Medical-Grade Silicone Coated with Rhamnolipid R89 Is Effective against Staphylococcus spp. Biofilms
Abstract
:1. Introduction
2. Results
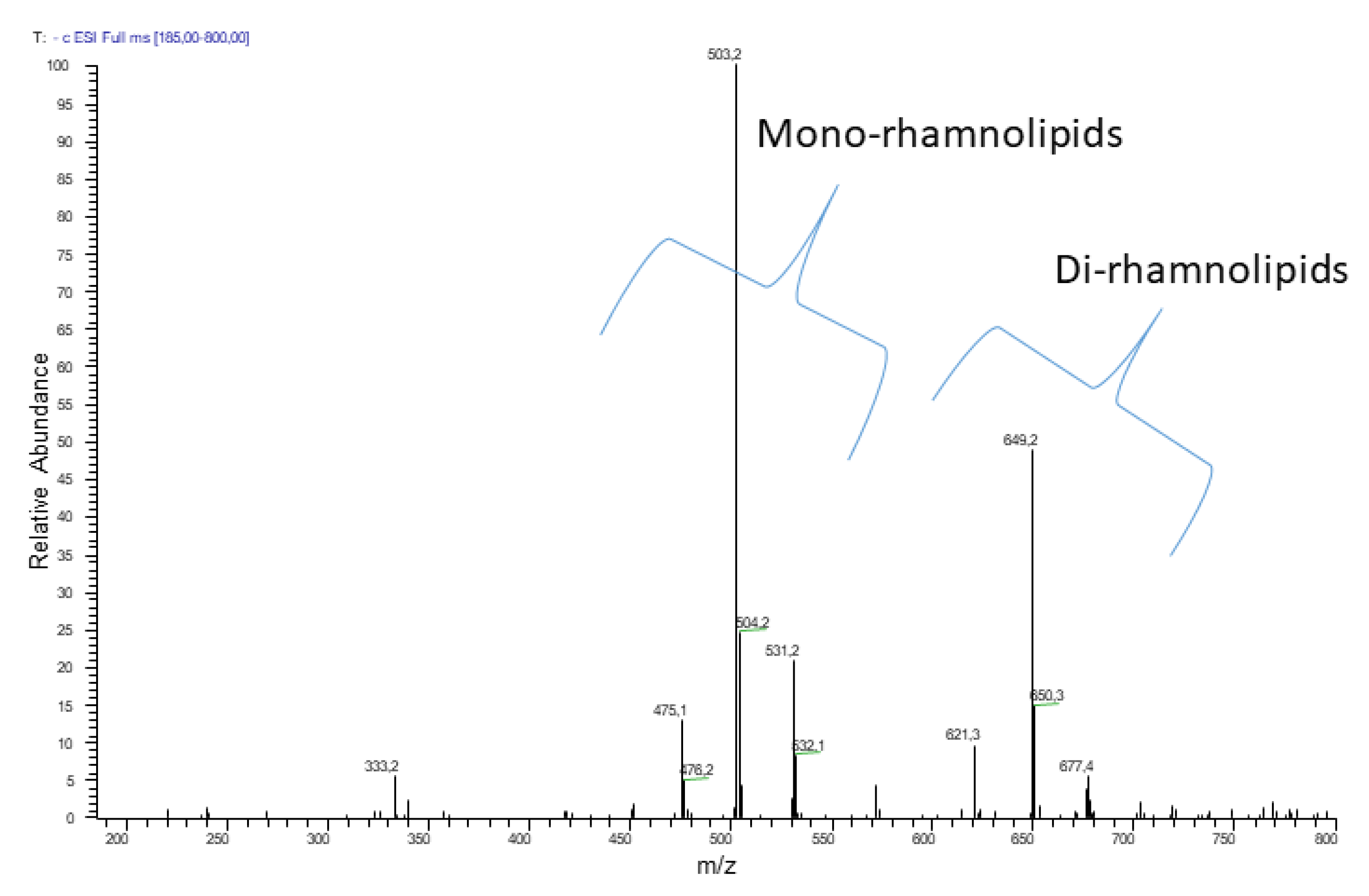
2.1. Chemical Characterization of R89BS
2.2. Antimicrobial Tests on Planktonic Cells
2.3. Disruption of Pre-Formed Biofilms
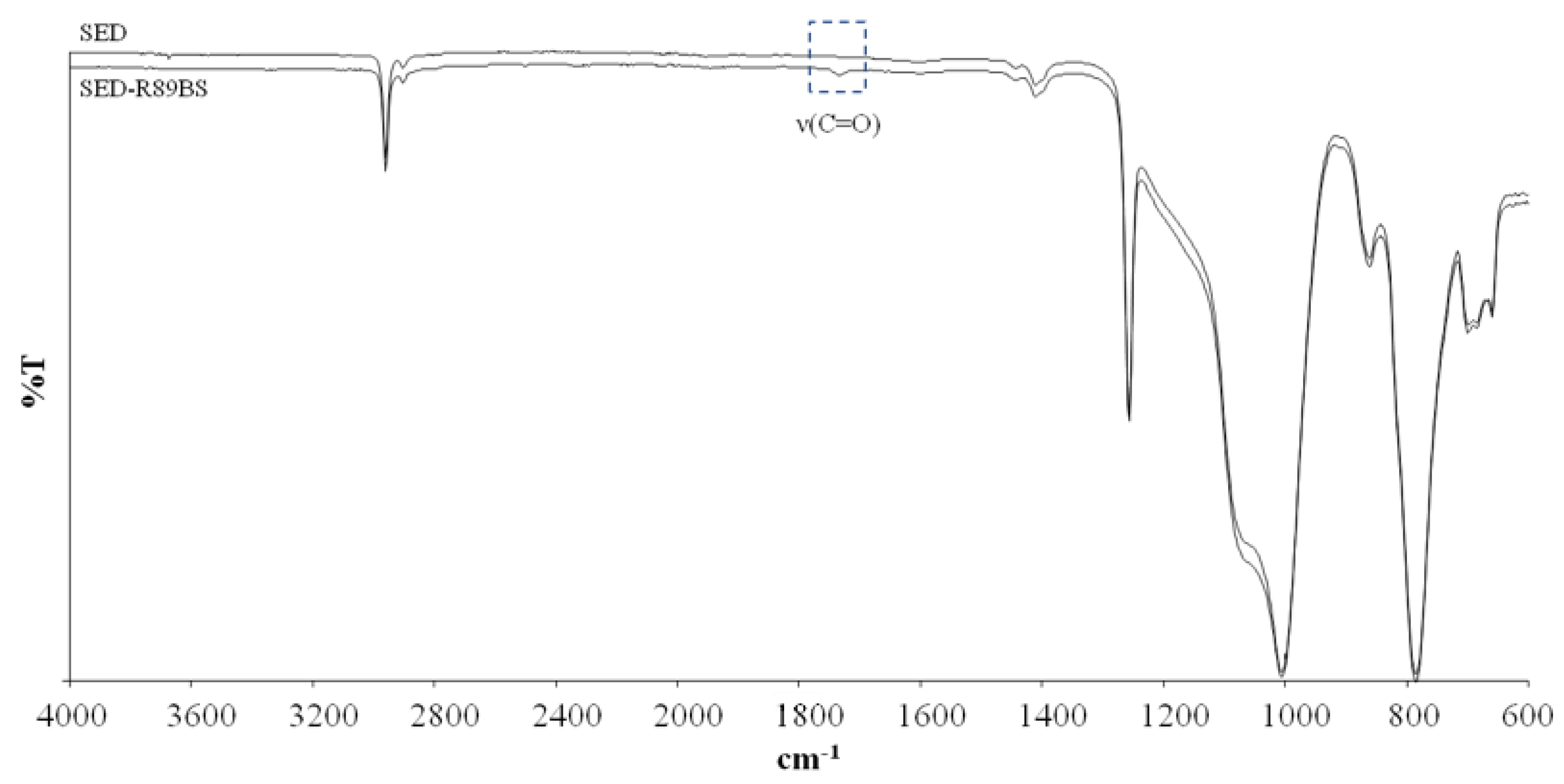
2.4. Surface Physicochemical Characterization
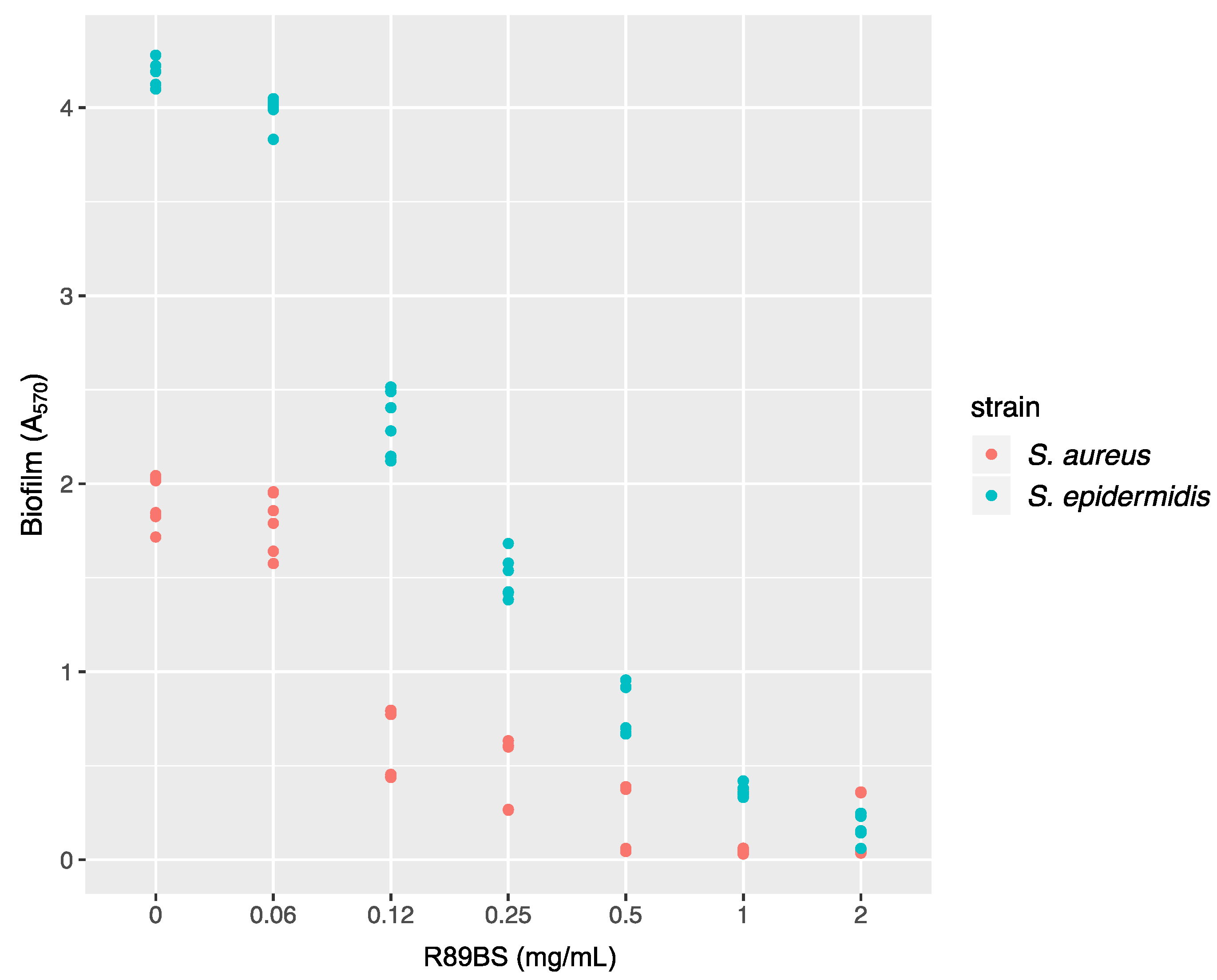
2.5. Anti-Biofilm Assay
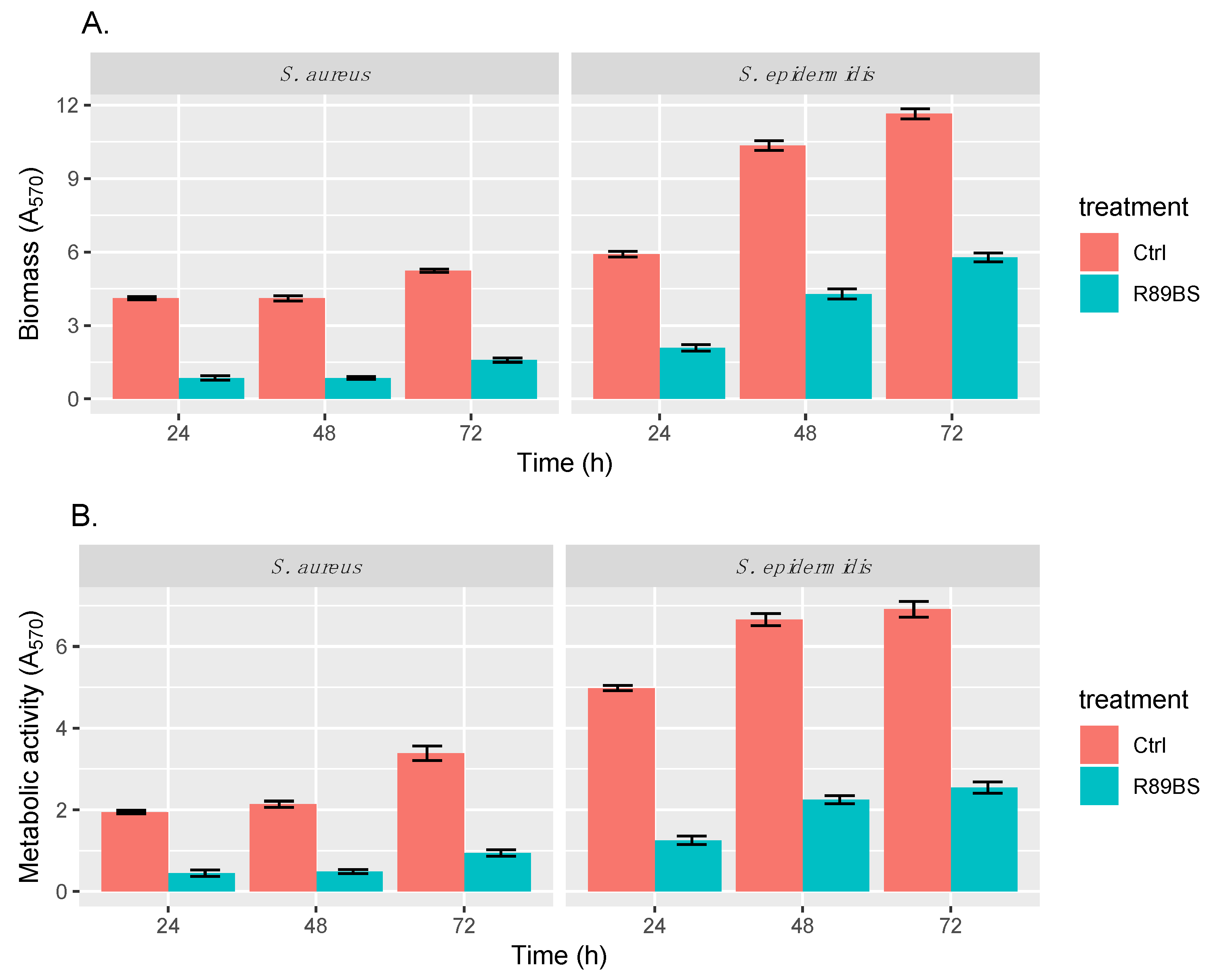
2.5.1. Biofilm Quantification
2.5.2. SEM Analysis of Bacterial Biofilms
2.6. Cytotoxicity Analysis
3. Discussion
4. Materials and Methods
4.1. Strains
4.2. Biosurfactant Production
4.3. Chemical Characterization of Biosurfactant R89
4.4. Antimicrobial Test on Planktonic Cells
4.5. Preparation of Medical-Grade Silicone Substrate
4.6. Disruption of Pre-Formed Biofilms
4.7. Silicone Surface Coating and Physicochemical Characterization
4.8. Anti-Biofilm Assays
4.8.1. Biofilm Quantification
4.8.2. Scanning Electron Microscopy Analysis of Bacterial Biofilms
4.9. R89BS and R89BS-Coated SED Cytotoxicity
4.10. Data Analysis and Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bryers, J.D. Medical biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Alam, A.; Rani, M.; Ehtesham, N.Z.; Hasnain, S.E. Biofilms: Survival and defense strategy for pathogens. Int. J. Med. Microbiol. 2017, 307, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Otto, M. Staphylococcal biofilms. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Vadyvaloo, V.; Otto, M. Molecular genetics of Staphylococcus epidermidis biofilms on indwelling medical devices. Int. J. Artif. Organs. 2005, 28, 1069–1078. [Google Scholar] [CrossRef]
- Sydnor, E.R.; Perl, T.M. Hospital epidemiology and infection control in acute-care settings. Clin Microbiol Rev. 2011, 24, 141–173. [Google Scholar] [CrossRef]
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of medical devices by staphylococci. Environ. Microbiol. 2018, 20, 3141–3153. [Google Scholar] [CrossRef] [Green Version]
- McCann, M.T.; Gilmore, B.F.; Gorman, S.P. Staphylococcus epidermidis device-related infections: Pathogenesis and clinical management. J. Pharm. Pharmacol. 2008, 60, 1551–1571. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef]
- John, T.; Rajpurkar, A.; Smith, G.; Fairfax, M.; Triest, J. Antibiotic pretreatment of hydrogel ureteral stent. J. Endourol. 2007, 21, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Abdelkefi, A.; Achour, W.; Ben, O.T.; Ladeb, S.; Torjman, L.; Lakhal, A.; Ben, H.A.; Hsairi, M.; Ben, A.A. Use of heparin-coated central venous lines to prevent catheter-related bloodstream infection. J. Support. Oncol. 2007, 5, 273–278. [Google Scholar] [PubMed]
- Darouiche, R.O. Prevention of infections associated with vascular catheters. Int. J. Artif. Organs. 2008, 31, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, M.; Elsner, J.J. Antibiotic-eluting medical devices for various applications. J. Control. Release 2008, 130, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Ghensi, P.; Bettio, E.; Maniglio, D.; Bonomi, E.; Piccoli, F.; Gross, S.; Caciagli, P.; Segata, N.; Nollo, G.; Tessarolo, F. Dental Implants with Anti-Biofilm Properties: A Pilot Study for Developing a New Sericin-Based Coating. Materials 2019, 12, 2429. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018, 4, e01067. [Google Scholar] [CrossRef] [Green Version]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and antimicrobial biomaterials: An overview. APMIS. 2017, 125, 392–417. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J. Recent Nanotechnology Approaches for Prevention and Treatment of Biofilm-Associated Infections on Medical Devices. Biomed. Res. Int. 2016, 2016, 1851242. [Google Scholar] [CrossRef]
- Wang, M.; Tang, T. Surface treatment strategies to combat implant-related infection from the beginning. J. Orthop. Translat. 2018, 17, 42–54. [Google Scholar] [CrossRef]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Vasilev, K.; Griesser, S.S.; Griesser, H.J. Antibacterial surfaces and coatings produced by plasma techniques. Plasma Process. Polym. 2011, 8, 1010–1023. [Google Scholar] [CrossRef]
- Fracchia, L.; Banat, J.J.; Cavallo, M.; Ceresa, C.; Banat, I.M. Potential therapeutic applications of microbial surface-active compounds. AIMS Bioeng. 2015, 2, 144–162. [Google Scholar] [CrossRef]
- Banat, I.M.; De Rienzo, M.A.; Quinn, G.A. Microbial biofilms: Biosurfactants as antibiofilm agents. Appl. Microbiol Biotechnol. 2014, 98, 9915–9929. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.K.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, T.; Rautenbach, M.; Vosloo, J.A.; Khan, S.; Khan, W. Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express. 2017, 7, 108. [Google Scholar] [CrossRef]
- Lotfabad, T.; Shahcheraghi, F.; Shooraj, F. Assessment of antibacterial capability of rhamnolipids produced by two indigenous Pseudomonas aeruginosa strains. Jundishapur J. Microbiol. 2012, 6, 29–35. [Google Scholar] [CrossRef]
- Rodrigues, L.R.; Banat, I.M.; van der Mei, H.C.; Teixeira, J.A.; Oliveira, R. Interference in adhesion of bacteria and yeasts isolated from explanted voice prostheses to silicone rubber by rhamnolipid biosurfactants. J. Appl. Microbiol. 2006, 100, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Perfumo, A.; Banat, I.M.; Canganella, F.; Marchant, R. Rhamnolipid production by a novel thermotolerant hydrocarbon-degrading Pseudomonas aeruginosa AP02-1. J. Appl. Microbiol. 2006, 75, 132–138. [Google Scholar]
- Raza, Z.A.; Khalid, Z.M.; Banat, I.M. Characterization of rhamnolipids produced by a Pseudomonas aeruginosa mutant strain grown on waste oils. J. Environ. Sci. Health. A Tox. Hazard. Subst. Environ. Eng. 2009, 44, 1367–1373. [Google Scholar] [CrossRef]
- Sotirova, A.V.; Spasova, D.I.; Galabova, D.N.; Karpenko, E.; Shulga, A. Rhamnolipid-biosurfactant permeabilizing effects on gram-positive and gram-negative bacterial strains. Curr. Microbiol. 2008, 56, 639–644. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Carmagnola, I.; Chiono, V.; Gentile, P.; Fracchia, L.; Ceresa, C.; Georgiev, G.; Ciardelli, G. Surface modification of poly(dimethylsiloxane) by two-step plasma treatment for further grafting with chitosan–Rose Bengal photosensitizer. Surf. Coat. Tech. 2013, 223, 92–97. [Google Scholar] [CrossRef]
- VanEpps, J.S.; Younger, J.G. Implantable Device-Related Infection. Shock. 2016, 46, 597–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, M. Staphylococcal infections: Mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Fracchia, L.; Ceresa, C.; Banat, I.M. Biosurfactants in cosmetic, biomedical and pharmaceutical industry. In Microbial Biosurfactants and their Environmental and Industrial Applications; Banat, I.M., Rengathavasi, T., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 258–288. [Google Scholar]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef]
- Fracchia, L.; Ceresa, C.; Franzetti, A.; Cavallo, M.; Gandolfi, I.; Van Hamme, J.D.; Gkorezis, P.; Marchant, R.; Banat, I.M. Industrial applications of biosurfactants. In Biosurfactant-Production and Utilization-Processes, Technologies, and Economics; Kosaric, N., Sukan, F.V., Eds.; CRS Press-Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 245–267. [Google Scholar]
- Ortiz, A.; Teruel, J.A.; Espuny, M.J.; Marqués, A.; Manresa, A.; Aranda, F.J. Interactions of a bacterial biosurfactant trehalose lipid with phosphatidylserine membranes. Chem. Phys. Lipids. 2009, 158, 46–53. [Google Scholar] [CrossRef]
- Zaragoza, A.; Aranda, F.J.; Espuny, M.J.; Teruel, J.A.; Marqués, A.; Manresa, A.; Ortiz, A. Mechanism of membrane permeabilization by a bacterial trehalose lipid biosurfactant produced by Rhodococcus sp. Langmuir. 2009, 25, 7892–7898. [Google Scholar] [CrossRef]
- Sánchez, M.; Aranda, F.J.; Teruel, J.A.; Espuny, M.J.; Marqués, A.; Manresa, A.; Ortiz, A. Permeabilization of biological and artificial membranes by a bacterial dirhamnolipid produced by Pseudomonas aeruginosa. J. Colloid Interface Sci. 2010, 341, 240–247. [Google Scholar] [CrossRef]
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Strategies for the prevention of microbial biofilm formation on silicone rubber voice prostheses. J. Biomed. Mater. Res. B. Appl. Biomater. 2007, 81B, 358–370. [Google Scholar] [CrossRef]
- Quinn, G.A.; Maloy, A.P.; Banat, M.M.; Banat, I.M. A comparison of effects of broad-spectrum antibiotics and biosurfactants on established bacterial biofilms. Curr. Microbiol. 2013, 67, 614–623. [Google Scholar] [CrossRef]
- Cameotra, S.S.; Makkar, R.S.; Kaur, J.; Mehta, S.K. Synthesis of biosurfactants and their advantages to microorganisms and mankind. Adv. Exp. Med. Biol. 2010, 672, 261–280. [Google Scholar]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Sekhon Randhawa, K.K.; Rahman, P.K. Rhamnolipid biosurfactants-past, present, and future scenario of global market. Front. Microbiol. 2014, 5, 454. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.; Li, Q. Microbial production of rhamnolipids: Opportunities, challenges and strategies. Microb. Cell Fact. 2017, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Rivardo, F.; Martinotti, M.G.; Turner, R.J.; Ceri, H. Synergistic effect of lipopeptide biosurfactant with antibiotics against Escherichia coli CFT073 biofilm. Int. J. Antimicrob. Agents. 2011, 37, 324–331. [Google Scholar] [CrossRef]
- Joshi-Navare, K.; Prabhune, A. A biosurfactant-sophorolipid acts in synergy with antibiotics to enhance their efficiency. Biomed. Res. Int. 2013, 2013, 512495. [Google Scholar] [CrossRef]
- Ceresa, C.; Rinaldi, M.; Fracchia, L. Synergistic activity of antifungal drugs and lipopeptide AC7 against Candida albicans biofilm on silicone. AIMS Bioeng. 2017, 4, 318–334. [Google Scholar] [CrossRef]
- Hage-Hülsmann, J.; Grünberger, A.; Thies, S.; Santiago-Schübel, B.; Klein, A.S.; Pietruszka, J.; Binder, D.; Hilgers, F.; Domröse, A.; Drepper, T.; et al. Natural biocide cocktails: Combinatorial antibiotic effects of prodigiosin and biosurfactants. PLoS ONE. 2018, 13, e0200940. [Google Scholar] [CrossRef]
- Aleksic, I.; Petkovic, M.; Jovanovic, M.; Milivojevic, D.; Vasiljevic, B.; Nikodinovic-Runic, J.; Senerovic, L. Anti-biofilm Properties of Bacterial Di-Rhamnolipids and Their Semi-Synthetic Amide Derivatives. Front. Microbiol. 2017, 8, 2454. [Google Scholar] [CrossRef]
- Nitschke, M.; Araújo, L.V.; Costa, S.G.; Pires, R.C.; Zeraik, A.E.; Fernandes, A.C.; Freire, D.M.; Contiero, J. Surfactin reduces the adhesion of food-borne pathogenic bacteria to solid surfaces. Lett. Appl. Microbiol. 2009, 49, 241–247. [Google Scholar] [CrossRef]
- Meylheuc, T.; van Oss, C.J.; Bellon-Fontaine, M.N. Adsorption of biosurfactant on solid surfaces and consequences regarding the bioadhesion of Listeria monocytogenes LO28. J. Appl. Microbiol. 2001, 91, 822–832. [Google Scholar] [CrossRef]
- Zeraik, A.E.; Nitschke, M. Biosurfactants as agents to reduce adhesion of pathogenic bacteria to polystyrene surfaces: Effect of temperature and hydrophobicity. Curr. Microbiol. 2010, 61, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Saharan, B.S. Functional characterization of biomedical potential of biosurfactant produced by Lactobacillus helveticus. Biotechnol. Rep. 2016, 11, 27–35. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, L.V.; Guimarães, C.R.; da Silva Marquita, R.L.; Santiago, V.M.; de Souza, M.P.; Nitschke, M.; Freire, D.M.G. Rhamnolipid and surfactin: Anti-adhesion/antibiofilm and antimicrobial effects. Food Control. 2016, 63, 171–178. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Ceresa, C.; Rinaldi, M.; Chiono, V.; Carmagnola, I.; Allegrone, G.; Fracchia, L. Lipopeptides from Bacillus subtilis AC7 inhibit adhesion and biofilm formation of Candida albicans on silicone. Antonie Van Leeuwenhoek. 2016, 109, 1375–1388. [Google Scholar] [CrossRef]
- Ceresa, C.; Tessarolo, F.; Caola, I.; Nollo, G.; Cavallo, M.; Rinaldi, M.; Fracchia, L. Inhibition of Candida albicans adhesion on medical-grade silicone by a Lactobacillus-derived biosurfactant. J. Appl. Microbiol. 2015, 118, 1116–1125. [Google Scholar] [CrossRef]
- Bressan, E.; Tessarolo, F.; Sbricoli, L.; Caola, I.; Nollo, G.; Di Fiore, A. Effect of chlorhexidine in preventing plaque biofilm on healing abutment: A crossover controlled study. Implant. Dent. 2014, 23, 64–68. [Google Scholar] [CrossRef]
- Hrubanova, K.; Krzyzanek, V.; Nebesarova, J.; Ruzicka, F.; Pilat, Z.; Samek, O. Monitoring Candida parapsilosis and Staphylococcus epidermidis Biofilms by a Combination of Scanning Electron Microscopy and Raman Spectroscopy. Sensors 2018, 18. [Google Scholar] [CrossRef]
- Ceresa, C.; Tessarolo, F.; Maniglio, D.; Caola, I.; Nollo, G.; Rinaldi, M.; Fracchia, L. Inhibition of Candida albicans biofilm by lipopeptide AC7 coated medical-grade silicone in combination with farnesol. AIMS Bioeng. 2018, 5, 192–208. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Test Strain | R89BS Concentration (mg/mL) | OD at 595 nm (n = 6) (mean ± SD) |
|---|---|---|
| S. aureus ATCC® 6538TM | Ctrl | 0.847 ± 0.013 |
| 0.03 | 0.772 ± 0.017 | |
| 0.06 | 0.004 ± 0.006 | |
| 0.12 | 0.001 ± 0.006 | |
| 0.25 | 0.001 ± 0.006 | |
| S. epidermidis ATCC® 35984TM | Ctrl | 0.918 ± 0.039 |
| 0.03 | 0.768 ± 0.065 | |
| 0.06 | 0.630 ± 0.062 | |
| 0.12 | 0.001 ± 0.004 | |
| 0.25 | 0.001 ± 0.007 |
| Time (h) | Strain | Total Biomass | Cell Metabolic Activity | ||
|---|---|---|---|---|---|
| 95% Confidence Interval for the Ratio Ctrl/R89BS | p-Value (t-Test) | 95% Confidence Interval for the Ratio Ctrl/R89BS | p-Value (t-Test) | ||
| 24 | S. aureus | (4.33, 5.35) | 1.50 × 10−7 | (3.65, 5.35) | 4.77 × 10−6 |
| S. epidermidis | (2.65, 3.04) | 2.22 × 10−8 | (3.65, 4.36) | 1.09 × 10−7 | |
| 48 | S. aureus | (4.50, 5.10) | 2.28 × 10−10 | (3.95, 4.89) | 3.27 × 10−8 |
| S. epidermidis | (2.30, 2.54) | 3.63 × 10−9 | (2.83, 3.10) | 4.30 × 10−11 | |
| 72 | S. aureus | (3.13, 3.49) | 6.36 × 10−9 | (3.29, 3.95) | 4.22 × 10−10 |
| S. epidermidis | (1.95, 2.08) | 6.43 × 10−11 | (2.56, 2.88) | 4.08 × 10−10 | |
| Time (h) | Strain | Biomass (CV) | Metabolic Activity (MTT) | Surface Coverage (SEM) |
|---|---|---|---|---|
| 24 | S. aureus | 79.2% | 77.1% | 69.9% |
| S. epidermidis | 64.6% | 74.9% | 40.3% | |
| 48 | S. aureus | 79.1% | 77.2% | 81.4% |
| S. epidermidis | 58.4% | 66.2% | 51.1% | |
| 72 | S. aureus | 69.6% | 72.3% | 78.0% |
| S. epidermidis | 50.2% | 63.1% | 52.8% |
| Time (h) | S. aureus | S. epidermidis | ||
|---|---|---|---|---|
| Control SEDs | R89BS-Coated SEDs | Control SEDs | R89BS-Coated SEDs | |
| 24 h | 3.06 ± 0.05 | 9.09 ± 0.19 | 18.74 ± 1.88 | 31.24 ± 4.05 |
| 48 h | 7.73 ± 0.63 | 9.79 ± 0.62 | 37.90 ± 5.12 | 45.73 ± 3.38 |
| 72 h | 8.47 ± 1.20 | 11.42 ± 1.58 | 51.09 ± 5.46 | 60.94 ± 2.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceresa, C.; Tessarolo, F.; Maniglio, D.; Tambone, E.; Carmagnola, I.; Fedeli, E.; Caola, I.; Nollo, G.; Chiono, V.; Allegrone, G.; et al. Medical-Grade Silicone Coated with Rhamnolipid R89 Is Effective against Staphylococcus spp. Biofilms. Molecules 2019, 24, 3843. https://doi.org/10.3390/molecules24213843
Ceresa C, Tessarolo F, Maniglio D, Tambone E, Carmagnola I, Fedeli E, Caola I, Nollo G, Chiono V, Allegrone G, et al. Medical-Grade Silicone Coated with Rhamnolipid R89 Is Effective against Staphylococcus spp. Biofilms. Molecules. 2019; 24(21):3843. https://doi.org/10.3390/molecules24213843
Chicago/Turabian StyleCeresa, Chiara, Francesco Tessarolo, Devid Maniglio, Erica Tambone, Irene Carmagnola, Emanuele Fedeli, Iole Caola, Giandomenico Nollo, Valeria Chiono, Gianna Allegrone, and et al. 2019. "Medical-Grade Silicone Coated with Rhamnolipid R89 Is Effective against Staphylococcus spp. Biofilms" Molecules 24, no. 21: 3843. https://doi.org/10.3390/molecules24213843
APA StyleCeresa, C., Tessarolo, F., Maniglio, D., Tambone, E., Carmagnola, I., Fedeli, E., Caola, I., Nollo, G., Chiono, V., Allegrone, G., Rinaldi, M., & Fracchia, L. (2019). Medical-Grade Silicone Coated with Rhamnolipid R89 Is Effective against Staphylococcus spp. Biofilms. Molecules, 24(21), 3843. https://doi.org/10.3390/molecules24213843







