Effect of Different Amino Acids and Heating Conditions on the Formation of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and Its Kinetics Formation Using Chemical Model System
Abstract
1. Introduction
- 2-amino-3-methylimidazo[4,5-f]quinoline (IQ)
- 2-amino-3,4-dimethyl-3H-imidazo[4,5-f]quinolone (MeIQ)
- 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx)
- 2-amino-3,7,8-trimethyl-3H-imidazo[4,5-f]quinoxaline (DiMeIQx)
- 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)
2. Results and Discussion
2.1. PhIP Identification and Quality Parameter
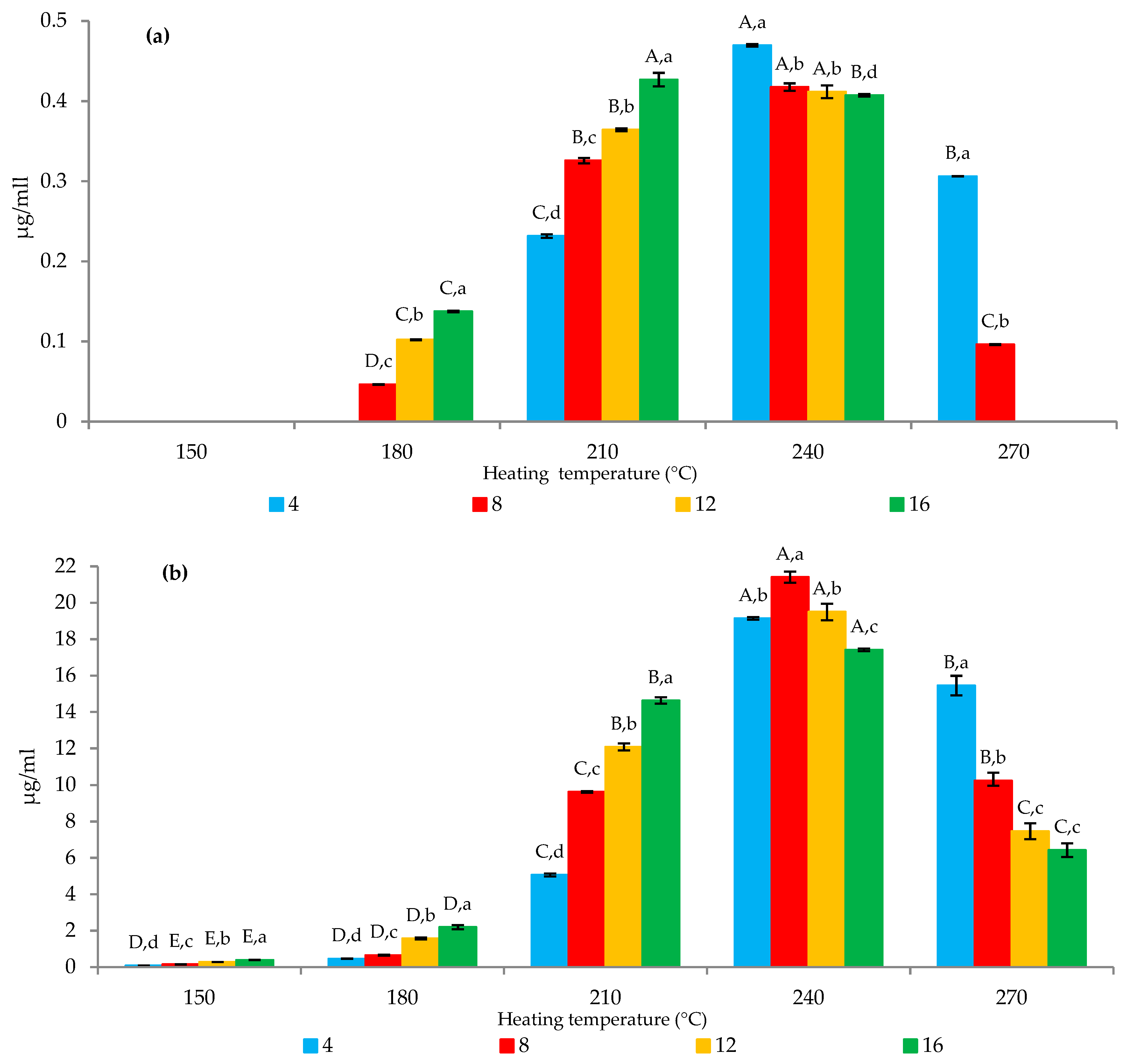
2.2. Effects of Time and Temperature
2.3. Effects of Amino Acid Precursors
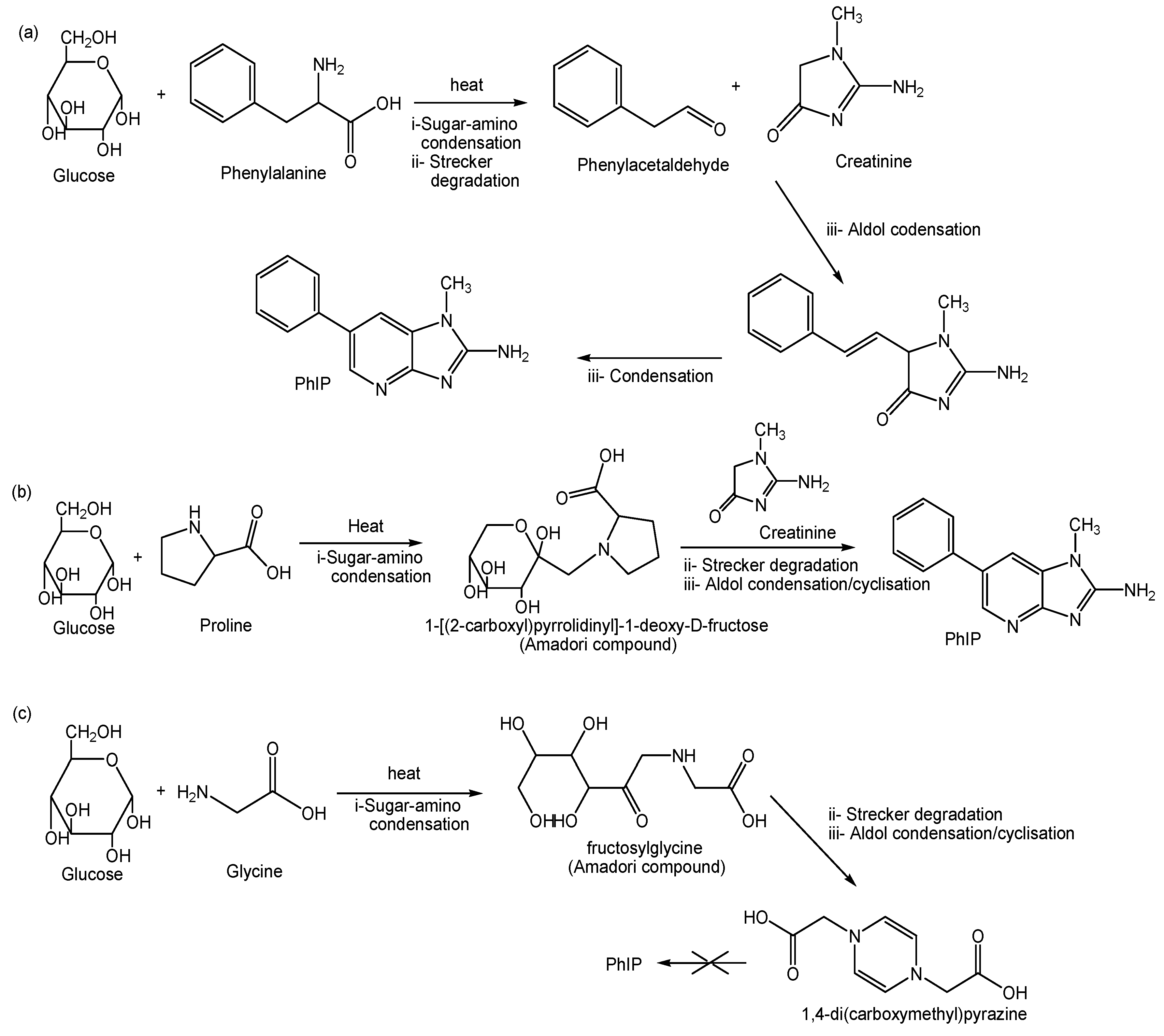
2.4. Kinetic Formation of PhIP
3. Materials and Methods
3.1. Chemicals
3.2. Model Systems
3.3. Instrument
3.4. Reaction Kinetics
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jahurul, M.H.A.; Jinap, S.; Ang, S.J.; Abdul-Hamid, A.; Hajeb, P.; Lioey, H.N.; Zaidul, I.S.M. Dietary exposure to heterocyclic amines in high-temperature cooked meat and fish in Malaysia. Food Addit. Contam. Part A 2010, 27, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Oz, F.; Kaban, G.; Kaya, M. Effects of cooking techniques and levels on the formation of heterocyclic aromatic amines in chicken and fish. J. Anim. Vet. Adv. 2010, 9, 1259–1264. [Google Scholar] [CrossRef][Green Version]
- Hasnol, N.D.; Jinap, S.; Sanny, M. Effect of different types of sugars in a marinating formulation on the formation of heterocyclic amines in grilled chicken. Food Chem. 2014, 145, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Murkovic, M. Formation of heterocyclic aromatic amines in model systems. J. Chromatogr. B 2004, 802, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Jägerstad, M.; Skog, K.; Arvidsson, P.; Solyakov, A. Chemistry, formation and occurrence of genotoxic heterocyclic amines identified in model systems and cooked foods. Zeitschrift für Lebensmitteluntersuchung und-Forschung A 1998, 207, 419–427. [Google Scholar] [CrossRef]
- Schut, H.A.J.; Snyderwine, E.G. DNA adducts of heterocyclic amine food mutagens: Implications for mutagenesis and carcinogenesis. Carcinogenesis 1999, 20, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Gibis, M. Heterocyclic aromatic amines in cooked meat products: Causes, formation, occurrence, and risk assessment. Compr. Rev. Food Sci. Food Saf. 2016, 15, 269–302. [Google Scholar] [CrossRef]
- IARC Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. In IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Human; International Agency for Research on Cancer: Lyon, France, 1993; Volume 56, pp. 245–395.
- Regulatory Summaries. Regul. Chem. Dir. 1995, 1647–1648. [CrossRef]
- Voutsinas, J.; Wilkens, L.R.; Franke, A.; Vogt, T.M.; Yokochi, L.A.; Decker, R.; Marchand, L.L. Heterocyclic amine intake, smoking, cytochrome P450 1A2 and N-acetylation phenotypes, and risk of colorectal adenoma in a multiethnic population. Gut 2013, 62, 416–422. [Google Scholar] [CrossRef]
- John, E.M.; Stern, M.C.; Sinha, R.; Jocelyn, K. Meat consumption, cooking practices, meat mutagens, and risk of prostate cancer. Nutr. Cancer 2011, 63, 525–537. [Google Scholar] [CrossRef]
- Kabat, G.C.; Cross, A.J.; Park, Y.; Schatzkin, A.; Hollenbeck, A.R.; Rohan, T.E.; Sinha, R. Meat intake and meat preparation in relation to risk of postmenopausal breast cancer in the NIH-AARP diet and health study. Int. J. Cancer 2009, 124, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.W.; Zhang, X.C.; Wang, M.F. Chemistry and safety of maillard reaction products III: Heterocyclic amines and amino acids derivatives. In Food Safety Chemistry: Toxicant Occurrence, Analysis and Mitigation; Yu, L., Wang, S., Sun, B.-G., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 69–88. [Google Scholar]
- Puangsombat, K.; Gadgil, P.; Houser, T.A.; Hunt, M.C.; Smith, J.S. Occurrence of heterocyclic amines in cooked meat products. Meat Sci. 2012, 90, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Skog, K.; Augustsson, K.; Steineck, G.; Stenberg, M.; Jägerstad, M. Polar and non-polar heterocyclic amines in cooked fish and meat products and their corresponding pan residues. Food Chem. Toxicol. 1997, 35, 555–565. [Google Scholar] [CrossRef]
- Iwasaki, M.; Kataoka, H.; Ishihara, J.; Takachi, R.; Hamada, G.S.; Sharma, S.; Le Marchand, L.; Tsugane, S. Heterocyclic amines content of meat and fish cooked by Brazilian methods. J. Food Compos. Anal. 2010, 23, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Jägerstad, M.; Skog, K.; Grivas, S.; Olsson, K. Formation of heterocyclic amines using model systems. Mutat. Res. 1991, 259, 219–233. [Google Scholar] [CrossRef]
- Skog, K.I.; Johansson, M.A.E.; Jägerstad, M.I. Carcinogenic heterocyclic amines in model systems and cooked foods: A review on formation, occurrence and intake. Food Chem. Toxicol. 1998, 36, 879–896. [Google Scholar] [CrossRef]
- Shioya, M.; Wakabayashi, K.; Sato, S.; Nagao, M.; Sugimura, T. Formation of a mutagen, 2-amino-1-methyl-6-phenylimidazo [4,5-b]-pyridine (PhIP) in cooked beef, by heating a mixture containing creatinine, phenylalanine and glucose. Mutat. Res. 1987, 191, 133–138. [Google Scholar] [CrossRef]
- Overvik, E.; Kleman, M.; Berg, I.; Gustafsson, J.A. Influence of creatine, amino acids and water on the formation of the mutagenic heterocyclic amines found in cooked meat. Carcinogenesis 1989, 10, 2293–2301. [Google Scholar] [CrossRef]
- Johansson, M.A.E.; Laurent, B.F.; Gross, G.A.; Olsson, K.; Jägerstad, M. Influence of amino acids on the formation of mutagenic/carcinogenic heterocyclic amines in a model system. Carcinogenesis 1995, 16, 2553–2560. [Google Scholar] [CrossRef]
- Arvidsson, P.; Van Boekel, M.A.J.S.; Skog, K.; Solyakov, A.; Jägerstad, M. Formation of heterocyclic amines in a meat juice model system. J. Food Sci. 1999, 64, 216–221. [Google Scholar] [CrossRef]
- Ahn, J.; Grün, I.U. Heterocyclic amines: 1. kinetics of formation of polar and nonpolar heterocyclic amines as a function of time and temperature. J. Food Sci. 2005, 70, C173–C179. [Google Scholar] [CrossRef]
- Hwang, D.K.; Ngadi, M. Kinetics of heterocyclic amines formation in meat emulsion at different fat contents. LWT Food Sci. Technol. 2002, 35, 600–606. [Google Scholar] [CrossRef]
- Moon, S.E.; Shin, H.S. Formation of genotoxic 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine (PhIP) and its kinetics in a model system. Food Sci. Biotechnol. 2013, 22, 137–145. [Google Scholar] [CrossRef]
- Arvidsson, P.; Van Boekel, M.A.J.S.; Skog, K.; Jägerstad, M. Kinetics of formation of polar heterocyclic amines in a meat model system. J. Food Sci. 1997, 62, 911–916. [Google Scholar] [CrossRef]
- Chiu, C.P.; Chen, B.H. Stability of heterocyclic amines during heating. Food Chem. 2000, 68, 267–272. [Google Scholar] [CrossRef]
- Parker, J.K. Thermal generation or aroma. In Flavour Development, Analysis and Perception in Food and Beverages; Parker, J., Elmore, J., Methven, L., Eds.; Elsevier Ltd.: London, UK, 2015; pp. 151–185. ISBN 9781782421030. [Google Scholar]
- Rizzi, G.P. The strecker degradation of amino acids: Newer avenues for flavor formation. Food Rev. Int. 2008, 24, 416–435. [Google Scholar] [CrossRef]
- Zochling, S.; Murkovic, M. Formation of the heterocyclic aromatic amine PhIP: Identification of precursors and intermediates. Food Chem. 2002, 79, 125–134. [Google Scholar] [CrossRef]
- Linghu, Z.; Karim, F.; Smith, J.S. Amino acids inhibitory effects and mechanism on 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) formation in the maillard reaction model systems. J. Food Sci. 2017, 82, 3037–3045. [Google Scholar] [CrossRef]
- Britt, P.F.; Buchanan, A.C.; Owens, C.V., Jr.; Skeen, J.T. Does glucose enhance the formation of nitrogen containing polycyclic aromatic compounds and polycyclic aromatic hydrocarbons in the pyrolysis of proline. Fuel 2004, 83, 1417–1432. [Google Scholar] [CrossRef]
- Kato, T.; Harashima, T.; Moriya, N.; Kikugawa, K.; Hiramoto, K. Formation of the mutagenic/carcinogenic imidazoquinoxaline-type heterocyclic amines through the unstable free radical Maillard intermediates and its inhibition by phenolic antioxidants. Carcinogenesis 1996, 17, 2469–2476. [Google Scholar] [CrossRef]
- Jokic, A.; Zimpel, Z.; Huang, P.M.; Mezey, P.G. Molecular shape analysis of a maillard reaction intermediate. SAR QSAR Environ. Res. 2001, 12, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, D.; Saito, Y.; Mizusaki, S. Isolation of 2-amino-3-methyl-imidazo-[4,5-f]quinoline as mutagen from the heated product of a mixture of creatine and proline. Agric. Biol. Chem. 1984, 48, 241–243. [Google Scholar]
- Skog, K.; Jägerstad, M. Effects of monosaccharides and disaccharides on the formation of food mutagens in model systems. Mutat. Res. 1990, 230, 263–272. [Google Scholar] [CrossRef]
- Gross, G.A.; Grüter, A. Quantitation of mutagmic/carcinogenic heterocyclic aromatic amines in food products. J. Chromatogr. 1992, 592, 271–278. [Google Scholar] [CrossRef]
- Jinap, S.; Mohd-Mokhtar, M.S.; Farhadian, A.; Hasnol, N.D.S.; Jaafar, S.N.; Hajeb, P. Effects of varying degrees of doneness on the formation of heterocyclic aromatic amines in chicken and beef satay. Meat Sci. 2013, 94, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Kamal, N.H.; Selamat, J.; Sanny, M. Simultaneous formation of polycyclic aromatic hydrocarbons (PAHs) and heterocyclic aromatic amines (HCAs) in gas-grilled beef satay at different temperatures. Food Addit. Contam. Part A 2018, 35, 848–869. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Temperature, °C | Model System | |
|---|---|---|
| Phenylalanine, µg/mL | Proline, µg/mL | |
| 150 | 0.28 ± 0.0004 | ND |
| 180 | 1.57 ± 0.04 a | 0.10 ± 0.0005 b |
| 210 | 12.08 ± 0.19 a | 0.36 ± 0.001 b |
| 240 | 19.50 ± 0.5 a | 0.41 ± 0.01 b |
| 270 | 7.47 ± 0.43 | ND |
| Model System | Temperature (°C) | A (ng/µL) | k (min−1) | to (min) | R2 |
|---|---|---|---|---|---|
| Proline | 150 | nd | nd | nd | nd |
| 180 * | 0.5098 | 0.0268 | 4.0951 | 0.99 | |
| 210 * | 0.4371 | 1.1757 | 0.00 | 0.99 | |
| 240 ** | - | 0.0122 | - | 0.99 | |
| 270 ** | - | 0.2897 | - | 0.99 | |
| Phenylalanine | 150 * | 10.4238 | 0.0024 | 0.6403 | 0.98 |
| 180 * | 24.2223 | 0.0059 | 0.3715 | 0.99 | |
| 210 * | 21.4828 | 0.0710 | 0.0334 | 0.99 | |
| 240 ** | - | 0.0258 | - | 0.97 | |
| 270 ** | - | 0.0738 | - | 0.95 |
| Model System | Ea (kJ/mol) | ∆H‡ (kJ/mol) | ∆S‡ (J/mol/K) |
|---|---|---|---|
| Proline | 114.12 | 110.23 | −35.25 |
| Phenylalanine | 95.36 | 91.61 | −83.95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishak, A.A.; Selamat, J.; Sulaiman, R.; Sukor, R.; Abdulmalek, E.; Jambari, N.N. Effect of Different Amino Acids and Heating Conditions on the Formation of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and Its Kinetics Formation Using Chemical Model System. Molecules 2019, 24, 3828. https://doi.org/10.3390/molecules24213828
Ishak AA, Selamat J, Sulaiman R, Sukor R, Abdulmalek E, Jambari NN. Effect of Different Amino Acids and Heating Conditions on the Formation of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and Its Kinetics Formation Using Chemical Model System. Molecules. 2019; 24(21):3828. https://doi.org/10.3390/molecules24213828
Chicago/Turabian StyleIshak, Ainaatul Asmaa, Jinap Selamat, Rabiha Sulaiman, Rashidah Sukor, Emilia Abdulmalek, and Nuzul Noorahya Jambari. 2019. "Effect of Different Amino Acids and Heating Conditions on the Formation of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and Its Kinetics Formation Using Chemical Model System" Molecules 24, no. 21: 3828. https://doi.org/10.3390/molecules24213828
APA StyleIshak, A. A., Selamat, J., Sulaiman, R., Sukor, R., Abdulmalek, E., & Jambari, N. N. (2019). Effect of Different Amino Acids and Heating Conditions on the Formation of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and Its Kinetics Formation Using Chemical Model System. Molecules, 24(21), 3828. https://doi.org/10.3390/molecules24213828





