G-Quadruplex-Forming Aptamers—Characteristics, Applications, and Perspectives
Abstract
1. Introduction
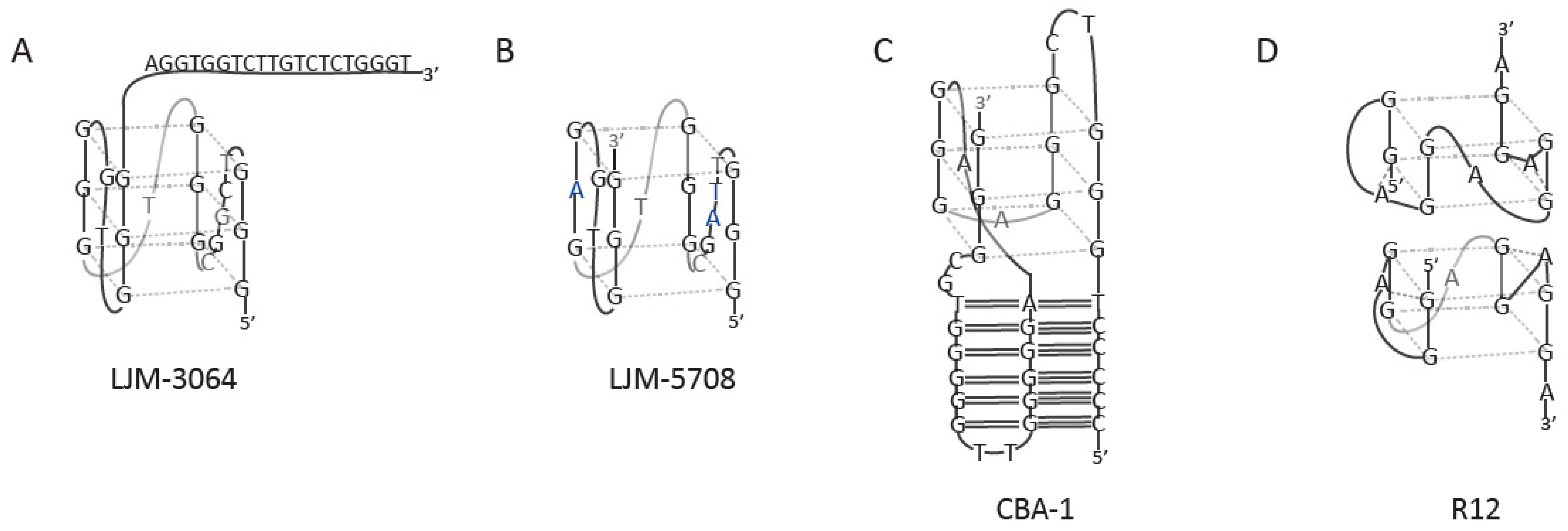
2. Anticoagulant Agents
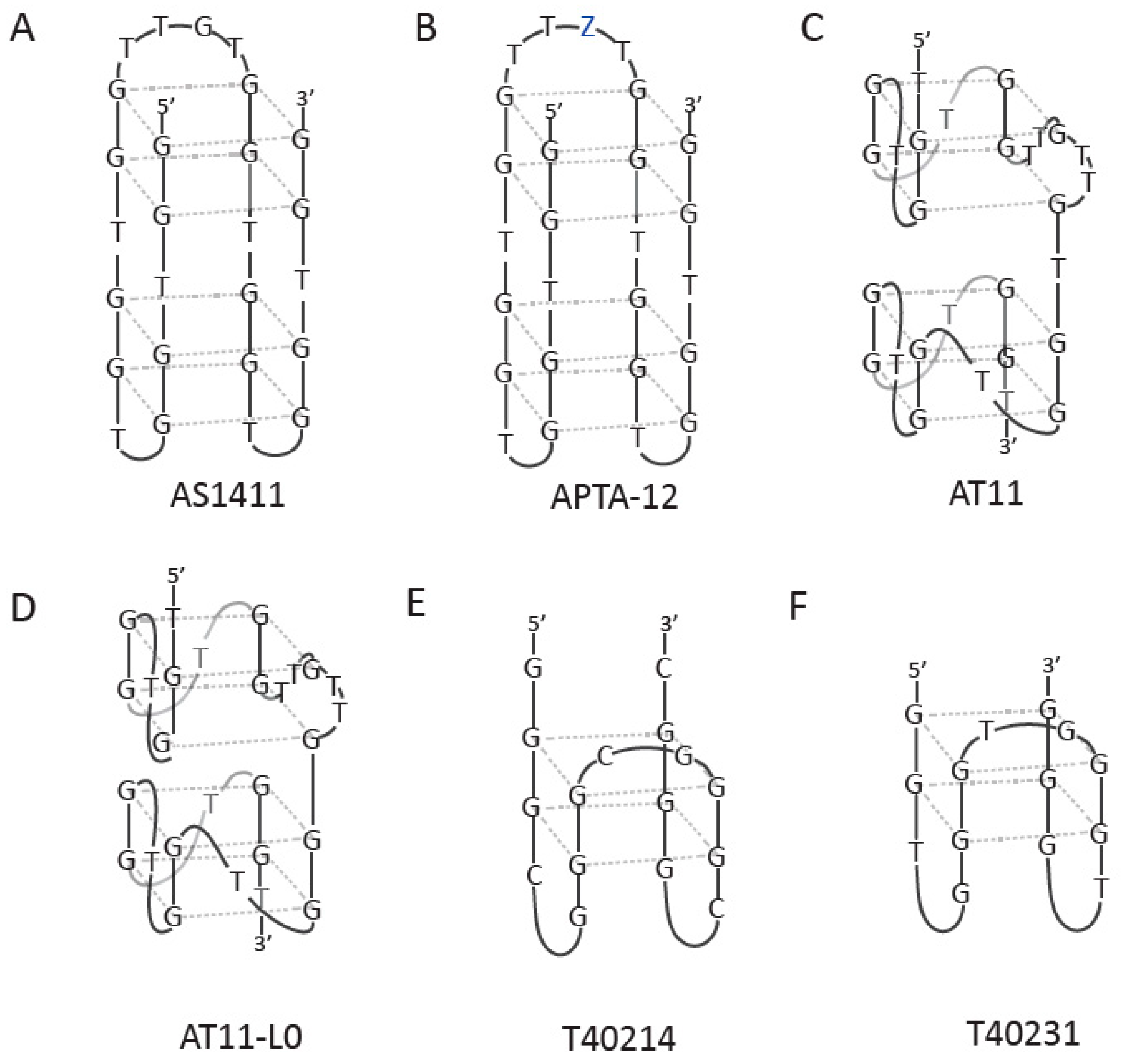
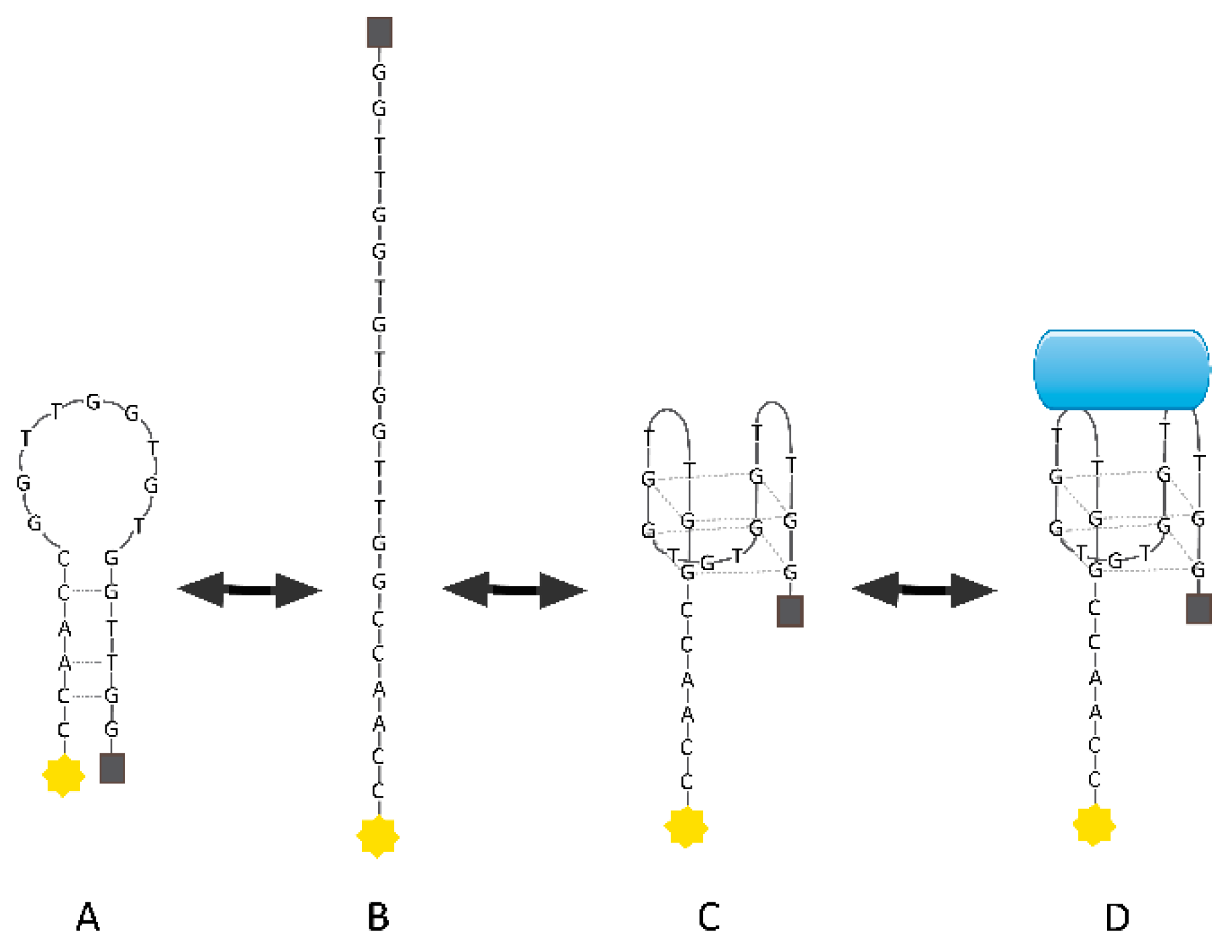
3. Anticancer Agents
4. Antiviral Agents
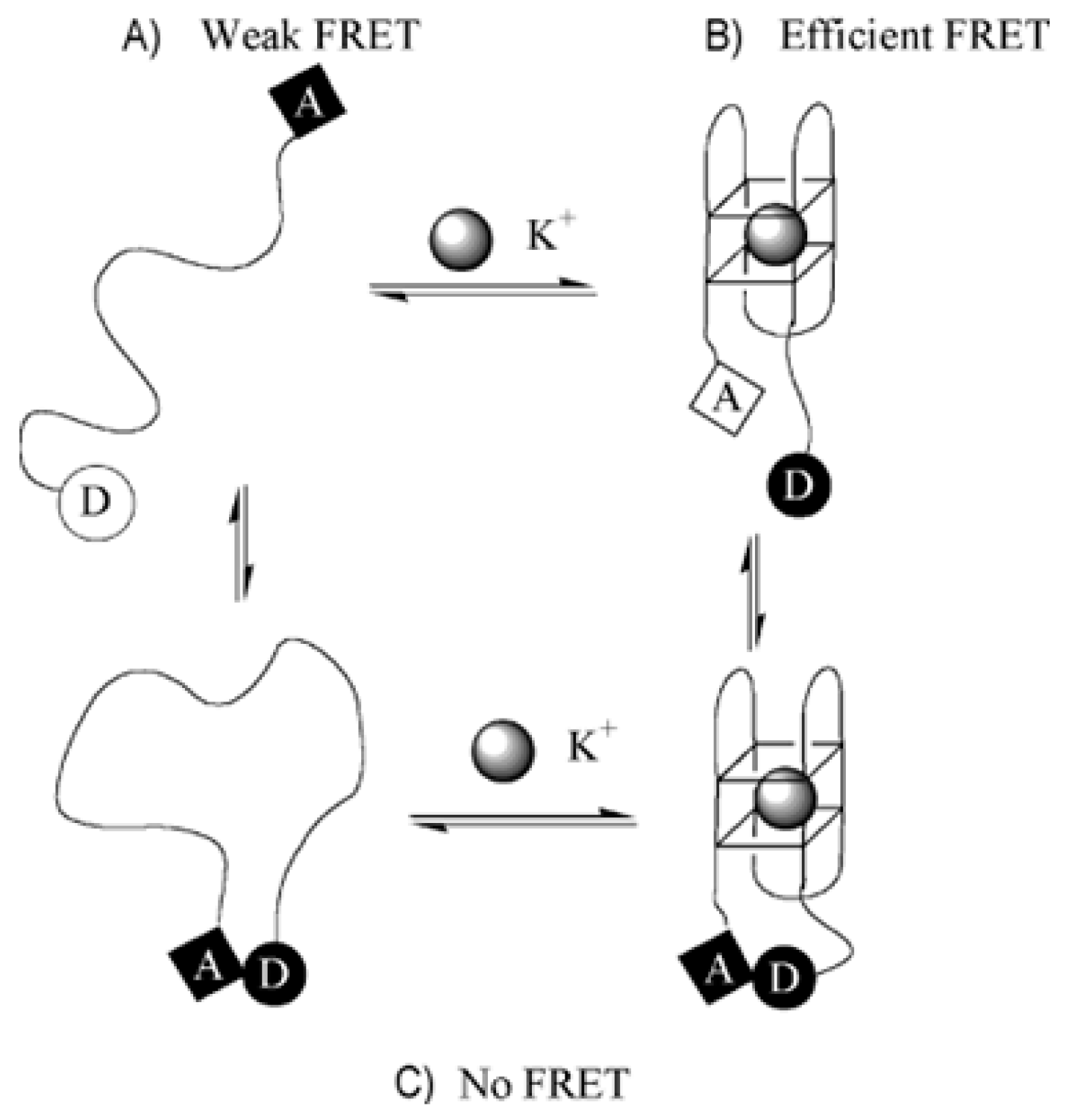
5. Aptasensors
6. Other Applications
7. Conclusions
Funding
Conflicts of Interest
References
- Ellington, A.D.; Szostak, J.W. In Vitro Selection of RNA Molecules That Bind Specific Ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Gatto, B.; Palumbo, M.; Sissi, C. Nucleic Acid Aptamers Based on the G-Quadruplex Structure: Therapeutic and Diagnostic Potential. Curr. Med. Chem. 2009, 16, 1248–1265. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichement: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Jenison, R.D.; Gill, S.C.; Pardi, A.; Polisky, B. High-Resolution Molecular Discrimination by RNA. Science 1994, 263, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Mendonsa, S.D.; Bowser, M.T. In Vitro Evolution of Functional DNA Using Capillary Electrophoresis. J. Am. Chem. Soc. 2004, 126, 20–21. [Google Scholar] [CrossRef]
- Hybarger, G.; Bynum, J.; Williams, R.F.; Valdes, J.J.; Chambers, J.P. A Microfluidic SELEX Prototype. Anal. Bioanal. Chem. 2006, 384, 191–198. [Google Scholar] [CrossRef]
- Hicke, B.J.; Marion, C.; Chang, Y.F.; Gould, T.; Lynott, C.K.; Parma, D.; Schmidt, P.G.; Warren, S. Tenascin-C Aptamers Are Generated Using Tumor Cells and Purified Protein. J. Biol. Chem. 2001, 276, 48644–48654. [Google Scholar] [CrossRef]
- Mi, J.; Liu, Y.; Rabbani, Z.N.; Yang, Z.; Urban, J.H.; Sullenger, A.; Clary, B.M.; Cone, M.; Hospital, M. In Vivo Selection of Tumor-Targeting RNA Motifs. Nat. Chem. Biol. 2010, 6, 22–24. [Google Scholar] [CrossRef]
- Cho, M.; Xiao, Y.; Nie, J.; Stewrat, R.; Csordas, A.T.; Oh, S.S.; Thomson, J.A.; Soh, H.T. Quantitative Selection of DNA Aptamers through Microfluidic Selection and High-Throughput Sequencing. Proc. Natl. Acad. Sci. USA 2011, 108, 4105–4110. [Google Scholar] [CrossRef]
- Albanese, C.M.; Suttapitugsakul, S.; Perati, S.; McGown, L.B. A Genome-Inspired, Reverse Selection Approach to Aptamer Discovery. Talanta 2018, 177, 150–156. [Google Scholar] [CrossRef]
- Rohloff, J.C.; Gelinas, A.D.; Jarvis, T.C.; Ochsner, U.A.; Schneider, D.J.; Gold, L.; Janjic, N. Nucleic Acid Ligands with Protein-like Side Chains: Modified Aptamers and Their Use as Diagnostic and Therapeutic Agents. Mol. Ther. Nucleic Acids 2014, 3, e201. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.O.; Shum, K.T.; Tanner, J.A. G-Quadruplex DNA Aptamers and Their Ligands: Structure, Function and Application. Curr. Pharm. Design 2012, 18, 2014–2026. [Google Scholar] [CrossRef] [PubMed]
- Rachwal, P.A.; Fox, K.R. Quadruplex Melting. Methods 2007, 43, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Burge, S.; Parkinson, G.N.; Hazel, P.; Todd, A.K.; Neidle, S. Quadruplex DNA: Sequence, Topology and Structure. Nucleic Acids Res. 2006, 34, 5402–5415. [Google Scholar] [CrossRef]
- Choi, E.W.; Nayak, L.V.; Bates, P.J. Cancer-Selective Antiproliferative Activity Is a General Property of Some G-Rich Oligodeoxynucleotides. Nucleic Acids Res. 2009, 38, 1623–1635. [Google Scholar] [CrossRef]
- Chang, T.; Qi, C.; Meng, J.; Zhang, N.; Bing, T.; Yang, X.; Cao, Z.; Shangguan, D. General Cell-Binding Activity of Intramolecular G-Quadruplexes with Parallel Structure. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Tasset, D.M.; Kubik, M.F.; Steiner, W. Oligonucleotide Inhibitors of Human Thrombin That Bind Distinct Epitopes. J. Mol. Biol. 1997, 272, 688–698. [Google Scholar] [CrossRef]
- Daei, P.; Ramezanpour, M.; Khanaki, K.; Tabarzad, M.; Nikokar, I.; Mojtaba Hedayati, C.H.; Elmi, A. Aptamer-Based Targeted Delivery of MiRNA Let-7d to Gastric Cancer Cells as a Novel Anti-Tumor Therapeutic Agent. Iran. J. Pharm. Res. 2018, 17, 1537–1549. [Google Scholar]
- Wilbanks, B.; Smestad, J.; Heider, R.M.; Warrington, A.E.; Rodriguez, M.; Maher, L.J. Optimization of a 40-Mer Antimyelin DNA Aptamer Identifies a 20-Mer with Enhanced Properties for Potential Multiple Sclerosis Therapy. Nucleic Acid Ther. 2019, 29, 126–135. [Google Scholar] [CrossRef]
- Hwang, D.W.; Ko, H.Y.; Lee, J.H.; Kang, H.; Ryu, S.H.; Song, I.C.; Lee, D.S.; Kim, S. A Nucleolin-Targeted Multimodal Nanoparticle Imaging Probe for Tracking Cancer Cells Using an Aptamer. J. Nucl. Med. 2010, 51, 98–105. [Google Scholar] [CrossRef]
- Wu, Y.; Midinov, B.; White, R.J. Electrochemical Aptamer-Based Sensor for Real-Time Monitoring of Insulin. ACS Sensors 2019, 4, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.P.R.; Royston, D. Thrombin Generation and Its Inhibition: A Review of the Scientific Basis and Mechanism of Action of Anticoagulant Therapies. Br. J. Anaesth. 2002, 88, 848–863. [Google Scholar] [CrossRef]
- Coppens, M.; Eikelboom, J.W.; Gustafsson, D.; Weitz, J.I.; Hirsh, J. Translational Success Stories: Development of Direct Thrombin Inhibitors. Circ. Res. 2012, 111, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Bock, L.C.; Griffin, L.C.; Latham, J.A.; Vermaas, E.H.; Toole, J.J. Selection of Single-Stranded DNA Molecules That Bind and Inhibit Humam Trombin. Nature 1992, 359, 710–713. [Google Scholar]
- Macaya, R.F.; Schultze, P.; Smith, F.W.; Roe, J.A.; Feigon, J. Thrombin-Binding DNA Aptamer Forms a Unimolecular Quadruplex Structure in Solution. Proc. Natl. Acad. Sci. USA 1993, 90, 3745–3749. [Google Scholar] [CrossRef]
- Russo Krauss, I.; Merlino, A.; Randazzo, A.; Novellino, E.; Mazzarella, L.; Sica, F. High-Resolution Structures of Two Complexes between Thrombin and Thrombin-Binding Aptamer Shed Light on the Role of Cations in the Aptamer Inhibitory Activity. Nucleic Acids Res. 2012, 40, 8119–8128. [Google Scholar] [CrossRef]
- Paborsky, L.R.; McCurdy, S.N.; Griffin, L.C.; Toole, J.J.; Leung, L.L.K. The Single-Stranded DNA Aptamer-Binding Site of Human Thrombin. J. Biol. Chem. 1993, 268, 20808–20811. [Google Scholar] [PubMed]
- Pica, A.; Russo Krauss, I.; Merlino, A.; Nagatoishi, S.; Sugimoto, N.; Sica, F. Dissecting the Contribution of Thrombin Exosite I in the Recognition of Thrombin Binding Aptamer. FEBS J. 2013, 280, 6581–6588. [Google Scholar] [CrossRef]
- Griffin, L.C.; Tidmarsh, G.F.; Bock, L.C.; Toole, J.J.; Leung, L.L.K. In Vivo Anticoagulant Properties. Blood 1993, 81, 3271–3276. [Google Scholar] [CrossRef]
- DeAnda, A.; Coutre, S.E.; Moon, M.R.; Vial, C.M.; Griffin, L.C.; Law, V.S.; Komeda, M.; Leung, L.L.K.; Miller, D.C. Pilot Study of the Efficacy of a Thrombin Inhibitor for Use during Cardiopulmonary Bypass. Ann. Thorac. Surg. 1994, 58, 344–350. [Google Scholar] [CrossRef]
- Li, W.X.; Kaplan, A.V.; Grant, G.W.; Toole, J.J.; Leung, L.L.K. A Novel Nucleotide-Based Thrombin Inhibitor Inhibits Clot-Bound Thrombin and Reduces Arterial Platelet Thrombus Formation. Blood 1994, 83, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Avino, A.; Fabrega, C.; Tintore, M.; Eritja, R. Thrombin Binding Aptamer, More than a Simple Aptamer: Chemically Modified Derivatives and Biomedical Applications. Curr. Pharm. Des. 2012, 18, 2036–2047. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, R.S.; Sullenger, B.A. Modulation of the Coagulation Cascade Using Aptamers. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Mayer, G.; Rohrbach, F.; Pötzsch, B.; Müller, J. Aptamer-Based Modulation of Blood Coagulation. Hamostaseologie 2011, 31, 258–263. [Google Scholar]
- He, G.X.; Krawczyk, S.H.; Swaminathan, S.; Shea, R.G.; Dougherty, J.P.; Terhorst, T.; Law, V.S.; Griffin, L.C.; Coutré, S.; Bischofberger, N. N2- and C8-Substituted Oligodeoxynucleotides with Enhanced Thrombin Inhibitory Activity in Vitro and in Vivo. J. Med. Chem. 1998, 41, 2234–2242. [Google Scholar] [CrossRef]
- Cai, B.; Yang, X.; Sun, L.; Fan, X.; Li, L.; Jin, H.; Wu, Y.; Guan, Z.; Zhang, L.; Zhang, L.; et al. Stability and Bioactivity of Thrombin Binding Aptamers Modified with D-/l-Isothymidine in the Loop Regions. Org. Biomol. Chem. 2014, 12, 8866–8876. [Google Scholar] [CrossRef]
- Mendelboum Raviv, S.; Horváth, A.; Aradi, J.; Bagoly, Z.; Fazakas, F.; Batta, Z.; Muszbek, L.; Hársfalvi, J. 4-Thio-Deoxyuridylate-Modified Thrombin Aptamer and Its Inhibitory Effect on Fibrin Clot Formation, Platelet Aggregation and Thrombus Growth on Subendothelial Matrix. J. Thromb. Haemost. 2008, 6, 1764–1771. [Google Scholar] [CrossRef]
- Virgilio, A.; Petraccone, L.; Vellecco, V.; Bucci, M.; Varra, M.; Irace, C.; Santamaria, R.; Pepe, A.; Mayol, L.; Esposito, V.; et al. Site-Specific Replacement of the Thymine Methyl Group by Fluorine in Thrombin Binding Aptamer Significantly Improves Structural Stability and Anticoagulant Activity. Nucleic Acids Res. 2015, 43, 10602–10611. [Google Scholar] [CrossRef][Green Version]
- Pasternak, A.; Hernandez, F.J.; Rasmussen, L.M.; Vester, B.; Wengel, J. Improved Thrombin Binding Aptamer by Incorporation of a Single Unlocked Nucleic Acid Monomer. Nucleic Acids Res. 2011, 39, 1155–1164. [Google Scholar] [CrossRef]
- Kotkowiak, W.; Lisowiec-Wachnicka, J.; Grynda, J.; Kierzek, R.; Wengel, J.; Pasternak, A. Thermodynamic, Anticoagulant, and Antiproliferative Properties of Thrombin Binding Aptamer Containing Novel UNA Derivative. Mol. Ther. Nucleic Acids 2018, 10, 304–316. [Google Scholar] [CrossRef]
- He, G.X.; Williams, J.P.; Postich, M.J.; Swaminathan, S.; Shea, R.G.; Terhorst, T.; Law, V.S.; Mao, C.T.; Sueoka, C.; Coutré, S.; et al. In Vitro and in Vivo Activities of Oligodeoxynucleotide-Based Thrombin Inhibitors Containing Neutral Formacetal Linkages. J. Med. Chem. 1998, 41, 4224–4231. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, A.; Petraccone, L.; Scuotto, M.; Vellecco, V.; Bucci, M.; Mayol, L.; Varra, M.; Esposito, V.; Galeone, A. 5-Hydroxymethyl-2′-Deoxyuridine Residues in the Thrombin Binding Aptamer: Investigating Anticoagulant Activity by Making a Tiny Chemical Modification. ChemBioChem 2014, 15, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Aaldering, L.J.; Poongavanam, V.; Langkjær, N.; Murugan, N.A.; Jørgensen, P.T.; Wengel, J.; Veedu, R.N. Development of an Efficient G-Quadruplex-Stabilised Thrombin-Binding Aptamer Containing a Three-Carbon Spacer Molecule. ChemBioChem 2017, 18, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Goji, S.; Matsui, J. Direct Detection of Thrombin Binding to 8-Bromodeoxyguanosine-Modified Aptamer: Effects of Modification on Affinity and Kinetics. J. Nucleic Acids 2011, 2011, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nallagatla, S.R.; Heuberger, B.; Haque, A.; Switzer, C. Combinatorial Synthesis of Thrombin-Binding Aptamers Containing Iso-Guanine. J. Comb. Chem. 2009, 11, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.G.; Damha, M.J. G-Quadruplex Induced Stabilization by 2′-Deoxy-2′-Fluoro-D-Arabinonucleic Acids (2′F-ANA). Nucleic Acids Res. 2007, 35, 4977–4988. [Google Scholar] [CrossRef] [PubMed]
- Schultze, P.; Macaya, R.F.; Feigon, J. Three-Dimensional Solution Structure of the Thrombin-Binding DNA Aptamer d(GGTTGGTGTGGTTGG). J. Mol. Biol. 1994, 235, 1532–1547. [Google Scholar] [CrossRef]
- Borbone, N.; Bucci, M.; Oliviero, G.; Morelli, E.; Amato, J.; D’Atri, V.; D’Errico, S.; Vellecco, V.; Cirino, G.; Piccialli, G.; et al. Investigating the Role of T7 and T12 Residues on the Biological Properties of Thrombin-Binding Aptamer: Enhancement of Anticoagulant Activity by a Single Nucleobase Modification. J. Med. Chem. 2012, 55, 10716–10728. [Google Scholar] [CrossRef]
- Jensen, T.B.; Henriksen, J.R.; Rasmussen, B.E.; Rasmussen, L.M.; Andresen, T.L.; Wengel, J.; Pasternak, A. Thermodynamic and Biological Evaluation of a Thrombin Binding Aptamer Modified with Several Unlocked Nucleic Acid (UNA) Monomers and a 2′-C-Piperazino-UNA Monomer. Bioorganic Med. Chem. 2011, 19, 4739–4745. [Google Scholar] [CrossRef]
- Pagano, B.; Martino, L.; Randazzo, A.; Giancola, C. Stability and Binding Properties of a Modified Thrombin Binding Aptamer. Biophys. J. 2008, 94, 562–569. [Google Scholar] [CrossRef]
- Martino, L.; Virno, A.; Randazzo, A.; Virgilio, A.; Esposito, V.; Giancola, C.; Bucci, M.; Cirino, G.; Mayol, L. A New Modified Thrombin Binding Aptamer Containing a 5′-5′ Inversion of Polarity Site. Nucleic Acids Res. 2006, 34, 6653–6662. [Google Scholar] [CrossRef] [PubMed]
- Esposito, V.; Russo, A.; Amato, T.; Vellecco, V.; Bucci, M.; Mayol, L.; Russo, G.; Virgilio, A.; Galeone, A. The “Janus Face” of the Thrombin Binding Aptamer: Investigating the Anticoagulant and Antiproliferative Properties through Straightforward Chemical Modifications. Bioorg. Chem. 2018, 76, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Kolganova, N.A.; Varizhuk, A.M.; Novikov, R.A.; Florentiev, V.L.; Pozmogova, G.E.; Borisova, O.F.; Shchyolkina, A.K.; Smirnov, I.P.; Kaluzhny, D.N.; Timofeev, E.N. Anomeric DNA Quadruplexes: Modified Thrombin Aptamers. Artif. DNA PNA XNA 2014, 5, e28422-1–e28422-8. [Google Scholar] [CrossRef] [PubMed]
- Esposito, V.; Scuotto, M.; Capuozzo, A.; Santamaria, R.; Varra, M.; Mayol, L.; Virgilio, A.; Galeone, A. A Straightforward Modification in the Thrombin Binding Aptamer Improving the Stability, Affinity to Thrombin and Nuclease Resistance. Org. Biomol. Chem. 2014, 12, 8840–8843. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Shafer, R.H. Covalent Ligation Studies on the Human Telomere Quadruplex. Nucleic Acids Res. 2005, 33, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Spiridonova, V.; Glinkina, K.; Gainutdinov, A.; Arutyunyan, A. Production of Thrombin Complexes with DNA Aptamers Containing G-Quadruplex and Different Duplexes. J. Nephrol. Ther. 2014, 4, 1–6. [Google Scholar]
- Mazurov, A.V.; Titaeva, E.V.; Khaspekova, S.G.; Storojilova, A.N.; Spiridonova, V.A.; Kopylov, A.M.; Dobrovolsky, A.B. Characteristics of a New DNA Aptamer, Direct Inhibitor of Thrombin. Bull. Exp. Biol. Med. 2011, 150, 422–425. [Google Scholar] [CrossRef]
- Spiridonova, V.A.; Barinova, K.V.; Glinkina, K.A.; Melnichuk, A.V.; Gainutdynov, A.A.; Safenkova, I.V.; Dzantiev, B.B. A Family of DNA Aptamers with Varied Duplex Region Length That Forms Complexes with Thrombin and Prothrombin. FEBS Lett. 2015, 589, 2043–2049. [Google Scholar] [CrossRef]
- Russo Krauss, I.; Spiridonova, V.; Pica, A.; Napolitano, V.; Sica, F. Different Duplex/Quadruplex Junctions Determine the Properties of Anti-Thrombin Aptamers with Mixed Folding. Nucleic Acids Res. 2016, 44, 983–991. [Google Scholar] [CrossRef]
- Kotkowiak, W.; Wengel, J.; Scotton, C.J.; Pasternak, A. Improved RE31 Analogues Containing Modified Nucleic Acid Monomers: Thermodynamic, Structural, and Biological Effects. J. Med. Chem. 2019, 62, 2499–2507. [Google Scholar] [CrossRef]
- Spiridonova, V.A.; Novikova, T.M.; Nikulina, D.M.; Shishkina, T.A.; Golubkina, E.V.; Dyukareva, O.S.; Trizno, N.N. Complex Formation with Protamine Prolongs the Thrombin-Inhibiting Effect of DNA Aptamer in Vivo. Biochimie 2018, 145, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Zavyalova, E.; Golovin, A.; Reshetnikov, R.; Mudrik, N.; Panteleyev, D.; Pavlova, G.; Kopylov, A. Novel Modular DNA Aptamer for Human Thrombin with High Anticoagulant Activity. Curr. Med. Chem. 2011, 18, 3343–3350. [Google Scholar] [CrossRef] [PubMed]
- Zavyalova, E.; Samoylenkova, N.; Revishchin, A.; Turashev, A.; Gordeychuk, I.; Golovin, A.; Kopylov, A.; Pavlova, G. The Evaluation of Pharmacodynamics and Pharmacokinetics of Anti-Thrombin DNA Aptamer RA-36. Front. Pharmacol. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zavyalova, E.; Samoylenkova, N.; Revishchin, A.; Golovin, A.; Pavlova, G.; Kopylov, A. Evaluation of Antithrombotic Activity of Thrombin DNA Aptamers by a Murine Thrombosis Model. PLoS ONE 2014, 9, 1–7. [Google Scholar] [CrossRef]
- Zavyalova, E.; Golovin, A.; Timoshenko, T.; Babiy, A.; Pavlova, G.; Kopylov, A. DNA Aptamers for Human Thrombin with High Anticoagulant Activity Demonstrate Target- and Species-Specificity. Curr. Med. Chem. 2012, 19, 5232–5237. [Google Scholar] [CrossRef] [PubMed]
- Amato, T.; Virgilio, A.; Pirone, L.; Vellecco, V.; Bucci, M.; Pedone, E.; Esposito, V.; Galeone, A. Investigating the Propertizes of TBA Variants with Twin Thrombin Binding Domains. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Troisi, R.; Napolitano, V.; Spiridonova, V.; Krauss, I.R.; Sica, F. Several Structural Motifs Cooperate in Determining the Highly Effective Anti-Thrombin Activity of NU172 Aptamer. Nucleic Acids Res. 2018, 46, 12177–12185. [Google Scholar] [CrossRef]
- Zavyalova, E.; Tagiltsev, G.; Reshetnikov, R.; Arutyunyan, A.; Kopylov, A. Cation Coordination Alters the Conformation of a Thrombin-Binding G-Quadruplex DNA Aptamer That Affects Inhibition of Thrombin. Nucleic Acid Ther. 2016, 26, 299–308. [Google Scholar] [CrossRef]
- Trapaidze, A.; Hérault, J.P.; Herbert, J.M.; Bancaud, A.; Gué, A.M. Investigation of the Selectivity of Thrombin-Binding Aptamers for Thrombin Titration in Murine Plasma. Biosens. Bioelectron. 2016, 78, 58–66. [Google Scholar] [CrossRef]
- Ni, X.; Castanares, M.; Mukherjee, A.; Lupold, S.E. Nucleic Acid Aptamers: Clinical Applications and Promising New Horizons. Curr Med. Chem. 2012, 18, 4206–4214. [Google Scholar] [CrossRef]
- Segers, K.; Dahlbäck, B.; Bock, P.E.; Tans, G.; Rosing, J.; Nicolaes, G.A.F. The Role of Thrombin Exosites I and II in the Activation of Human Coagulation Factor V*,S. Bone 2008, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Derszniak, K.; Przyborowski, K.; Matyjaszczyk, K.; Moorlag, M.; De Laat, B.; Nowakowska, M.; Chlopicki, S. Comparison of Effects of Anti-Thrombin Aptamers HD1 and HD22 on Aggregation of Human Platelets, Thrombin Generation, Fibrin Formation, and Thrombus Formation under Flow Conditions. Front. Pharmacol. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Freitag, D.; Mayer, G.; Pötzsch, B. Anticoagulant Characteristics of HD1-22, a Bivalent Aptamer That Specifically Inhibits Thrombin and Prothrombinase. J. Thromb. Haemost. 2008, 6, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Dailey, M.M.; Clarke Miller, M.; Bates, P.J.; Lane, A.N.; Trent, J.O. Resolution and Characterization of the Structural Polymorphism of a Single Quadruplex-Forming Sequence. Nucleic Acids Res. 2010, 38, 4877–4888. [Google Scholar] [CrossRef]
- Bates, P.J.; Kahlon, J.B.; Thomas, S.D.; Trent, J.O.; Miller, D.M. Antiproliferative Activity of G-Rich Oligonucleotides Correlates with Protein Binding. J. Biol. Chem. 1999, 274, 26369–26377. [Google Scholar] [CrossRef]
- Wu, X.; Chen, J.; Wu, M.; Zhao, J.X. Aptamers: Active Targeting Ligands for Cancer Diagnosis and Therapy. Theranostics 2015, 5, 322–344. [Google Scholar] [CrossRef]
- Soundararajan, S.; Wang, L.; Sridharan, V.; Chen, W.; Courtenay-Luck, N.; Jones, D.; Spicer, E.K.; Fernandes, D.J. Plasma Membrane Nucleolin Is a Receptor for the Anticancer Aptamer AS1411 in MV4-11 Leukemia Cells. Mol. Pharmacol. 2009, 76, 984–991. [Google Scholar] [CrossRef]
- Reyes-Reyes, E.M.; Šalipur, F.R.; Shams, M.; Forsthoefel, M.K.; Bates, P.L. Mechanistic Studies of Anticancer Aptamer AS1411 Reveal a Novel Role for Nucleolin in Regulating Rac1 Activation. Mol. Oncol. 2015, 9, 1392–1405. [Google Scholar] [CrossRef]
- Métifiot, M.; Amrane, S.; Mergny, J.; Andreola, M. Biochimie Anticancer Molecule AS1411 Exhibits Low Nanomolar Antiviral Activity against HIV-1. Biochimie 2015, 118, 173–175. [Google Scholar] [CrossRef]
- Bates, P.J.; Reyes-reyes, E.M.; Malik, M.T.; Murphy, E.M.; Toole, M.G.O.; Trent, J.O. Biochimica et Biophysica Acta G-Quadruplex Oligonucleotide AS1411 as a Cancer-Targeting Agent: Uses and Mechanisms ☆. BBA Gen. Subj. 2017, 1861, 1414–1428. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, G.; Zhang, S.; Nigim, F.; Zhou, G.; Yu, Z.; Song, Y.; Chen, Y.; Li, Y. AS1411-Induced Growth Inhibition of Glioma Cells by Up-Regulation of P53 and Down- Regulation of Bcl-2 and Akt1 via Nucleolin. PLoS ONE 2016, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, F.; Kefayat, A.; Shahbazi-gahrouei, D. AS1411 Aptamer-Targeted Gold Nanoclusters Effect on the Enhancement of Radiation Therapy Efficacy in Breast Tumor-Bearing Mice. Nanomedicine 2018, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Cho, Y.L.; Chae, J.R.; Moon, S.H.; Cho, W.G.; Choi, Y.J.; Lee, S.J.; Kang, W.J. Gemcitabine-Incorporated G-Quadruplex Aptamer for Targeted Drug Delivery into Pancreas Cancer. Mol. Ther. Nucleic Acids 2018, 12, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Do, N.Q.; Chung, W.J.; Truong, T.H.A.; Heddi, B.; Phan, A.T. G-Quadruplex Structure of an Anti-Proliferative DNA Sequence. Nucleic Acids Res. 2017, 45, 7487–7493. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.; Lopes-Nunes, J.; Lopes, A.C.; Cabral Campello, M.P.; Paulo, A.; Queiroz, J.A.; Cruz, C. Aptamer-Guided Acridine Derivatives for Cervical Cancer. Org. Biomol. Chem. 2019, 17, 2992–3002. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.; Paiva, A.; Cabral Campello, M.P.; Paulo, A.; Mergny, J.-L.; Salgado, G.F.; Queiroz, J.A.; Cruz, C. Aptamer-Based Targeted Delivery of a G-Quadruplex Ligand in Cervical Cancer Cells. Sci. Rep. 2019, 9, 7945. [Google Scholar] [CrossRef]
- Jing, N.; Li, Y.; Xiong, W.; Sha, W.; Jing, L.; Tweardy, D.J. G-Quartet Oligonucleotides: A New Class of Signal Transducer and Activator of Transcription 3 Inhibitors That Suppresses Growth of Prostate and Breast Tumors through Induction of Apoptosis. Cancer Res. 2004, 64, 6603–6609. [Google Scholar] [CrossRef]
- Jing, N.; Zhu, Q.; Yuan, P.; Li, Y.; Mao, L.; Tweardy, D.J. Targeting Signal Transducer and Activator of Transcription 3 with G-Quartet Oligonucleotides: A Potential Novel Therapy for Head and Neck Cancer. Mol. Cancer Ther. 2006, 5, 279–286. [Google Scholar] [CrossRef]
- Weerasinghe, P.; Garcia, G.E.; Zhu, Q.; Yuan, P.; Feng, L.; Mao, L.I.; Jing, N. Inhibition of Stat3 Activation and Tumor Growth Suppression of Non-Small Cell Lung Cancer by G-Quartet Oligonucleotides. Int. J. Oncol. 2007, 31, 129–136. [Google Scholar] [CrossRef]
- Hu, J.; Wu, J.; Li, C.; Zhu, L.; Zhang, W.Y.; Kong, G.; Lu, Z.; Yang, C.J. A G-Quadruplex Aptamer Inhibits the Phosphatase Activity of Oncogenic Protein Shp2 in Vitro. ChemBioChem 2011, 12, 424–430. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, Z.; Liu, Q.; Ye, M.; Tan, W. Study of the Function of G-Rich Aptamers Selected for Lung Adenocarcinoma. Chem. An. Asian J. 2015, 10, 1519–1525. [Google Scholar] [CrossRef]
- Dapić, V.; Abdomerović, V.; Marrington, R.; Peberdy, J.; Rodger, A.; Trent, J.O.; Bates, P.J. Biophysical and Biological Properties of Quadruplex Oligodeoxyribonucleotides. Nucleic Acids Res. 2003, 31, 2097–2107. [Google Scholar] [CrossRef]
- Scuotto, M.; Rivieccio, E.; Varone, A.; Corda, D.; Bucci, M.; Vellecco, V.; Cirino, G.; Virgilio, A.; Esposito, V.; Galeone, A.; et al. Site Specific Replacements of a Single Loop Nucleoside with a Dibenzyl Linker May Switch the Activity of TBA from Anticoagulant to Antiproliferative. Nucleic Acids Res. 2015, 43, 7702–7716. [Google Scholar] [CrossRef]
- Esposito, V.; Russo, A.; Vellecco, V.; Bucci, M.; Russo, G.; Mayol, L.; Virgilio, A.; Galeone, A. Thrombin Binding Aptamer Analogues Containing Inversion of Polarity Sites Endowed with Antiproliferative and Anti-Motility Properties against Calu-6 Cells. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2645–2650. [Google Scholar] [CrossRef]
- Gaddes, E.R.; Lee, D.; Gydush, G.; Wang, Y.; Dong, C. Regulation of Fibrin-Mediated Tumor Cell Adhesion to the Endothelium Using Anti-Thrombin Aptamer. Exp. Cell Res. 2015, 339, 417–426. [Google Scholar] [CrossRef]
- Perry, C.M.; Balfour, J.A.B. Fomivirsen. Drugs 1999, 57, 375–380. [Google Scholar] [CrossRef]
- Ng, E.W.M.; Shima, D.T.; Calias, P.; Cunningham, E.T.; Guyer, D.R.; Adamis, A.P. Pegaptanib, a Targeted Anti-VEGF Aptamer for Ocular Vascular Disease. Nat. Rev. Drug Discov. 2006, 5, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, J.R.; Vickers, T.A.; Robersont, J.L.; Buckheit, R.W.; Klimkaitt, T.; Debaets, E.; Davis, P.W.; Rayner, B.; Imbach, J.L.; Ecker, D.J. Combinatorially Selected Guanosine-Quartet Structure Is a Potent Inhibitor of Human Immunodeficiency Virus Envelope-Mediated Cell Fusion. Biochemistry 1994, 91, 1356–1360. [Google Scholar] [CrossRef]
- Yoon, V.; Fridkis-Hareli, M.; Munisamy, S.; Lee, J.; Anastasiades, D.; Stevceva, L. The GP120 Molecule of HIV-1 and Its Interaction with T Cells. Curr. Med. Chem. 2010, 17, 741–749. [Google Scholar] [CrossRef]
- Onofrio, J.D.; Petraccone, L.; Erra, E.; Martino, L.; Di Fabio, G.; De Napoli, L.; Ii, F.; Ii, F.; Universitario, C. 5′ -Modified G-Quadruplex Forming Oligonucleotides Endowed with Anti-HIV Activity: Synthesis and Biophysical Properties. Bioconjug. Chem. 2007, 18, 1194–1204. [Google Scholar] [CrossRef]
- Pedersen, E.B.; Nielsen, J.T.; Nielsen, C.; Filichev, V.V. Enhanced Anti-HIV-1 Activity of G-Quadruplexes Comprising Locked Nucleic Acids and Intercalating Nucleic Acids. Nucleic Acids Res. 2011, 39, 2470–2481. [Google Scholar] [CrossRef]
- Perrone, R.; Butovskaya, E.; Lago, S.; Garzino-Demo, A.; Pannecouque, C.; Palù, G.; Richter, S.N. The G-Quadruplex-Forming Aptamer AS1411 Potently Inhibits HIV-1 Attachment to the Host Cell. Int. J. Antimicrob. Agents 2016, 47, 311–316. [Google Scholar] [CrossRef]
- Mukundan, V.T.; Do, N.Q.; Tua, A. HIV-1 Integrase Inhibitor T30177 Forms a Stacked Dimeric G-Quadruplex Structure Containing Bulges. Nucleic Acids Res. 2011, 39, 8984–8991. [Google Scholar] [CrossRef]
- Ojwang, J.O.; Buckheit, R.W.; Pommier, Y.; Mazumder, A.; Reymen, D.; Pallansch, L.A.; Vreese, K.D.E.; Lackman-smith, C.; Wallace, T.L.; Clercq, E.D.E.; et al. T30177, an Oligonucleotide Stabilized by an Intramolecular Guanosine Octet, Is a Potent Inhibitor of Laboratory Strains and Clinical Isolates of Human Immunodeficiency Virus Type 1. Antimicrob. Agents Chemother. 1995, 39, 2426–2435. [Google Scholar] [CrossRef]
- Urata, H.; Kumashiro, T.; Kawahata, T.; Otake, T.; Akagi, M. Anti-HIV-1 Activity and Mode of Action of Mirror Image Oligodeoxynucleotide Analogue of Zintevir. Biochem. Biophys. Res. Commun. 2004, 313, 55–61. [Google Scholar] [CrossRef]
- Andreola, M.-L.; Calmels, C.; Ventura, M.; Tarrago-litvak, L.; Toulme, J. DNA Aptamers Selected against the HIV-1 RNase H Display in Vitro Antiviral. Biochemistry 2001, 40, 10087–10094. [Google Scholar] [CrossRef]
- De Soultrait, V.R.; Lozach, P.; Altmeyer, R.; Tarrago-litvak, L.; Litvak, S.; Andreola, M. DNA Aptamers Derived from HIV-1 RNase H Inhibitors Are Strong Anti-Integrase Agents. J. Mol. Biol. 2002, 324, 195–203. [Google Scholar] [CrossRef]
- Faure-Perraud, A.; Métifiot, M.; Reigadas, S.; Recordon-Pinson, P.; Parissi, V.; Ventura, M.; Andréola, M.L. The Guanine-Quadruplex Aptamer 93del Inhibits HIV-1 Replication Ex Vivo by Interfering with Viral Entry, Reverse Transcription and Integration. Antivir. Ther. 2011, 16, 383–394. [Google Scholar] [CrossRef]
- Michalowski, D.; Chitima-Matsiga, R.; Held, D.M.; Burke, D.H. Novel Bimodular DNA Aptamers with Guanosine Quadruplexes Inhibit Phylogenetically Diverse HIV-1 Reverse Transcriptases. Nucleic Acids Res. 2008, 36, 7124–7135. [Google Scholar] [CrossRef]
- Woo, H.M.; Kim, K.S.; Lee, J.M.; Shim, H.S.; Cho, S.J.; Lee, W.K.; Ko, H.W.; Keum, Y.S.; Kim, S.Y.; Pathinayake, P.; et al. Single-Stranded DNA Aptamer That Specifically Binds to the Influenza Virus NS1 Protein Suppresses Interferon Antagonism. Antiviral Res. 2013, 100, 337–345. [Google Scholar] [CrossRef]
- Blaum, B.S.; Wünsche, W.; Benie, A.J.; Kusov, Y.; Peters, H.; Gauss-Müller, V.; Peters, T.; Sczakiel, G. Functional Binding of Hexanucleotides to 3C Protease of Hepatitis A Virus. Nucleic Acids Res. 2012, 40, 3042–3055. [Google Scholar] [CrossRef]
- Jones, L.A.; Clancy, L.E.; Rawlinson, W.D.; White, P.A. High-Affinity Aptamers to Subtype 3a Hepatitis C Virus Polymerase Display Genotypic Specificity. Antimicrob. Agents Chemother. 2006, 50, 3019–3027. [Google Scholar] [CrossRef]
- Magbanua, E.; Zivkovic, T.; Hansen, B.; Beschorner, N.; Lorenzen, I.; Grötzinger, J.; Hauber, J.; Andrew, E.; Mayer, G.; Rose-john, S.; et al. D(GGGT)4 and r(GGGU)4 Are Both HIV-1 Inhibitors and Interleukin-6 Receptor Aptamers. RNA Biol. 2013, 10, 216–227. [Google Scholar] [CrossRef]
- Soto Rodriguez, P.E.D.; Calderon Nash, V.I. Aptamer-Based Strategies for Diagnostics. In Nucleic Acid Nanotheranostics; Elsevier Inc.: New York, NY, USA, 2019; pp. 189–211. [Google Scholar]
- Saleh, S.M.; Ali, R.; Ali, I.A.I. A Novel, Highly Sensitive, Selective, Reversible and Turn-on Chemi-Sensor Based on Schiff Base for Rapid Detection of Cu(II). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 225–231. [Google Scholar] [CrossRef]
- Bahreyni, A.; Ramezani, M.; Alibolandi, M.; Hassanzadeh, P.; Abnous, K.; Taghdisi, S.M. High Affinity of AS1411 toward Copper; Its Application in a Sensitive Aptasensor for Copper Detection. Anal. Biochem. 2019, 575, 1–9. [Google Scholar] [CrossRef]
- Wen, S.; Miao, X.; Fan, G.C.; Xu, T.; Jiang, L.P.; Wu, P.; Cai, C.; Zhu, J.J. Aptamer-Conjugated Au Nanocage/Sio 2 Core-Shell Bifunctional Nanoprobes with High Stability and Biocompatibility for Cellular Sers Imaging and near-Infrared Photothermal Therapy. ACS Sensors 2019, 4, 301–308. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, C.; Chen, M. A Novel Fluorometric Method for Inorganic Pyrophosphatase Detection Based on G-Quadruplex-Thioflavin, T. Mol. Cell. Probes 2019, 43, 29–33. [Google Scholar] [CrossRef]
- Srinivasan, S.; Ranganathan, V.; DeRosa, M.C.; Murari, B.M. Comparison of Turn-on and Ratiometric Fluorescent G-Quadruplex Aptasensor Approaches for the Detection of ATP. Anal. Bioanal. Chem. 2019, 411, 1319–1330. [Google Scholar] [CrossRef]
- Moccia, F.; Platella, C.; Musumeci, D.; Batool, S.; Zumrut, H.; Bradshaw, J.; Mallikaratchy, P.; Montesarchio, D. The Role of G-Quadruplex Structures of LIGS-Generated Aptamers R1.2 and R1.3 in IgM Specific Recognition. Int. J. Biol. Macromol. 2019, 133, 839–849. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yokohama, K. Development of a Fluorescent Peptide for the Detection of Vascular Endothelial Growth Factor (VEGF). ChemBioChem 2009, 10, 1793–1795. [Google Scholar] [CrossRef]
- Nonaka, Y.; Yoshida, W.; Abe, K.; Ferri, S.; Schulze, H.; Bachmann, T.T.; Ikebukuro, K. Affinity Improvement of a VEGF Aptamer by in Silico Maturation for a Sensitive VEGF-Detection System. Anal. Chem. 2013, 85, 1132–1137. [Google Scholar] [CrossRef]
- Yoshida, W.; Mochizuki, E.; Takase, M.; Hasegawa, H.; Morita, Y. Selection of DNA Aptamers against Insulin and Construction of an Aptameric Enzyme Subunit for Insulin Sensing. Biosens. Bioelectron. J. 2009, 24, 1116–1120. [Google Scholar] [CrossRef]
- Lee, M.; Walt, D.R. A Fiber-Optic Microarray Biosensor Using Aptamers as Receptors. Anal. Biochem. 2000, 282, 142–146. [Google Scholar] [CrossRef]
- Hamaguchi, N.; Ellington, A.; Stanton, M. Aptamer Beacons for the Direct Detection of Proteins. Anal. Biochem. 2001, 294, 126–131. [Google Scholar] [CrossRef]
- Na, W.; Liu, X.; Wang, L.; Su, X. Label-Free Aptamer Biosensor for Selective Detection of Thrombin. Anal. Chim. Acta 2015, 899, 85–90. [Google Scholar] [CrossRef]
- Basnar, B.; Elnathan, R.; Willner, I. Following Aptamer-Thrombin Binding by Force Measurements. Anal. Chem. 2006, 78, 3638–3642. [Google Scholar] [CrossRef]
- Vasilescu, A.; Gaspar, S.; Mihai, I.; Tache, A.; Litescu, S.C. Development of a Label-Free Aptasensor for Monitoring the Self-Association of Lysozyme. Analyst 2013, 138, 3530–3537. [Google Scholar] [CrossRef]
- Pagba, C.V.; Lane, S.M.; Cho, H.; Wachsmann-Hogiu, S. Direct Detection of Aptamer-Thrombin Binding via Surface-Enhanced Raman Spectroscopy. J. Biomed. Opt. 2010, 15, 1–8. [Google Scholar] [CrossRef]
- Ocaña, C.; del Valle, M. Three Different Signal Amplification Strategies for the Impedimetric Sandwich Detection of Thrombin. Anal. Chim. Acta 2016, 912, 117–124. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, R.; Chai, Y.; Yuan, Y.; Bai, L. In Situ Enzymatic Silver Enhancement Based on Functionalized Graphene Oxide and Layer-by-Layer Assembled Gold Nanoparticles for Ultrasensitive Detection of Thrombin. Biosens. Bioelectron. 2012, 38, 50–54. [Google Scholar] [CrossRef]
- Xie, S.; Ye, J.; Yuan, Y.; Chai, Y.; Yuan, R. A Multifunctional Hemin@metal-Organic Framework and Its Application to Construct an Electrochemical Aptasensor for Thrombin Detection. Nanoscale 2015, 7, 18232–18238. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, Y.; Yuan, R.; Yuan, Y.; Bai, L.; Xie, S. A Highly Sensitive Electrochemical Aptasensor for Thrombin Detection Using Functionalized Mesoporous Silica@multiwalled Carbon Nanotubes as Signal Tags and DNAzyme Signal Amplification. Analyst 2013, 138, 6938–6945. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Zhang, Y.; Deng, D.; He, H.; Luo, L.; Wang, Z. A Label-Free Electrochemical Aptasensor Based on Graphene Oxide/Double-Stranded DNA Nanocomposite. Colloids Surf. B Biointerfaces 2016, 145, 160–166. [Google Scholar] [CrossRef]
- Shangguan, L.; Zhu, W.; Xue, Y.; Liu, S. Construction of Photoelectrochemical Thrombin Aptasensor via Assembling Multilayer of Graphene-CdS Nanocomposites. Biosens. Bioelectron. 2015, 64, 611–617. [Google Scholar] [CrossRef]
- Nagatoishi, S.; Nojima, T.; Galezowska, E.; Juskowiak, B.; Takenaka, S. G Quadruplex-Based FRET Probes with the Thrombin-Binding Aptamer (TBA) Sequence Designed for the Efficient Fluorometric Detection of the Potassium Ion. ChemBioChem 2006, 7, 1730–1737. [Google Scholar] [CrossRef]
- Nagatoishi, S.; Nojima, T.; Juskowiak, B.; Takenaka, S. A Pyrene-Labeled G-Quadruplex Oligonucleotide as a Fluorescent Probe for Potassium Ion Detection in Biological Applications. Angew. Chemie Int. Ed. 2005, 44, 5067–5070. [Google Scholar] [CrossRef]
- Shum, K.T.; Lui, E.L.H.; Wong, S.C.K.; Yeung, P.; Sam, L.; Wang, Y.; Watt, R.M.; Tanner, J.A. Aptamer-Mediated Inhibition of Mycobacterium Tuberculosis Polyphosphate Kinase 2. Biochemistry 2011, 50, 3261–3271. [Google Scholar] [CrossRef]
- Kalra, P.; Mishra, S.K.; Kaur, S.; Kumar, A.; Prasad, H.K.; Sharma, T.K.; Tyagi, J.S. G-Quadruplex-Forming DNA Aptamers Inhibit the DNA-Binding Function of HupB and Mycobacterium Tuberculosis Entry into Host Cells. Mol. Ther. Nucleic Acids 2018, 13, 99–109. [Google Scholar] [CrossRef]
- Smestad, J.; James Maher, L. Ion-Dependent Conformational Switching by a DNA Aptamer That Induces Remyelination in a Mouse Model of Multiple Sclerosis. Nucleic Acids Res. 2013, 41, 1329–1342. [Google Scholar] [CrossRef]
- Shum, K.T.; Chan, C.; Leung, C.; Tanner, J.A. Identification of a DNA Aptamer That Inhibits Sclerostin ’ s Antagonistic Effect on Wnt Signalling. Biochem. J. 2011, 501, 493–501. [Google Scholar] [CrossRef]
- Rusconi, C.P.; Roberts, J.D.; Pitoc, G.A.; Nimjee, S.M.; White, R.R.; Quick, G.; Scardino, E.; Fay, W.P.; Sullenger, B.A. Antidote-Mediated Control of an Anticoagulant Aptamer in Vivo. Nat. Biotechnol. 2004, 22, 1423–1428. [Google Scholar] [CrossRef]
- Oney, S.; Lam, R.T.S.; Bompiani, K.M.; Blake, C.M.; Quick, G.; Heidel, J.D.; Liu, J.Y.C.; MacK, B.C.; Davis, M.E.; Leong, K.W.; et al. Development of Universal Antidotes to Control Aptamer Activity. Nat. Med. 2009, 15, 1224–1228. [Google Scholar] [CrossRef]
- Vahed, M.; Ahmadian, G.; Ameri, N.; Vahed, M. G-Rich VEGF Aptamer as a Potential Inhibitor of Chitin Trafficking Signal in Emerging Opportunistic Yeast Infection. Comput. Biol. Chem. 2019, 80, 168–176. [Google Scholar] [CrossRef]
- Marušič, M.; Veedu, R.N.; Wengel, J.; Plavec, J. G-Rich VEGF Aptamer with Locked and Unlocked Nucleic Acid Modifications Exhibits a Unique G-Quadruplex Fold. Nucleic Acids Res. 2013, 41, 9524–9536. [Google Scholar] [CrossRef]
- Orava, E.W.; Jarvik, N.; Shek, Y.L.; Sidhu, S.S.; Garie, J. A Short DNA Aptamer That Recognizes TNFα and Blocks Its Activity in Vitro. ACS Chem. Biol. 2013, 8, 170–178. [Google Scholar] [CrossRef]
- Bing, T.; Zheng, W.; Zhang, X.; Shen, L.; Liu, X.; Wang, F.; Cui, J.; Cao, Z.; Shangguan, D. Triplex-Quadruplex Structural Scaffold: A New Binding Structure of Aptamer. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Collie, G.W.; Parkinson, G.N. The Application of DNA and RNA G-Quadruplexes to Therapeutic Medicines. Chem. Soc. Rev. 2011, 40, 5867–5892. [Google Scholar] [CrossRef]
- Mashima, T.; Matsugami, A.; Nishikawa, F.; Nishikawa, S.; Katahira, M. Unique Quadruplex Structure and Interaction of an RNA Aptamer against Bovine Prion Protein. Nucleic Acids Res. 2009, 37, 6249–6258. [Google Scholar] [CrossRef]
- Mashima, T.; Nishikawa, F.; Kamatari, Y.O.; Fujiwara, H.; Saimura, M.; Nagata, T.; Kodaki, T.; Nishikawa, S.; Kuwata, K.; Katahira, M. Anti-Prion Activity of an RNA Aptamer and Its Structural Basis. Nucleic Acids Res. 2013, 41, 1355–1362. [Google Scholar] [CrossRef]
- Lévesque, D.; Beaudoin, J.-D.; Roy, S.; Perreault, J.-P. In Vitro Selection and Characterization of RNA Aptamers Binding Thyroxine Hormone. Biochem. J. 2007, 403, 129–138. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roxo, C.; Kotkowiak, W.; Pasternak, A. G-Quadruplex-Forming Aptamers—Characteristics, Applications, and Perspectives. Molecules 2019, 24, 3781. https://doi.org/10.3390/molecules24203781
Roxo C, Kotkowiak W, Pasternak A. G-Quadruplex-Forming Aptamers—Characteristics, Applications, and Perspectives. Molecules. 2019; 24(20):3781. https://doi.org/10.3390/molecules24203781
Chicago/Turabian StyleRoxo, Carolina, Weronika Kotkowiak, and Anna Pasternak. 2019. "G-Quadruplex-Forming Aptamers—Characteristics, Applications, and Perspectives" Molecules 24, no. 20: 3781. https://doi.org/10.3390/molecules24203781
APA StyleRoxo, C., Kotkowiak, W., & Pasternak, A. (2019). G-Quadruplex-Forming Aptamers—Characteristics, Applications, and Perspectives. Molecules, 24(20), 3781. https://doi.org/10.3390/molecules24203781







