. [Google Scholar] [CrossRef]
- West, R.; Sohn, H.; Powell, D.R.; Müller, T.; Apeloig, Y. The dianion of tetraphenylgermole is aromatic. Angew. Chem. Int. Ed. 1996, 35, 1002–1004. [Google Scholar] [CrossRef]
- Choi, S.-B.; Boudjouk, P.; Hong, J.-H. Unique bis-η5/η1-bonding in a dianionic germole. Synthesis and structural characterization of the dilithium salt of the 2,3,4,5-tetraethyl germole dianion. Organometallics 1999, 18, 2919–2921. [Google Scholar] [CrossRef]
- Freeman, W.P.; Tilley, T.D.; Liable-Sands, L.M.; Rheingold, A.L. Synthesis and study of cyclic π-systems containing silicon and germanium. The question of aromaticity in cyclopentaidenyl analogues. J. Am. Chem. Soc. 1996, 118, 10457–10468. [Google Scholar] [CrossRef]
- Goldfuss, B.; Schleyer, P.V.R.; Hampel, F. Aromaticity in silole dianions: Structural, energetic, and magnetic aspects. Organometallics 1996, 15, 1755–1757. [Google Scholar] [CrossRef]
- Goldfuss, B.; Schleyer, P.V.R. Aromaticity in group 14 metalloles: Structural, energetic, and magnetic criteria. Organometallics 1997, 16, 1543–1552. [Google Scholar] [CrossRef]
- Aubouy, L.; Huby, N.; Hirsch, L.; Van Der Lee, A.; Gerbier, P. Molecular engineering to improve the charge carrier balance in single-layer silole-based OLEDs. New J. Chem. 2009, 33, 1290. [Google Scholar] [CrossRef]
- Bankwitz, U.; Sohn, H.; Powell, D.R.; West, R. Synthesis, soild-state structure, and reduction of 1,1-dichloro-2,3,4,5-tetramethylsilole. J. Organomet. Chem. 1995, 499, C7–C9. [Google Scholar] [CrossRef]
- Hong, J.-H.; Pan, Y.; Boudjouk, P. A novel lithocenophane derivative of a trisgermole dianion: [Li(thf)(tmeda)][2,3,4,5-Et4-Ge,Ge-{Li(2,3,4,5-Et4C4Ge)2}C4Ge]. Angew. Chem. Int. Ed. 1996, 35, 186–188. [Google Scholar] [CrossRef]
- Sohn, H.; Powell, D.R.; West, R.; Hong, J.-H.; Joo, W.-C. Dimerization of the silole anion [C4Ph4SiMe]− to a tricyclic diallylic dianion. Organometallics 1997, 16, 2770–2772. [Google Scholar] [CrossRef]
- Pan, Y.; Hong, J.-H.; Choi, S.-B.; Boudjouk, P. Surprising reaction of non-nucleophilic bases with 1-hydrosiloles: addition and not deprotonation1. Organometallics 1997, 16, 1445–1451. [Google Scholar] [CrossRef]
- Hong, J.-H.; Boudjouk, P. Synthesis and characterization of a novel pentavalent silane: 1-Methyl-1,1-dihydrido-2,3,4,5-tetraphenyl-1-silacyclopentadiene silicate, [Ph4C4SiMeH2−]•[K+]. Organometallics 1995, 14, 574–576. [Google Scholar] [CrossRef]
- Hong, J.-H. Synthesis and NMR-study of the 2,3,4,5-tetraethylsilole dianion [SiC4Et4]2−•2[Li]+. Molecules 2011, 16, 8033–8040. [Google Scholar] [CrossRef]
- Hong, J.-H. Dissociation of the disilatricyclic diallylic dianion [(C4Ph4SiMe)2]−2 to the silole anion [MeSiC4Ph4]− by halide ion coordination or halide ion nucleophilic substitution at the silicon atom. Molecules 2011, 16, 8451–8462. [Google Scholar] [CrossRef]
- Zhan, X.; Risko, C.; Amy, F.; Chan, C.; Zhao, W.; Barlow, S.; Kahn, A.; Brédas, J.-L.; Marder, S.R. Electron affinities of 1,1-diaryl-2,3,4,5-tetraphenylsiloles: Direct measurements and comparison with experimental and theoretical estimates. J. Am. Chem. Soc. 2005, 127, 9021–9029. [Google Scholar] [CrossRef]
- Tandura, S.N.; Kolesnikov, S.P.; Nosov, K.S.; Lee, V.Y.; Egorov, M.P.; Nefedov, O.M. Electron density distributions in substituted 2,3,4,5-tetraphenyl-1-germacyclopenta-2,4-dienes studied by NMR spectroscopy. Russ. Chem. Bull. 1997, 46, 1859–1861. [Google Scholar] [CrossRef]
- Fekete, C.; Nyulászi, L.; Kovács, I. Synthesis and NMR characterization of 2,5-bis(Trimethylsilyl)-3,4-diphenyl-1-silacyclopentadienyl dianion. Phosphorus Sulfur Silicon Relat. Elem. 2014, 189, 1076–1083. [Google Scholar] [CrossRef]
- Dong, Z.; Reinhold, C.R.W.; Schmidtmann, M.; Müller, T. Trialkylsilyl-substituted silole and germole dianions. Organometallics 2018, 37, 4736–4743. [Google Scholar] [CrossRef]
- Fekete, C.; Kovács, I.; Nyulászi, L.; Holczbauer, T. Planar lithium silolide: aromaticity, with significant contribution of non-classical resonance structures. Chem. Commun. 2017, 53, 11064–11067. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Xie, K.; Ma, X.; Lu, X. Synthesis and characterization of a 2, 5-bis(trimethylsilyl)-substituted bis(1, 1′-silolide) dianion. Phosphorus, Sulfur, Silicon Relat. Elements 2018, 193, 1–15. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, W.-X.; Xi, Z. The aromatic dianion metalloles. Chem. Sci. 2018, 9, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Toulokhonova, I.S.; Stringfellow, T.C.; Ivanov, S.A.; Masunov, A.; West, R. A disilapentalene and a stable diradical from the reaction of a dilithiosilole with a dichlorocyclopropene. J. Am. Chem. Soc. 2003, 125, 5767–5773. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.; Merritt, J.; Powell, D.R.; West, R. A new spirocyclic system: Synthesis of a silaspirotropylidene. Organometallics 1997, 16, 5133–5134. [Google Scholar] [CrossRef]
- Toulokhonova, I.S.; Guzei, I.A.; West, R. Synthesis of a silene from 1,1-dilithiosilole and 2-adamantanone. J. Am. Chem. Soc. 2004, 126, 5336–5337. [Google Scholar] [CrossRef]
- Toulokhonova, I.S.; Friedrichsen, D.R.; Hill, N.J.; Müller, T.; West, R. Unusual reaction of 1,1-dilithio-2,3,4,5-tetraphenylsilole with 1,3-dienes yielding spirosilanes and elemental lithium. Angew. Chem. Int. Ed. 2006, 45, 2578–2581. [Google Scholar] [CrossRef]
- Toulokhonova, I.S.; Timokhin, V.I.; Bunck, D.N.; Guzei, I.; West, R.; Müller, T. Silylene and germylene intermediates in the reactions of silole and germole dianions with N,N′-di-tert-butylethylenediimine. Eur. J. Inorg. Chem. 2008, 2008, 2344–2349. [Google Scholar] [CrossRef]
- Jutzi, P.; Karl, A. Synthese und reaktionen einiger 1R, 1R′, 2, 3, 4, 5-tetraphenyl-1-silacyclopentadiene. J. Organomet. Chem. 1981, 214, 289–302. [Google Scholar] [CrossRef]
- McCann, S.D.; Stahl, S.S. ChemInform abstract: Copper-catalyzed aerobic oxidations of organic molecules: Pathways for two-electron oxidation with a four-electron oxidant and a one-electron redox-active catalyst. Acc. Chem. Res. 2015, 46, 1756–1766. [Google Scholar] [CrossRef]
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Rout, L. Recent advances in copper-catalyzed oxidation of organic compounds. Co-ord. Chem. Rev. 2008, 252, 134–154. [Google Scholar] [CrossRef]
- Allen, S.E.; Walvoord, R.R.; Padilla-Salinas, R.; Kozlowski, M.C. Aerobic copper-catalyzed organic reactions. Chem. Rev. 2013, 113, 6234–6458. [Google Scholar] [CrossRef] [PubMed]
- Wendlandt, A.E.; Suess, A.M.; Stahl, S.S. Copper-catalyzed aerobic oxidative C—H functionalizations: Trends and mechanistic insights. Angew. Chem. Int. Ed. 2011, 50, 11062–11087. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, C.; Tang, C.; Jiao, N. Recent advances in transition-metal catalyzed reactions using molecular oxygen as the oxidant. Chem. Soc. Rev. 2012, 41, 3381. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I. Organocopper Reagents: A Practical Approach; Taylor, R.J.K., Ed.; Oxford University Press: Oxford, UK, 1994; Chapter 12; p. 257. [Google Scholar]
- Zhang, C.; Tang, C.; Jiao, N. Recent advances in copper-catalyzed dehydrogenative functionalization via a single electron transfer (SET) process. Chem. Soc. Rev. 2012, 41, 3464. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.A.; Knauber, T.; Li, C.-J. The cross-dehydrogenative coupling of C (sp3)-H bonds: a versatile strategy for CC bond formations. Angew. Chem. Int. Ed. 2014, 53, 74–100. [Google Scholar] [CrossRef]
- Yang, L.; Lu, Z.; Stahl, S.S. Regioselective copper-catalyzed chlorination and bromination of arenes with O2 as the oxidant. Chem. Commun. 2009, 42, 6460–6462. [Google Scholar] [CrossRef]
- Chen, X.; Goodhue, C.E.; Hao, X.-S.; Yu, J.-Q.; Hao, X.; Yu, J. Cu(II)-catalyzed functionalizations of aryl C—H bonds using O2 as an oxidant. J. Am. Chem. Soc. 2006, 37, 6790–6791. [Google Scholar] [CrossRef]
- Uemura, T.; Imoto, S.; Chatani, N. Amination of the ortho C–H bonds by the Cu(OAc)2-mediated reaction of 2-phenylpyridines with anilines. Chem. Lett. 2006, 35, 842–843. [Google Scholar] [CrossRef]
- Menini, L.; Gusevskaya, E.V. Novel highly selective catalytic oxychlorination of phenols. Chem. Commun. 2006, 2, 209–211. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Jin, R.-Z.; Tamao, K.; Shiro, M. Silicon-catenated silole oligomers: Oligo(1,1-silole)s. Organometallics 1997, 16, 2486–2488. [Google Scholar] [CrossRef]
- Liu, Y.; Stringfellow, T.C.; Ballweg, D.; Guzei, I.A.; West, R. Structure and chemistry of 1-silafluorenyl dianion, its derivatives, and an organosilicon diradical dianion. J. Am. Chem. Soc. 2002, 124, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ballweg, D.; Müller, T.; Guzei, I.A.; Clark, R.W.; West, R. Chemistry of the aromatic 9-germafluorenyl dianion and some related silicon and carbon species. J. Am. Chem. Soc. 2002, 124, 12174–12181. [Google Scholar] [CrossRef] [PubMed]
- Menini, L.; Gusevskaya, E.V. Aerobic oxychlorination of phenols catalyzed by copper(II) chloride. Appl. Catal. A Gen. 2006, 309, 122–128. [Google Scholar] [CrossRef]
- Menini, L.; Santos, J.C.D.C.; Gusevskaya, E.V. Copper-catalyzed oxybromination and oxychlorination of primary aromatic amines using LiBr or LiCl and molecular oxygen. Adv. Synth. Catal. 2008, 350, 2052–2058. [Google Scholar] [CrossRef]
- Fleming, I.; Hill, J.H.M.; Parker, D.; Waterson, D. Diastereoselectivity in the alkylation and protonation of some β-silyl enolates. Chem. Commun. 1985, 6, 318–321. [Google Scholar] [CrossRef]
- Wilkinson, J.R.; Nuyen, C.E.; Carpenter, T.S.; Harruff, S.R.; Van Hoveln, R. Copper-catalyzed carbon–silicon bond formation. ACS Catal. 2019, 9, 8961–8979. [Google Scholar] [CrossRef]
- Cowley, A.H.; Elkins, T.M.; Jones, R.A.; Nunn, C.M. Phosphane-stabilized CuI and AgI silanes. Angew. Chem. Int. Ed. 1988, 27, 1349–1350. [Google Scholar] [CrossRef]
- Heine, A.; Herbst-Irmer, R.; Stalke, D. [Cu2R2BrLi(thf)3], R=Si(SiMe3)3–a complex containing five-coordinate silicon in a three-centre two-electron bond (thf= tetrahydrofuran). Chem. Commun. 1993, 23, 1729–1731. [Google Scholar] [CrossRef]
- Klinkhammer, K.W. Synthesis and crystal structure of the two lithium hypersilylcuprates LiCu2[Si(SiMe3)3]3[Li7 (O-tBu)6][Cu2{Si(SiMe3) 3}3]. Z. Anorg. Allg. Chem. 2000, 626, 1217–1223. [Google Scholar] [CrossRef]
- Lerner, H.-W.; Scholz, S.; Bolte, M. The sodium cuprate (tBu3Si)2CuNa: Formation and X-ray crystal structure analysis. Organometallics 2001, 20, 575–577. [Google Scholar] [CrossRef]
- Klinkhammer, K.W.; Klett, J.; Xiong, Y.; Yao, S. Homo- and heteroleptic hypersilylcuprates—Valuable reagents for the synthesis of molecular compounds with a Cu−Si bond. Eur. J. Inorg. Chem. 2003, 2003, 3417–3424. [Google Scholar] [CrossRef]
- Klett, J.; Klinkhammer, K.W.; Niemeyer, M. Ligand exchange between arylcopper compounds and bis(hypersilyl)tin or bis(hypersilyl)lead: Synthesis and characterization of hypersilylcopper and a stannanediyl complex with a Cu−Sn bond. Chem. Eur. J. 1999, 5, 2531–2536. [Google Scholar] [CrossRef]
- Heine, A.; Stalke, D. Preparation and structure of two highly reactive intermediates: [Li(thf)4][Cu5Cl4R2] and [Li(thf)4][AlCl3R], R=Si(SiMe3)3. Angew. Chem. Int. Ed. 1993, 32, 121–122. [Google Scholar] [CrossRef]
- So, J.H.; Boudjouk, P. A convenient synthesis of solvated and unsolvated anhydrous metal chlorides via dehydration of metal chloride hydrates with trimethylchlorosilane. Inorg. Chem. 1990, 29, 1592–1593. [Google Scholar] [CrossRef]
- Sohn, H.; Huddleston, R.R.; Powell, D.R.; West, R.; Oka, K.; Yonghua, X. An electroluminescent polysilole and some dichlorooligosiloles. J. Am. Chem. Soc. 1999, 121, 2935–2936. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.-H. Synthesis of 1,1′-Bis(1-Methyl/Chloro-2,3,4,5-Tetraphenyl-1-Silacyclopentadienyl) [Ph4C4Si(Me/Cl)-(Me/Cl)SiC4Ph4] from Silole Anion [MeSiC4Ph4]−•[Li+ or Na+] and Silole Dianion [SiC4Ph4]2−•2[Li+]; Oxidative Coupling of Silole Anion [MeSiC4Ph4]−•[Li+ or Na+] by Ferrous Chloride (FeCl2) and Oxidative Coupling and Chlorination of Silole Dianion [SiC4Ph4]2−•2[Li+] by Cupric Chloride (CuCl2). Molecules 2019, 24, 3772. https://doi.org/10.3390/molecules24203772
Hong J-H. Synthesis of 1,1′-Bis(1-Methyl/Chloro-2,3,4,5-Tetraphenyl-1-Silacyclopentadienyl) [Ph4C4Si(Me/Cl)-(Me/Cl)SiC4Ph4] from Silole Anion [MeSiC4Ph4]−•[Li+ or Na+] and Silole Dianion [SiC4Ph4]2−•2[Li+]; Oxidative Coupling of Silole Anion [MeSiC4Ph4]−•[Li+ or Na+] by Ferrous Chloride (FeCl2) and Oxidative Coupling and Chlorination of Silole Dianion [SiC4Ph4]2−•2[Li+] by Cupric Chloride (CuCl2). Molecules. 2019; 24(20):3772. https://doi.org/10.3390/molecules24203772
Chicago/Turabian StyleHong, Jang-Hwan. 2019. "Synthesis of 1,1′-Bis(1-Methyl/Chloro-2,3,4,5-Tetraphenyl-1-Silacyclopentadienyl) [Ph4C4Si(Me/Cl)-(Me/Cl)SiC4Ph4] from Silole Anion [MeSiC4Ph4]−•[Li+ or Na+] and Silole Dianion [SiC4Ph4]2−•2[Li+]; Oxidative Coupling of Silole Anion [MeSiC4Ph4]−•[Li+ or Na+] by Ferrous Chloride (FeCl2) and Oxidative Coupling and Chlorination of Silole Dianion [SiC4Ph4]2−•2[Li+] by Cupric Chloride (CuCl2)" Molecules 24, no. 20: 3772. https://doi.org/10.3390/molecules24203772





