Research Advances of Purple Sweet Potato Anthocyanins: Extraction, Identification, Stability, Bioactivity, Application, and Biotransformation
Abstract
1. The Extraction Methods of PSPAs
1.1. Solvent Extraction
1.2. Enzymatic Extraction
1.3. High Voltage Pulse Electric Field Method
1.4. Microwave-Assisted Extraction
1.5. Ultrasound-Assisted Extraction
1.6. Accelerated Solvent Extraction
2. The Structural Characterization of Purple Sweet Potato
2.1. Ultraviolet-Visible Spectroscopy
2.2. High-Performance Liquid Chromatography-Mass Spectrometry
2.3. Nuclear Magnetic Resonance
3. The Stability of PSPAs
3.1. Effect of Chemical Structure on Stability
3.2. Effect of Processing Method on Stability
3.3. Effect of Other Factors on Stability
4. Functional Activity of PSPAs
4.1. Antioxidant Activity
4.2. Antimutagenic and Anti-Tumor Activities
4.3. Liver Protection
4.4. Other Function
5. Application of PSPAs
5.1. Application of PSPAs in Food Industry
5.2. Application of PSPAs in Cosmetics Industry
5.3. Application of PSPAs in Pharmaceutical Industry
6. Biotransformation of PSPAs
7. Application Prospects
Funding
Conflicts of Interest
References
- Bovell-Benjamin, A.C. Sweet potato: A review of its past, present, and future role in human nutrition. Adv. Food Nutr. Res. 2007, 52, 1–59. [Google Scholar]
- Teow, C.C.; Truong, V.; McFeeters, R.F.; Thompson, R.L.; Pecota, K.V.; Yencho, G.C. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007, 103, 829–838. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Steed, L.E.; Truong, V.D. Anthocyanin content, antioxidant activity, and selected physical properties of flowable purple-fleshed sweetpotato purees. J. Food Sci. 2008, 73, S215–S221. [Google Scholar] [CrossRef] [PubMed]
- Oki, T.; Osame, M.; Masuda, M.; Furuta, S.; Nishiba, Y.; Terahara, N.; Suda, I. Simple and rapid spectrophotometric method for selecting purple-fleshed sweet potato cultivars with a high radical-scavenging activity. Food Chem. Toxicol. 2003, 53, 101–107. [Google Scholar] [CrossRef]
- Yoshinaga, M.; Yamakawa, O.; Nakatani, M. Genotypic diversity of anthocyanin content and composition in purple-fleshed sweet potato (Ipomoea batatas (L.) Lam). Breed. Sci. 2010, 49, 43–47. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Lim, S.; Griffin, J.; Carey, E.; Katz, B.; Tomich, J.; Smith, J.S.; Wang, W. Characterisation and stability of anthocyanins in purple-fleshed sweet potato P40. Food Chem. 2015, 186, 90–96. [Google Scholar] [CrossRef]
- Enicole, B.; Maris, C.; Vanden, T. Extraction of anthocyanins from industrial purple-fleshed sweet potatoes and enzymatic hydrolysis of residues for fermentable sugars. Ind. Crops Prod. 2010, 32, 613–620. [Google Scholar]
- Gisela, J.; Wilhelm, F. Coloured potatoes (Solanum tuberosum L.)-anthocyanin content and tuber quality. Genet. Resour. Crop Evol. 2006, 53, 1321. [Google Scholar]
- Odake, K.; Hatanaka, A.; Kajiwara, T.; Muroi, T.; Nishiyama, K.; Yamakawa, O.; Terahara, N.; Yamaguchi, M. Evaluation method and breeding of purple sweet potato “YAMAGAWA MURASAKI” (Ipomoea batatas POIR.) for raw material of food colorants. Nippon Shokuhin Kagaku Kogaku Kaishi 1994, 41, 287–293. [Google Scholar] [CrossRef]
- Fan, G.; Han, Y.; Gu, Z.; Chen, D. Optimizing conditions for anthocyanins extraction from purple sweet potato using response surface methodology (RSM). LWT Food Sci. Technol. 2008, 41, 155–160. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, S.; Zheng, Y.; Lu, J.; Wu, D.; Shan, Q.; Hu, B. Purple sweet potato color attenuates oxidative stress and inflammatory response induced by d-galactose in mouse liver. Food Chem. Toxicol. 2009, 47, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, M.; Okuno, S.; Yamaguchi, M.; Yamakawa, O. Antimutagenicity of deacylated anthocyanins in purple-fleshed sweet potato. Biosci. Biotechnol. Biochem. 2001, 65, 1652–1655. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yan, Q.; Lu, L.; Zhang, Y. In vivo antioxidant, hypoglycemic, and anti-tumor activities of anthocyanin extracts from purple sweet potato. Nutr. Res. Pract. 2013, 7, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Choi, J.H.; Yun, H.J.; Han, E.H.; Kim, H.G.; Kim, J.Y.; Park, B.H.; Khanal, T.; Choi, J.M.; Chung, Y.C.; et al. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem. Toxicol. 2011, 49, 93–99. [Google Scholar] [CrossRef]
- Jing, P.; Giusti, M.M. Effects of extraction conditions on improving the yield and quality of an anthocyanin-rich purple corn (Zea mays L.) color extract. J. Food Sci. 2007, 72, C363–C368. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Salgado, J.M.; Domínguez, J.M.; Cortés-Diéguez, S. Comparison of soxhlet, accelerated solvent and supercritical fluid extraction techniques for volatile (GC–MS and GC/FID) and phenolic compounds (HPLC–ESI/MS/MS) from Lamiaceae species. Phytochem. Anal. 2015, 26, 61–71. [Google Scholar] [CrossRef]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016, 197, 266–272. [Google Scholar] [CrossRef]
- Han, Y. Extraction, Compositoion Analysis, Stability and Antioxidant Activity of Anthocyanins from Purple Sweet Potato. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2007. (In Chinese). [Google Scholar]
- Lu, G.; Shi, Z.; Wu, H.; Huang, H. Extraction purification and component analysis of anthocyanins from purple sweet potato. Nat. Product Res. Devel. 1997, 9, 48–51. (In Chinese) [Google Scholar]
- Wu, Q. Advances in the extraction process of purple sweet potato fuchsia. Today Sci. Technol. 2003, 6, 43–44. (In Chinese) [Google Scholar]
- Santos, D.T.; Vegg, P.C.; Meireles, M.A.A. Extraction of antioxidant compounds from jabuticaba (Myrciaria cauliflora) skins: Yield, composition and economical evaluation. J. Food Eng. 2010, 101, 23–31. [Google Scholar] [CrossRef]
- Liu, X.; Mu, T.; Sun, H.; Zhang, M.; Chen, J. Optimisation of aqueous two-phase extraction of anthocyanins from purple sweet potatoes by response surface methodology. Food Chem. 2013, 141, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, L.; Tu, Z.; Cao, F. Study on the extraction of purple sweet potato color glycosides by bioenzyme method. Jiangxi Food Ind. 2009, 4, 25–27. (In Chinese) [Google Scholar]
- Cheng, B.; Li, L.; Tang, H.; Shen, J. Enzymatic extraction and purification of anthocyanins from purple sweet potato. Food Sci. 2012, 33, 59–62. (In Chinese) [Google Scholar]
- Liu, X.; Sun, J.; Li, J.; Liao, X. Review on novel application of pulsed electric fields in food processing. Food Ferment. Ind. 2010, 36, 138–142. (In Chinese) [Google Scholar]
- Donsì, F.; Ferrari, G.; Pataro, G. Applications of pulsed electric field treatments for the enhancement of mass transfer from vegetable tissue. Food Eng. Rev. 2010, 2, 109–130. [Google Scholar] [CrossRef]
- Knorr, D.; Froehling, A.; Jaeger, H.; Reineke, K.; Schlueter, O.; Schoessler, K. Emerging technologies in food processing. Annu. Rev. Food Sci. Technol. 2011, 2, 203–235. [Google Scholar] [CrossRef]
- Puértolas, E.; Luengo, E.; Álvarez, I.; Raso, J. Improving mass transfer to soften tissues by pulsed electric fields: Fundamentals and applications. Annu. Rev. Food Sci. Technol. 2012, 3, 263–282. [Google Scholar] [CrossRef]
- Puértolas, E.; Cregenzán, O.; Luengo, E.; Álvarez, I.; Raso, J. Pulsed-electric-field-assisted extraction of anthocyanins from purple-fleshed potato. Food Chem. 2013, 136, 1330–1336. [Google Scholar] [CrossRef]
- Pensado, L.; Casais, C.; Mejuto, C.; Cela, R. Optimization of the extraction of polycyclic aromatic hydrocarbons from wood samples by the use of microwave energy. J. Chromatogr. A 2000, 869, 505–513. [Google Scholar] [CrossRef]
- Navas, M.J.; Jiménez-Moreno, A.M.; Bueno, J.M.; Saez-Plaza, P.; Asuero, A.G. Analysis and antioxidant capacity of anthocyanin pigments. Part IV: Extraction of anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 313–342. [Google Scholar] [CrossRef]
- Delazar, A.; Nahar, L.; Hamedeyazdan, S.; Sarker, S.D. Microwave-assisted extraction in natural products isolation. Methods Mol. Biol. 2012, 864, 89–115. [Google Scholar] [PubMed]
- Xu, Z.; Gao, Y.; Shi, S.; Liu, X. Study on the extraction of pigment from purple sweet potato powder by microwave-assisted technique. Food Sci. 2005, 26, 234–239. [Google Scholar]
- Zhu, Z.; Guan, Q.; Koubaa, M.; Barba, F.J.; Roohinejad, S.; Cravotto, G.; Yang, X.; Li, S.; He, J. HPLC-DAD-ESI-MS2 analytical profile of extracts obtained from purple sweet potato after green ultrasound-assisted extraction. Food Chem. 2017, 215, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.D.; Hu, Z.; Thompson, R.L.; Yencho, G.C.; Pecota, K.V. Pressurized liquid extraction and quantification of anthocyanins in purple-fleshed sweet potato genotypes. J. Food Compos. Anal. 2012, 26, 96–103. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, B.; Williams, P.; Pace, R.D. Identification of anthocyanins in muscadine grapes with HPLC-ESI-MS. LWT Food Sci. Technol. 2009, 42, 819–824. [Google Scholar] [CrossRef]
- Andersen, O.M.; Jordheim, M. The Anthocyanins. Flavonoids: Chemistry, Biochemistry, and Applications; CRC Press: Boca Raton, FL, USA, 2006; pp. 471–530. [Google Scholar]
- Zhu, H.M.; Zhao, M. Study on chemical constituents and antioxidant activity of anthocyanins from Ipomoea batatas L. (purple sweet potato). Chem. Ind. Forest Products 2009, 29, 39–45. (In Chinese) [Google Scholar]
- Pascualteresa, S.D.; Sanchezballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Harborne, J.B.; Grayer, R.J. The Flavonoids: The Anthocyanins; Chapman and Hall Ltd.: London, UK, 1988. [Google Scholar]
- Chen, W.; Pei, Z.; Liu, X.; Ma, N.; Zhang, Y.; Wang, H. The research of determination method of purple potato anthocyanins by LC—MS/MS. China Food Addit. 2015, 3, 191–194. (In Chinese) [Google Scholar]
- Qi, M. Characteristics and Anthocyanins Process Stability of Purple Sweet Potatoes Used for Fresh Cooking or Manufacturing. Master’s Thesis, Wuhan Polytechnic University, Wuhan, China, 2015. (In Chinese). [Google Scholar]
- Chen, J. Study on Extraction, Purification and Stability of Purple Sweet Potato Pigment. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2011. (In Chinese). [Google Scholar]
- Kuang, M.; Qi, M.; He, J.; Li, S.; Liu, G.; Zhu, Z.; Cai, H.; Feng, J. Identification of anthocyanins in Brassica campestris L. and their stability and antioxidant activity. Sci. Agric. Sin. 2014, 47, 4067–4077. (In Chinese) [Google Scholar]
- Li, J.; Li, X.; Zhang, Y.; Zheng, Z.; Qu, Z.; Liu, M.; Zhu, S.; Liu, S.; Wang, M. Identification and thermal stability of purple-fleshed sweet potato anthocyanins in aqueous solutions with various pH values and fruit juices. Food Chem. 2013, 136, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.D.; Deighton, N.; Thompson, R.T.; McFeeters, R.F.; Dean, L.O.; Pecota, K.V.; Yencho, G.C. Characterization of anthocyanins and anthocyanidins in purple-fleshed sweet potatoes by HPLC-DAD/ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, N.S.; Shin, S.; Shin, S.; Lim, S.; Suh, D.; Baek, I.; Park, K.; Ha, T.J. Characterisation of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS analysis. Food Chem. 2009, 112, 226–231. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, Q.; Xue, R.; Zhang, J.; Zhang, Y. Isolation and identification of colourless caffeoyl compounds in purple sweet potato by HPLC-DAD–ESI/MS and their antioxidant activities. Food Chem. 2014, 161, 22–26. [Google Scholar] [CrossRef]
- Kirby, C.W.; Wu, T.; Tsao, R.; McCallum, J.L. Isolation and structural characterization of unusual pyranoanthocyanins and related anthocyanins from Staghorn sumac (Rhustyphina L.) via UPLC-ESI-MS, 1H, 13C, and 2D NMR spectroscopy. Phytochemistry 2013, 94, 284–293. [Google Scholar] [CrossRef]
- Terahara, N.; Matsui, T.; Minoda, K.; Nasu, K.; Kikuchi, R.; Fukui, K.; Ono, H.; Matusumoto, K. Functional new acylatedsophoroses and deglucosylated anthocyanins in a fermented red vinegar. J. Agric. Food Chem. 2009, 57, 8331–8338. [Google Scholar] [CrossRef]
- Goda, Y.; Shimizu, T.; Kato, Y.; Nakamura, M.; Maitani, T.; Yamada, T.; Terahara, N.; Yamaguchi, M. Two acylated anthocyanins from purple sweet potato. Phytochemistry 1997, 44, 183–186. [Google Scholar] [CrossRef]
- Terahara, N.; Shimizu, T.; Kato, Y.; Nakamura, M.; Maitani, T.; Yamaguchi, M.; Goda, Y. Six diacylated anthocyanins from the storage roots of purple sweet potato, Ipomoea batatas. J. Agric. Chem. Soc. Jpn. 1999, 63, 1420–1424. [Google Scholar] [CrossRef][Green Version]
- Shi, Z.; Bassa, I.A.; Gabriel, S.L.; Francis, F.J. Anthocyanin pigments of sweet potatoes—Ipomoea batatas. J. Food Sci. 2010, 57, 755–757. [Google Scholar] [CrossRef]
- Odake, K. Characteristics of food color pigments derived from Ayamurasaki. In Proceedings of the International Workshop on Sweet Potato Production System toward the 21st Century, Miyakonojo, Japan, 9–10 December 1997; Labonte, D.R., Yamashita, M., Mochida, H., Eds.; pp. 303–309. [Google Scholar]
- Yoshinaga, M. Breeding of purple fleshed sweet potato. In Proceedings of the International Workshop on Sweet Potato Production System toward the 21st Century, Miyakonojo, Japan, 9–10 December 1997; Labonte, D.R., Yamashita, M., Mochida, H., Eds.; pp. 193–199. [Google Scholar]
- Odake, K.; Terahara, N.; Saito, N.; Toki, K.; Honda, T. Chemical structures of two anthocyanins from purple sweet potato, Ipomoea batatas. Phytochemistry 1992, 31, 2127–2130. [Google Scholar] [CrossRef]
- Yang, Z.; Cai, Z. Structural identification of anthocyanin from sweet potato. Food Sci. 1999, 26, 182–192. (In Chinese) [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Winefield, C.; Davies, K.; Gould, K. Anthocyanins: Biosynthesis, Functions, and Applications; Springer: New York, NY, USA, 2009. [Google Scholar]
- Qi, M.; Guo, Y.; Li, S.; He, J. Effect of different Chinese cooking hot processing on anthocyanin content in fresh food and processed purple sweet potato. Food Nutr. China 2015, 21, 28–31. (In Chinese) [Google Scholar]
- Kim, H.W.; Kim, J.B.; Cho, S.M.; Chung, M.N.; Lee, Y.M.; Chu, S.M.; Che, J.H.; Kim, S.N.; Kim, S.Y.; Cho, Y.S.; et al. Anthocyanin changes in the Korean purple-fleshed sweet potato, Shinzami, as affected by steaming and baking. Food chem. 2012, 130, 966–972. [Google Scholar] [CrossRef]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Vamanu, E. Polyphenolic nutraceuticals to combat oxidative stress through microbiota modulation. Front. Pharmacol. 2019, 10, 492. [Google Scholar] [CrossRef]
- Kano, M.; Tskayanagi, T.; Harada, K.; Makino, K.; Ishikawa, F. Antioxidative activity of anthocyanins from purple sweet potato, Ipomera Batatas Cultivar Ayamurasaki. Biosci. Biotechnol. Biochem. 2005, 69, 979–988. [Google Scholar] [CrossRef]
- Suda, I.; Furuta, S.; Nishiba, Y.; Yamakawa, O.; Matsugano, K.; Sugita, K. Reduction of liver injury induced by carbon tetrachloride in rats administered purple-colored sweet potato juice. Nippon Shokuhin Kogyo Gakkaishi 1997, 44, 315–318. [Google Scholar] [CrossRef][Green Version]
- Suda, I.; Yamakawa, O.; Matsugano, K.; Sugita, K.; Takeguma, Y.; Irisa, K.; Tokumaru, F. Changes of serum Y-GTP, GOT and GPT levels in hepatic function-weakling subjects by ingestion of high anthocyanin sweet potato juice. Nippon Shokuhin Kogyo Gakkaishi 1998, 45, 611–617. [Google Scholar] [CrossRef]
- Suda, I.; Ishikawa, F.; Hatakeyama, M.; Miyawaki, M.; Kudo, T.; Hirano, K.; Ito, A.; Yamakawa, O.; Horiuchi, S. Intake of purple sweet potato beverage affects on serum hepatic biomarker levels of healthy adult men with borderline hepatitis. Eur. J. Clin. Nutr. 2008, 62, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Shimada, K.; Sekikawa, M.; Fukushima, M. Anthocyanin-rich red potato flakes affect serum lipid peroxidation and hepatic SOD mRNA level in rats. Biosci. Biotech. Biochem. 2007, 71, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Choi, J.H.; Choi, J.M.; Chung, Y.C.; Jeong, H.G. Protective mechanisms of anthocyanins from purple sweet potato against tert-butyl hydroperoxide-induced hepatotoxicity. Food Chem. Toxicol. 2011, 49, 2081–2089. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, J.; Zheng, Y.; Wu, D.; Hu, B.; Shan, Q.; Cheng, W.; Li, M.; Sun, Y. Purple sweet potato color attenuates hepatic insulin resistance via blocking oxidative stress and endoplasmic reticulum stress in high-fat-diet-treated mice. J. Nutr. Biochem. 2013, 24, 1008–1018. [Google Scholar] [CrossRef]
- Cai, Z.; Song, L.; Qian, B.; Xu, W.; Ren, J.; Jing, P.; Oey, I. Understanding the effect of anthocyanins extracted from purple sweet potatoes on alcohol-induced liver injury in mice. Food Chem. 2018, 245, 463–470. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Lu, J.; Chen, J.; Wang, X.; Feng, J.; Ruan, J.; Sun, X.; Li, C.; Sun, Q. Purple sweet potato color suppresses lipopolysaccharide-induced acute inflammatory response in mouse brain. Neurochem. Int. 2010, 56, 424–430. [Google Scholar] [CrossRef]
- Li, W.; Ji, G.; Zhang, X.; Yu, H. The influence and mechanisms of purple sweet potato anthocyanins on the growth of bladder cancer BIU87 cell. Zhonghua Yi Xue Za Zhi 2018, 98, 457–459. [Google Scholar]
- Matsui, T.; Ebuchi, S.; Kobayashi, M.; Fukul, K.; Sugita, K.; Terahara, N.; Matsumoto, K. Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the alpha-glucosidase inhibitory action. J. Agric. Food Chem. 2002, 50, 7244–7248. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Wu, Z.; Weng, P. The modulatory effect of anthocyanins from purple sweet potato on human intestinal microbiota in vitro. J. Agric. Food Chem. 2016, 64, 2582–2590. [Google Scholar] [CrossRef]
- Sun, W. Extraction, Deodorization, Stability and Initial Structure Identification of Anthocyanins from Purple Sweet Potato. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2015. (In Chinese). [Google Scholar]
- Suda, I.; Oki, T.; Masuda, M.; Kobayashi, M.; Nishiba, Y.; Furuta, S. Physiological functionality of purple-fleshed sweet potatoes containing anthocyanins and their utilization in foods. JARQ Jpn. Agric. Res. Q. 2003, 37, 167–173. [Google Scholar] [CrossRef]
- Bassa, L.A.; Francis, F.J. Stability of anthocyanins from sweet potatoes in a model beverage. J. Food Sci. 1987, 52, 1753–1754. [Google Scholar] [CrossRef]
- Tensiska, T.; Marta, H.; Cahyana, Y.; Amirah, N.S. Application of encapsulated anthocyanin pigments from purple sweet potato (Ipomoea Batatas L.) in jelly drink. KnE Life Sci. 2017, 2, 482–493. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Dong, N. Research progress of purple sweet potato anthocyanin. Cereal Feed Ind. 2011, 12, 38–40. (In Chinese) [Google Scholar]
- Li, Y.; Lu, S.; Huang, Y.; Wu, C. Application prospect of the purple sweet potato anthocyanin. J. Anhui Agric. Univ. 2008, 36, 12641–12642. (In Chinese) [Google Scholar]
- Fang, J. Some anthocyanins could be efficiently absorbed across the gastrointestinal mucosa: Extensive presystemic metabolism reduces apparent bioavailability. J. Agric. Food Chem. 2014, 62, 3904–3908. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Zhao, T.; Zhang, Z.; Mao, J.; Feng, W.; Wu, X.; Yang, L. Biotransformation and metabolism of three mulberry anthocyanin monomers by rat gut microflora. Food Chem. 2017, 237, 887–894. [Google Scholar] [CrossRef]
- Aura, A.M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A 13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef]
- Flores, G.; del Castillo, M.L.R.; Costabile, A.; Klee, A.; Guergoletto, K.B.; Gibson, G.R. In vitro fermentation of anthocyanins encapsulated with cyclodextrins: Release, metabolism and influence on gut microbiota growth. J. Funct. Foods 2015, 16, 50–57. [Google Scholar] [CrossRef]
- Kubow, S.; Iskandar, M.M.; Sabally, K.; Azadi, B.; Ekbatan, S.S.; Kumarathasan, P.; Das, D.D.; Prakash, S.; Burgos, G.; Felde, T.Z. Biotransformation of anthocyanins from two purple-fleshed sweet potato accessions in a dynamic gastrointestinal system. Food Chem. 2016, 192, 171–177. [Google Scholar] [CrossRef] [PubMed]


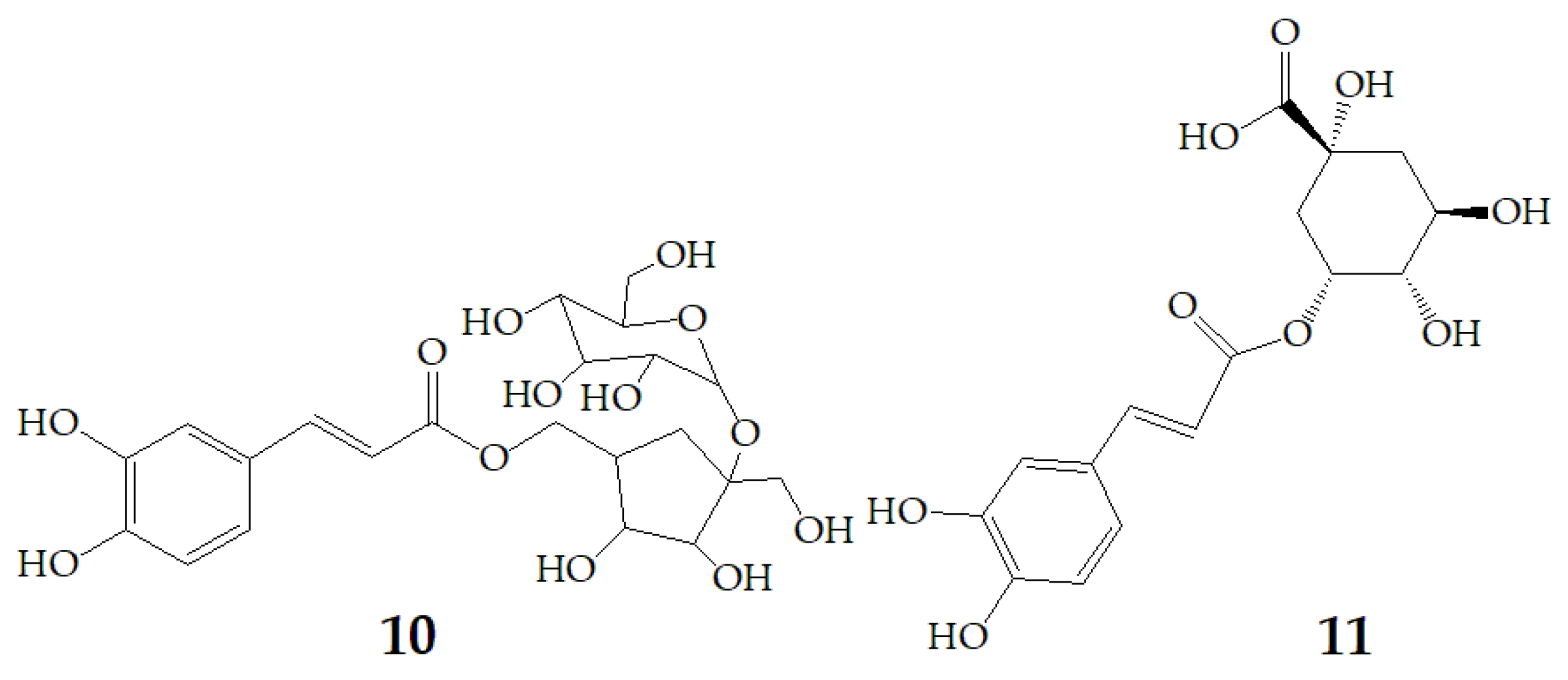
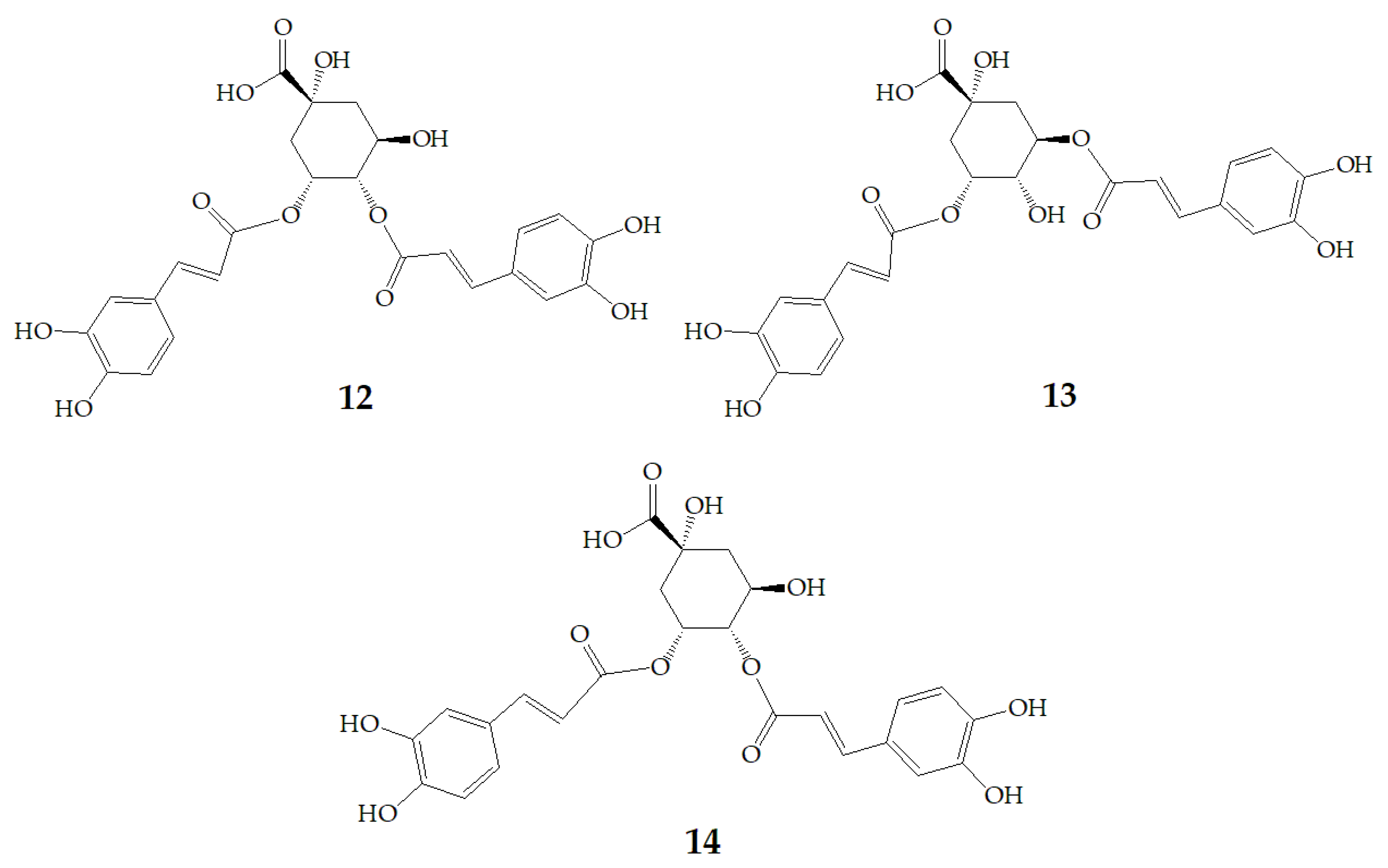
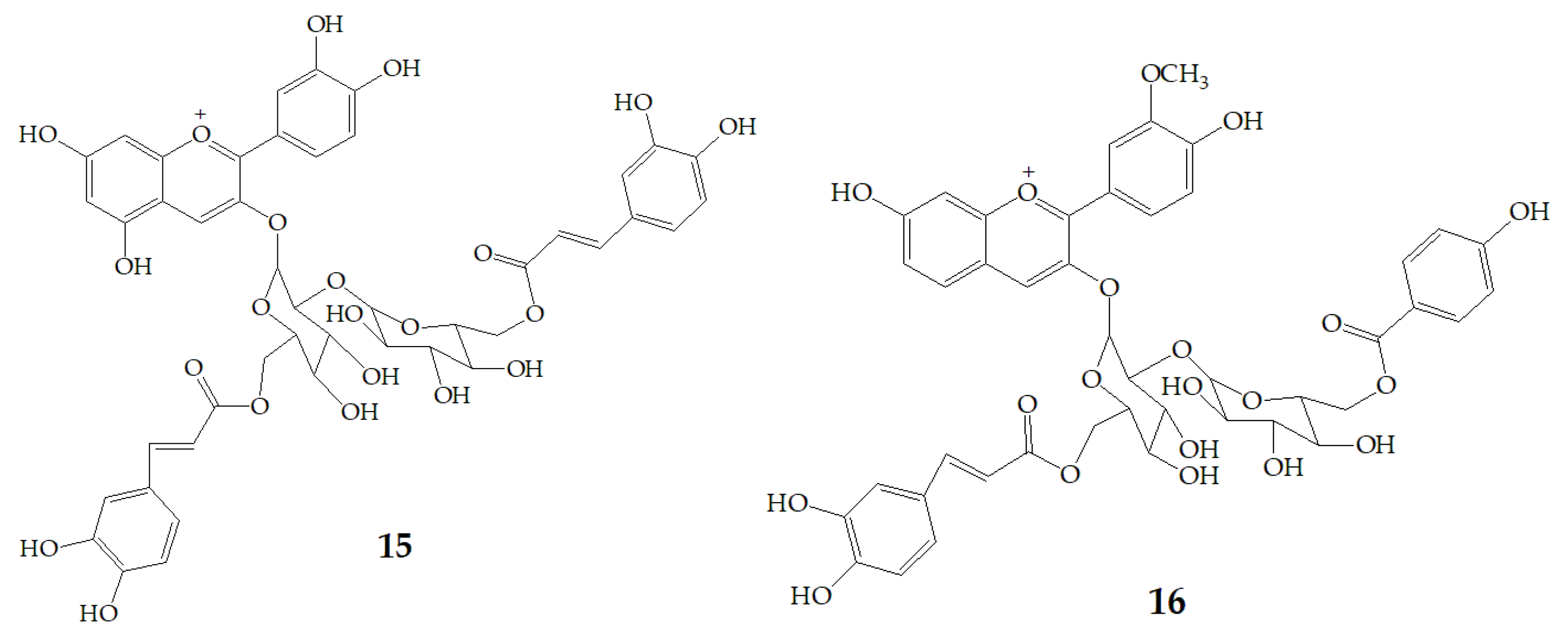
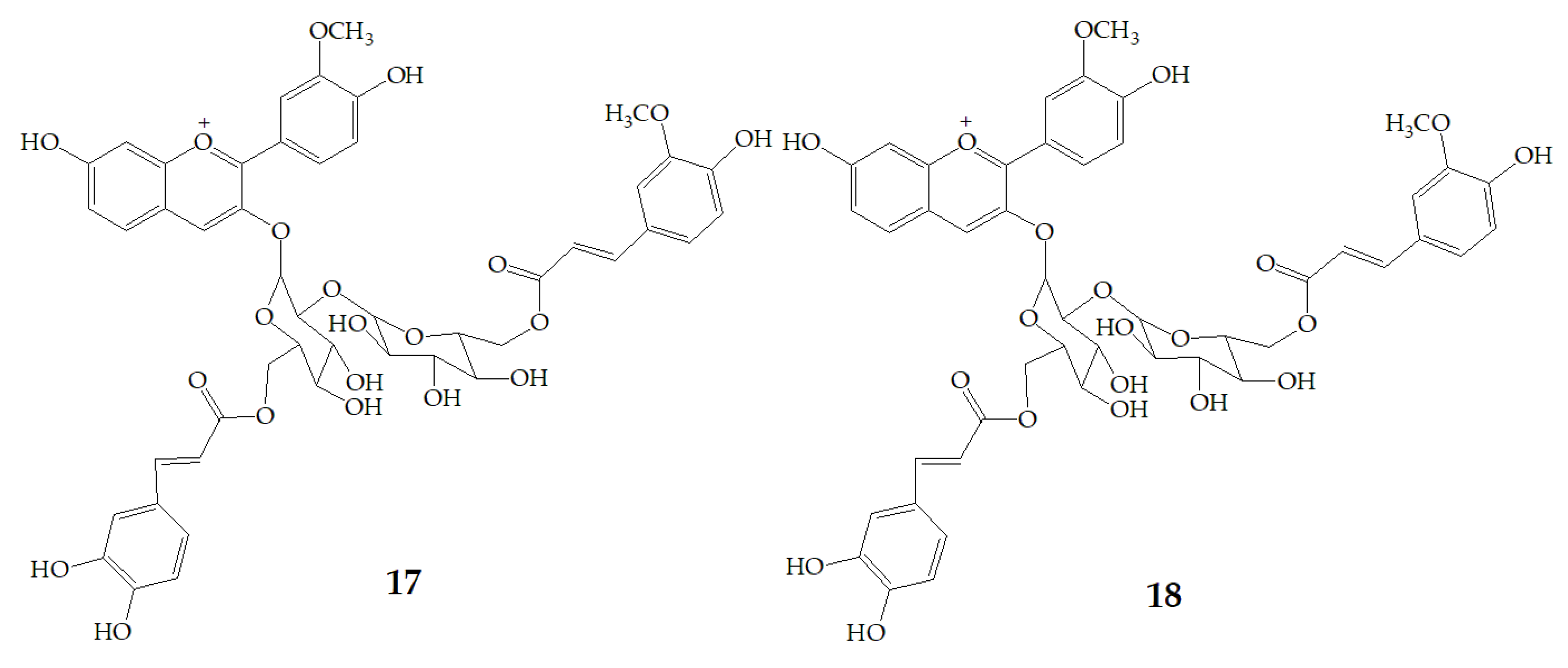
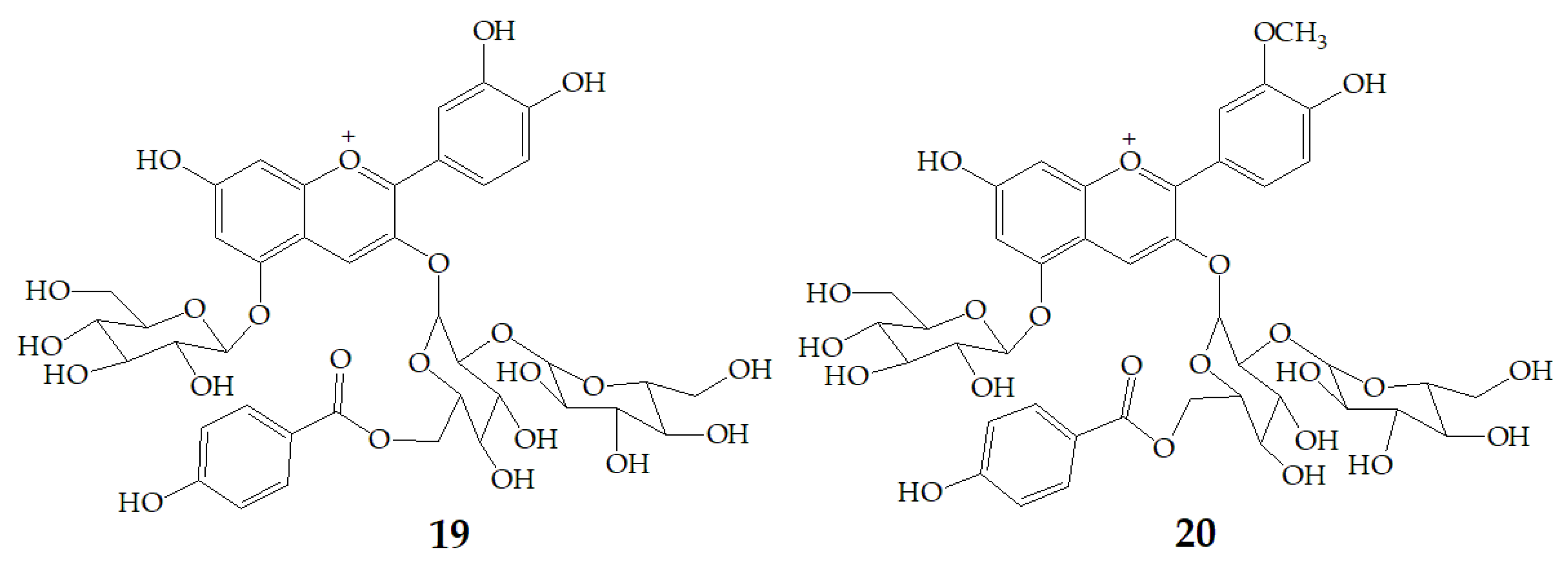
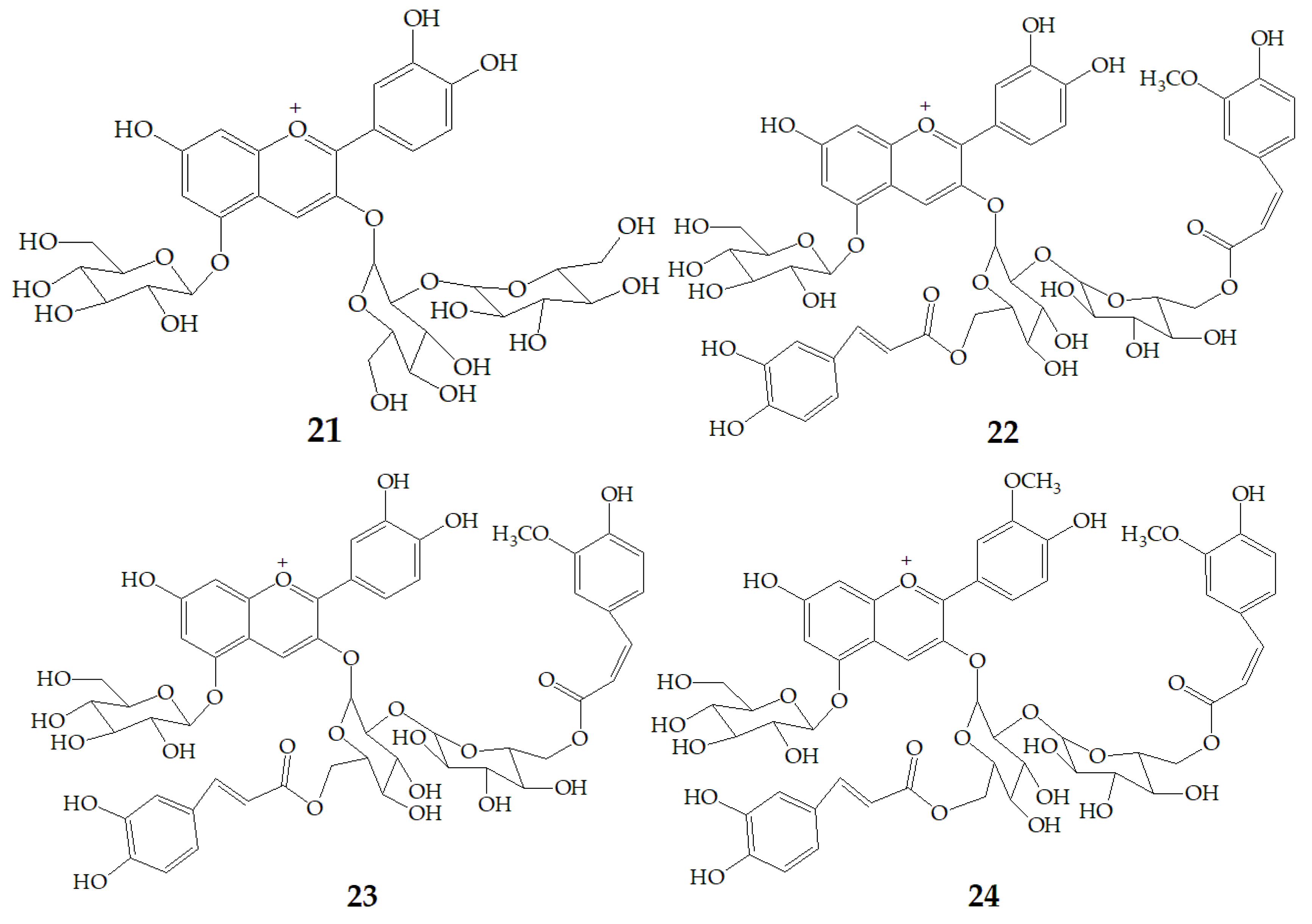

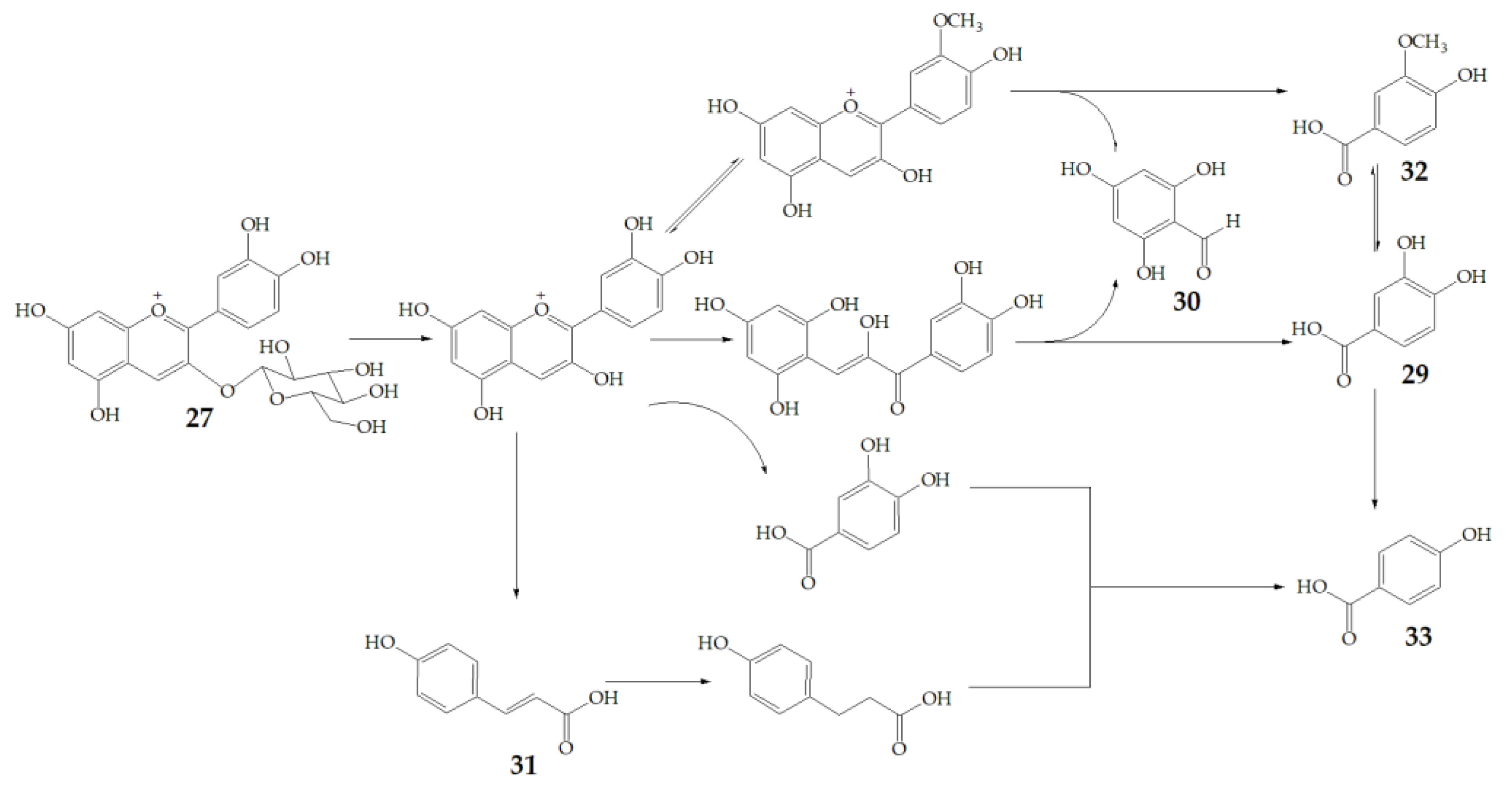
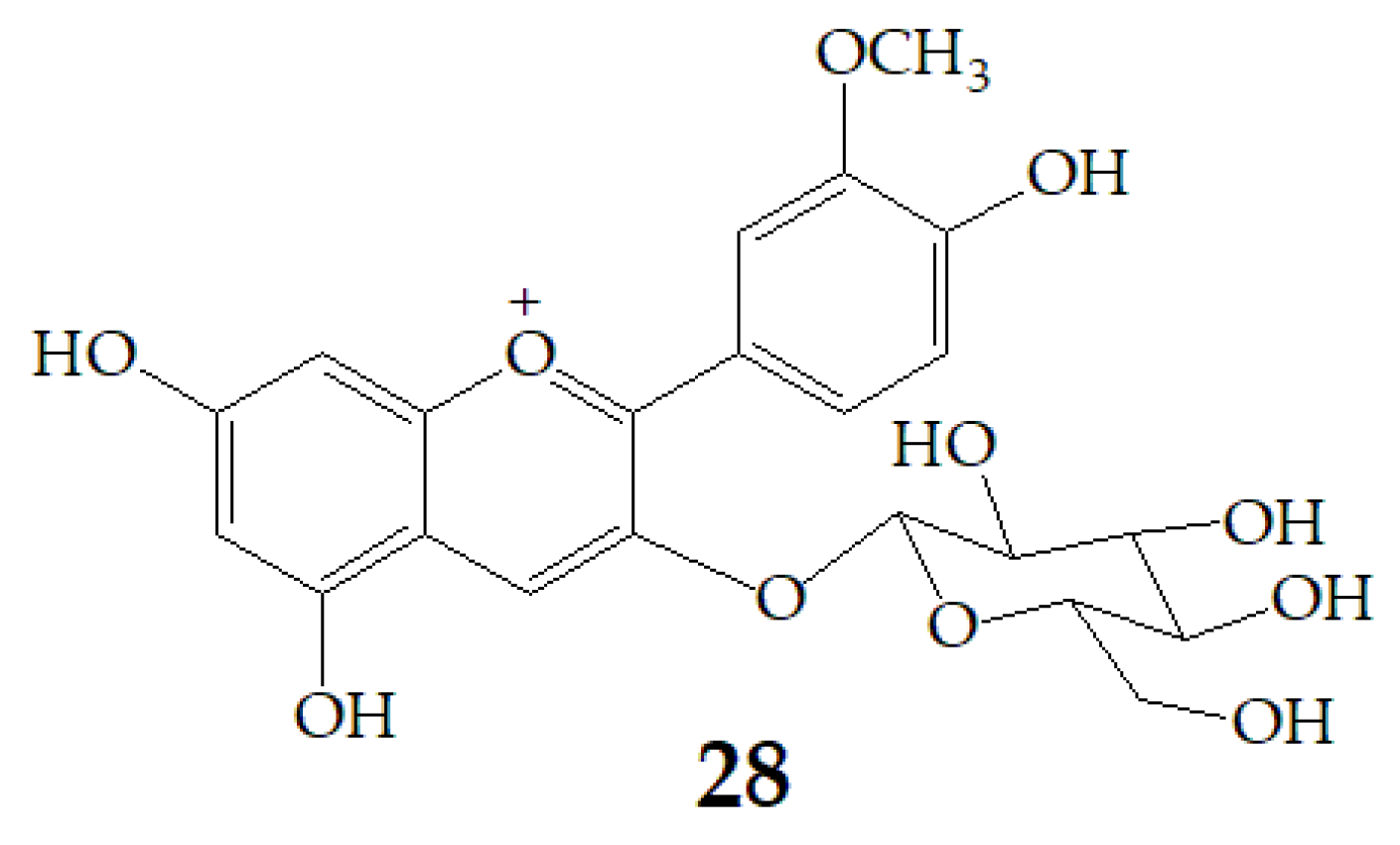
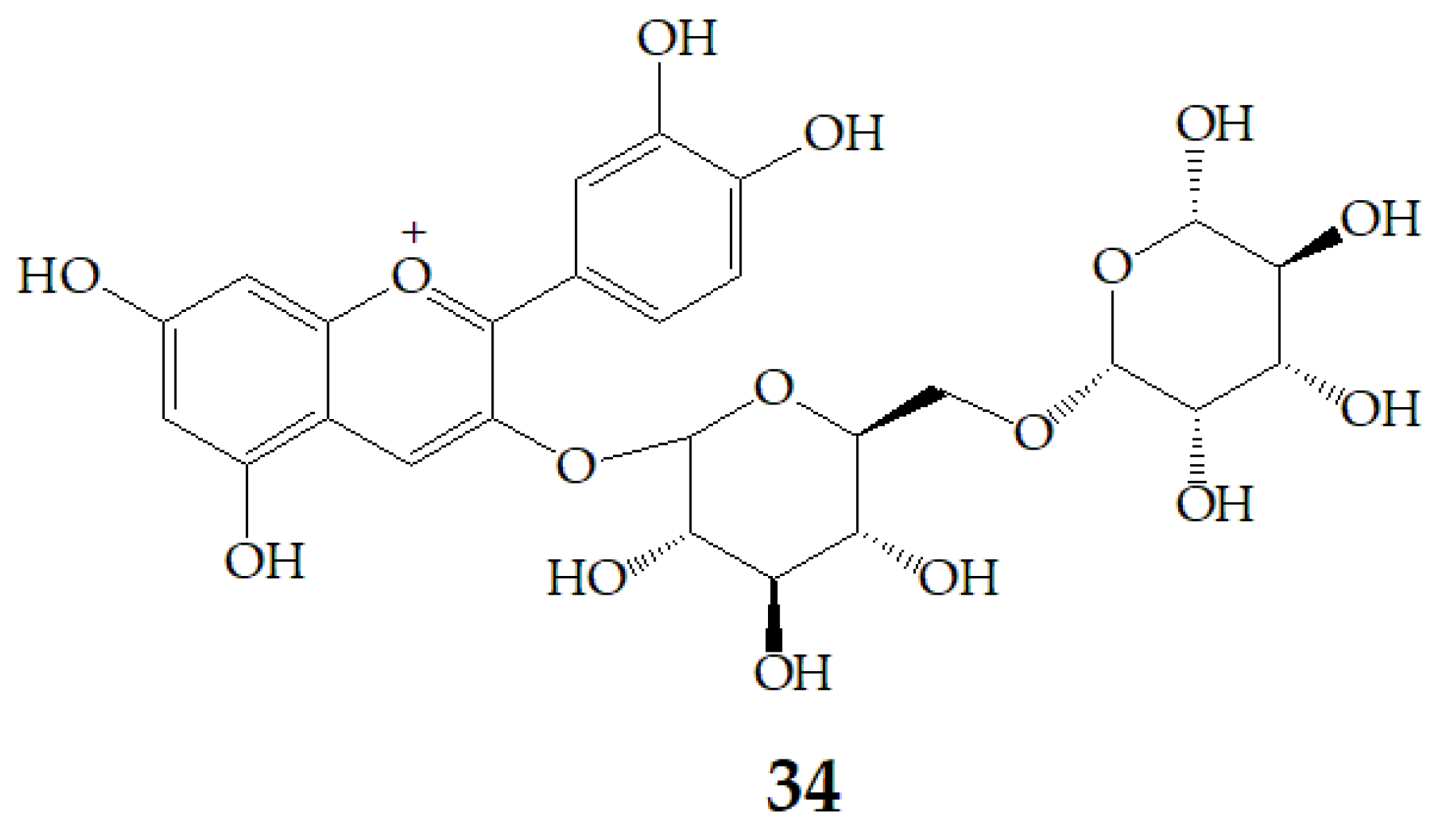

| Name | Structure | Fragment Ion Peak |
|---|---|---|
| Glucose | 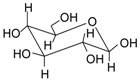 | 162 |
| Sophorose | 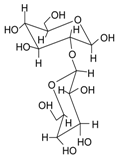 | 324 |
| Ferulic acid | 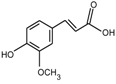 | 176 |
| Caffeic acid | 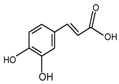 | 162 |
| P-coumaric acid | 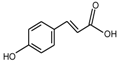 | 146 |
| Sinapic acid | 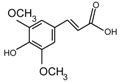 | 206 |
| Gallic acid | 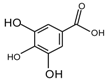 | 152 |
| Malic acid | 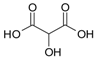 | 116 |
| Tartaric acid | 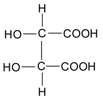 | 132 |
| Oxalate | 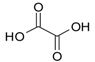 | 72 |
| Propanedioic acid | 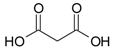 | 86 |
| Succinic acid | 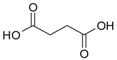 | 100 |
| P-hydroxybenzoic acid |  | 120 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.; Xiao, R.; He, S.; An, X.; He, Y.; Wang, C.; Yin, S.; Wang, B.; Shi, X.; He, J. Research Advances of Purple Sweet Potato Anthocyanins: Extraction, Identification, Stability, Bioactivity, Application, and Biotransformation. Molecules 2019, 24, 3816. https://doi.org/10.3390/molecules24213816
Li A, Xiao R, He S, An X, He Y, Wang C, Yin S, Wang B, Shi X, He J. Research Advances of Purple Sweet Potato Anthocyanins: Extraction, Identification, Stability, Bioactivity, Application, and Biotransformation. Molecules. 2019; 24(21):3816. https://doi.org/10.3390/molecules24213816
Chicago/Turabian StyleLi, Aoran, Ruoshi Xiao, Sijia He, Xiaoyu An, Yi He, Chengtao Wang, Sheng Yin, Bin Wang, Xuewei Shi, and Jingren He. 2019. "Research Advances of Purple Sweet Potato Anthocyanins: Extraction, Identification, Stability, Bioactivity, Application, and Biotransformation" Molecules 24, no. 21: 3816. https://doi.org/10.3390/molecules24213816
APA StyleLi, A., Xiao, R., He, S., An, X., He, Y., Wang, C., Yin, S., Wang, B., Shi, X., & He, J. (2019). Research Advances of Purple Sweet Potato Anthocyanins: Extraction, Identification, Stability, Bioactivity, Application, and Biotransformation. Molecules, 24(21), 3816. https://doi.org/10.3390/molecules24213816








