Abstract
Chitin, an insoluble linear polymer of β-1,4-N-acetyl-d-glucosamine (GlcNAc; A), can be converted to chitosan, a soluble heteropolymer of GlcNAc and d-glucosamine (GlcN; D) residues, by partial deacetylation. In nature, deacetylation of chitin is catalyzed by enzymes called chitin deacetylases (CDA) and it has been proposed that CDAs could be used to produce chitosan. In this work, we show that CDAs can remove up to approximately 10% of N-acetyl groups from two different (α and β) chitin nanofibers, but cannot produce chitosan.
1. Introduction
Chitin, the second most abundant biopolymer in nature, is a crystalline linear polysaccharide containing β-1,4 linked N-acetyl glucosamine (GlcNAc, A) residues. It is a common structural component in cell walls of yeast and fungi and in the exoskeletons of insects, crustaceans, and parasitic nematodes [1,2]. Alpha-chitin is the most common chitin form. It contains GlcNAc chains in an antiparallel orientation [3], and it is found in insects, crustaceans, fungi, and yeast. Beta-chitin is derived from squid pens and exists in parallel GlcNAc chains [1,4]. In γ-chitin, the rarest crystalline form of chitin found in insects and in the stomach of the Loligo squid, two GlcNAc chains are oriented in one direction and the third runs in the opposite direction [3,5]. The individual chitin chains are held together by hydrogen bonds [3].
Partial deacetylation of chitin yields chitosan, a heteropolymer of (1-4)-linked (GlcNAc) and glucosamine (GlcN, D) units with the GlcN units randomly distributed along the polymer chain [6]. The name chitosan refers to a continuum of soluble polymeric chitin derivatives that can be described and classified according to several characteristics where the degree of polymerization (DP) and the fraction of N-acetylated residues (FA) are the most important. In weak acidic media, the GlcN units are charged, making chitosan, unlike chitin, water-soluble [1,7]. Solubility in weak aqueous media requires the FA to be below approximately 0.65 [8]. Due to its non-toxicity, biodegradability, and biocompatibility, combined with interesting biological and physicochemical properties, chitosan has various applications in agriculture, industry, medicine, water treatment, and biotechnology [1,7]. The most common method for conversion of chitin to chitosan is usually done by demineralization and deproteination of chitin-rich biomass at high temperature using aqueous NaOH as a base [9]. This is a relatively uncontrolled process that generates basic wastewater that may lead to environmental pollution [10].
In nature, enzymes called chitin deacetylases (CDAs) that belong to the family 4 of carbohydrate esterases (CE4), according to the CAZy classification system (www.cazy.org) [11], catalyze the removal of N-acetyl groups from chitin. This esterase family comprises enzymes that de-N- or de-O-acetylate chitin, acetyl xylan and peptidoglycan. Ever since the discovery of the characterization of these enzymes, it has been speculated that CDAs could be used to convert chitin into chitosan, in what would be an environmentally-friendly and potentially cost-efficient approach. While seemingly attractive, from a theoretical point of view, it may not seem very likely that CDAs would be able to solubilize chitin (further discussed below). In this study, we have investigated the possibility of several CDAs to solubilize chitin, focusing on a chitin deacetylase from Vibrio cholerae (VcCDA) as a deacetylation catalyst [12]. As a substrate, we assessed both α- and β-chitin nanofibers with a high surface to volume ratio, which presumably would make these fibers particularly suited for enzymatic deacetylation.
2. Results and Discussion
Initially, three enzymes with known chitin-deacetylating activity, VcCDA from Vibrio cholera [12,13], AnCDA9 from Aspergillus nidulans [14], and SpPgdA from Streptococcus pneumoniae [15] were assessed for their ability to deacetylate chitin nanofibers using the methods described below. Incubation with chitin nanofibers for 48 h displayed an ability of the deacetylases to remove 3–6 % of the N-acetyl groups (results not shown). For further investigation, we chose to focus on VcCDA since this appeared to most efficient of the three enzymes.
Prior to in-depth studies with VcCDA, untreated α- and β-chitin nanofibers were subjected to 1H NMR to determine the FA of the starting materials according to Einbu and Vårum (Figure 1) [16]. The FA was calculated using Equation (1):
where IαH1A represent the integral of the H-1 protons of the α-anomer at the reducing end of GlcNAc residues, IβH1A+H1D represents the integral of the H-1 protons of the β-anomer at the reducing end of GlcNAc and GlcN residues, respectively, IH1A represents the integral of H-1 protons of GlcNAc residues within the polymer chain, and IH2D represents the integral of H-2 protons of GlcN residues with the polymer chain [16]. By subtracting the integral from H-2 from GlcN units (IH2D) from the integral representing all H-1 protons (IαH1A + IβH1A+H1D + IH1A), the integral representing only acetylated unit is obtained. Thus, Equation (1) implies that the FA is determined by dividing the integral of only acetylated units by the integral of all H-1 protons [16]. As expected for polymeric chitin, no significant peak was observed for the α-conformation at the C1. This approach yielded FA values of 0.950 and 0.895 for α- and β-chitin, respectively (Figure 1). This implies that the chitin nanofibers contain an inherent small fraction of deacetylated units, in accordance with previous observations [16]. The fraction of acetylated units (FA) in both α- and β-chitin nanofibers after incubation with VcCDA at 37 °C was determined at different time points (Figure 2). After 10 days, the FA values reached minima of 0.85, for α-chitin, and 0.83 for β-chitin as observed as a stopped decline in FA values with respect to time (depicted in Figure 2).
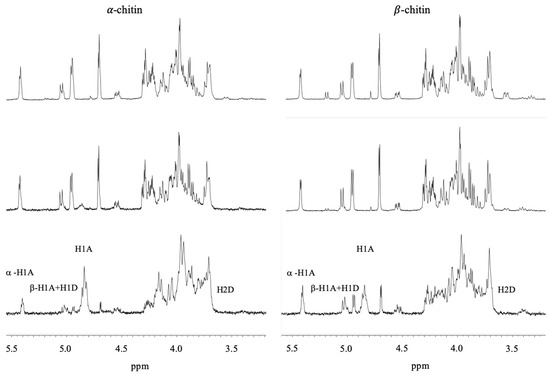
Figure 1.
1H NMR spectrum of untreated α-chitin (bottom left, FA = 0.950) and β-chitin (bottom right, FA = 0.895) nanofibers before addition of VcCDA and after 24 h (α-chitin middle left, FA = 0.945) and (β-chitin middle right, FA = 0.873), and 48 h (α-chitin middle left, FA = 0.898) and (β-chitin middle right, FA = 0.852) of incubation with VcCDA. The peaks of βH1 and H1D come at ppm of 5.05 and 5.07, respectively, H1A comes at ppm 4.91, and H2D comes at ppm 3.44 [16].
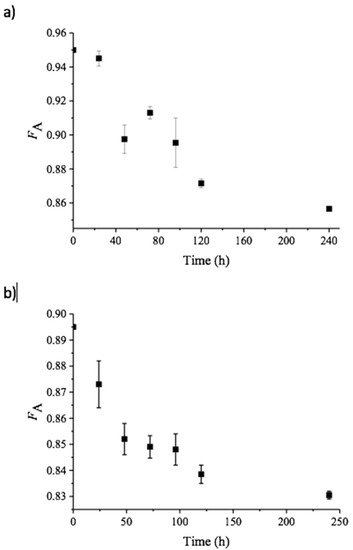
Figure 2.
Change in FA over time upon incubation of α-chitin (a) or β-chitin (b) with VcCDA at pH 8.0 and t = 37 °C, as determined by 1H NMR spectroscopy. The points show average values derived from three independent experiments with standard deviations. Note that starting FA values were 0.950 and 0.895 for α- and β-chitin, respectively.
To ensure that the decrease in the rate of deacetylation was not due to the inactivation of VcCDA. A new batch of the enzyme was added after 240 h, thus doubling the total amount of added enzyme from 0.5 µM to 1.0 µM and incubated for another 24 h. This did not lead to a significant further increase in deacetylation (FA of 0.857 vs. 0.854 for α-chitin and 0.831 vs. 0.824 for β-chitin, respectively), indicating that essentially no more acetyl groups are available as a substrate for VcCDA.
In another control experiment, soluble N,N-diacetyl chitobiose (to a final concentration of 1 mM) was added to the reaction mixtures after 120 h of incubation with the chitin substrates and was allowed to incubate for 24 h with the chitobiose substrate. VcCDA catalyzes the hydrolysis of the N-acetyl groups attached to the penultimate GlcNAc residue from the non-reducing end [13], and mass spectrometry analysis showed that 24 h after its addition, all chitobiose had lost an N-acetyl group (Figure S1). It is thus clear that the stagnation in the deacetylation is not due to enzyme deactivation. The binding of VcCDA to β-chitin nanofibers was also assessed to see if the low degree of maximum deacetylation could be due to strong, perhaps unproductive binding of VcCDA to the substrate, which could make the enzyme incapable of performing catalysis. The plot of bound protein versus free protein was fitted to Equation (2) yielding a Kd of 5 ± 2 µM and a Bmax of 8.4 μM, in the fit, which corresponds to 0.1 μmol/gram chitin fiber (Figure 3).
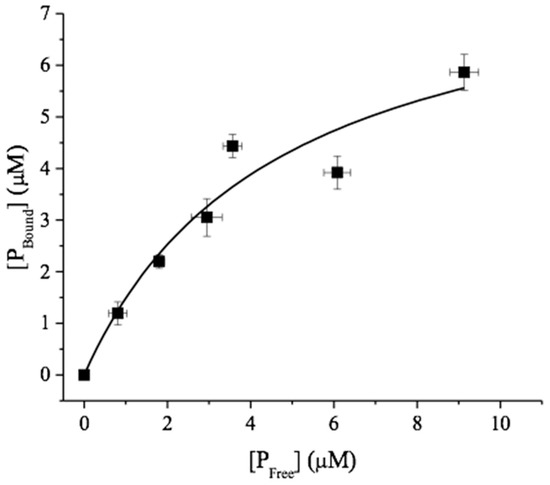
Figure 3.
Equilibrium isotherm for the adsorption of VcCDA to β-chitin nanofibers at pH 8.0 and t = 37 °C.
3. Materials and Methods
3.1. Materials
Both α- and β-chitin nanofibers, derived from crab shells, were kindly donated by S. Ifuku, Department of Chemistry and Biotechnology, Tottori University Japan (Tottori City, Japan) [17,18]. Dialyzed chitin nanofibers DH2O were lyophilized using a Christ Alpha 2-4 LD plus freeze dryer. N,N-Diacetyl chitobiose was purchased from Megazyme (Wicklow, Ireland). All further chemicals and solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification.
3.2. Production of AnCDA9, SpPgdA, and VcCDA
AnCDA9 from Aspergillus nidulans was produced in Escherichia coli and purified as described previously [14]. SpPgdA from Streptococcus pneumoniae in E. coli and purified as described previously [15].
The gene encoding VcCDA (accession code AAF94439) was ordered from Gen-Script (Piscataway, NJ, USA). The gene, without the nucleotides encoding the signal peptide, was amplified by PCR using a forward (5′-TTAAGAAGGAGATATACTATGAATAGCACCCCGAAAGGCA C-3′) and reverse (5′-AATGGTGGTGATGATGGTGCGCCAGCGCGGTGAACAGGGTA-3′) primer (Eurofins, Ebersberg, Germany). The underlined nucleotides represent over-hang sequences. The amplified PCR product was subsequently cloned into the pNIC-CH vector [19] utilizing ligation-independent cloning (LIC) [20]. As a result of this cloning strategy, the N-terminus of the (signal peptide-free) protein was extended with methionine on the N-terminus, while a seven residue His-tag (AHHHHHH) was added at the C-terminus. The pNIC-CH vector containing the VcCDA gene sequence was then transformed into chemically competent XL1-Blue cells (Agilent, CA, USA) by heat shock. The host strain was allowed to proliferate in Super Optimal broth with catabolite repression (SOC) for 60 min prior to plating on lysogenic broth (LB) agar containing 50 µg/mL kanamycin and 5% sucrose. After incubation overnight at 37 °C, single transformant colonies were inoculated in liquid LB containing 50 µg/mL kanamycin and incubated overnight at 37 °C. The plasmid was isolated from transformants using a NucleoSpin Plasmid kit (Macherey-Nagel), and the gene sequence of VcCDA was verified by Sanger sequencing (GATC, Konstanz, Germany). The isolated plasmid was subsequently transformed by heat shock into chemically competent OneShot BL-21 StarTM (DE3) E. coli cells (Invitrogen) and grown in SOC media as described above, before plating on LB agar containing 50 µg/mL kanamycin and overnight incubation at 37 °C. A transformant colony was inoculated in 5 mL LB (50 µg/mL kanamycin) and incubated at 37 °C overnight. The pre-culture was then grown in 0.5 L Terrific Brothcontaining 50 µg/mL kanamycin using a Harbinger system (Harbinger Biotechnology and Engineering, Markham, Canada) at 21 °C. After 24 h, the culture was induced with isopropyl-β-d-thiogalactopyranoside (final concentration 0.2 mM), followed by incubation at 21 °C for 24 h. The cells were harvested by centrifugation (8000 rpm, 20 min at 4 °C) and the pellet resuspended in 30 mL 20 mM Tris-HCl pH 8, 20 mM imidazole, 500 mM NaCl before the cells were lysed using a Vibra-cell sonicator (Sonics and Materials Inc., Newtown, CT, USA) with 5 s on/off pulses for 4 min at 28% amplitude while kept on ice. The cell debris was removed by centrifugation at 12,000 rpm for 15 min and the cell-free protein extract was filtrated using a 0.45 µm syringe filter (Sarstedt, Nümbrecht, Germany). Proteins were purified from the resulting cell-free extract using a column packed with Ni-NTA Agarose (Qiagen, Venlo, The Netherlands, 1.5 cm in diameter, 5 mL stationary phase in total). The column was pre-equilibrated in a buffer containing 20 mM Tris-HCl, 20 mM imidazole, and 500 mM NaCl, pH 8.0, before the cell extracts were applied. After washing with a buffer containing 20 mM Tris-HCl and 500 mM NaCl, pH 8.0, the enzyme was eluted with a buffer containing 20 mM Tris-HCl, 250 mM imidazole, and 500 mM NaCl, pH 8.0. A flow rate of 2.5 mL/min was used at all times. Enzyme purity of all CDAs was verified by SDS-PAGE (Supplementary Figure S1) and fractions containing purified enzymes were concentrated and transferred to 20 mM potassium phosphate buffer pH 6.0 by centrifugal ultrafiltration (Macrosep Advance Centrifugal Device, 10 kDa cutoff, Pall Corporation, Port Washington, NY, USA). Protein concentration was determined by using the Bradford Protein Assay from Bio-Rad (Hercules, CA, USA).
After purification, the enzymes were checked to have similar activity as reported values [13,14,15] by initial acetate release from N,N-diacetyl chitobiose (1 mM) measured by IC using a Dionex ICS3000 system with suppressed conductivity detection as described by Liu et al. [14].
3.3. Enzymatic Deacetylation of Chitin Nanofibers
Both α- and β-chitin nanofibers (5 mg/mL) were incubated with CDA (0.5 µM) in Tris-HCl (25 mM, pH 8.0) and CoCl2 (0.1 mM) at 37 °C, with shaking at 800 rpm. Samples (13 mL) were taken at different time points and enzyme activity was immediately quenched by adding an equal volume of acetonitrile. After removal of the organic solvent under reduced pressure, the materials were dialyzed to H2O, using Spectra/Por 6 dialysis membranes with a cutoff of 100 Da, followed by lyophilization using a Christ Alpha 2–4 LD plus freeze dryer, which rendered the chitin fibers colorless solids. Three parallels of each sample were prepared for 1H NMR analysis.
3.4. 1H NMR of α- and β-Chitin Nanofibers
1H NMR sample preparation was conducted as described by Einbu and Vårum [16]. The lyophilized chitin nanofiber samples were first wetted with 100 µL 1% DCl in D2O (99.9 atom % D, contains 0.05 wt% TMS as internal standard) followed by dissolving in 700 µL 37% DCl. 1H NMR spectra were recorded at 25 °C with 64 scans using a Bruker AscendTM 400 MHz spectrometer. The chemical shifts are reported in parts per million (ppm) relative to TMS.
The fraction of acetylated units (FA) was calculated according to Einbu and Vårum [16] using the integrals of H1- and H2 protons of GlcNAc and GlcN residues as described in the text.
3.5. Enzyme Activity Test
Remaining chitin deacetylase activity after five days of incubation with chitin nanofibers was measured by adding of N,N-diacetyl chitobiose to the reaction mixture to a final concentration of 1 mM. After 24 h, deacetylation products were analyzed by MALDI-TOF, as described by Cederkvist et al. [21].
3.6. Binding Assay
Enzyme binding to β-chitin nanofibers was assessed as described by Zakariassen et al. [22]. VcCDA was diluted to various concentrations (0–20 µM) in Tris-HCl (25 mM, pH 8.0) and 0.1 mM CoCl2. The A280 of these solutions was measured, and a standard curve was created. The reaction volume was 1.0 mL, the concentration of β-chitin WAS 5 mg/mL, and the VcCDA concentration in the mixtures was 0, 2, 4, 6, 8, 10, and16 µM, respectively. The mixtures were incubated at 37 °C and 300 rpm. After 2 h, the β-chitin nanofibers were spun down for 20 min at 13,000 rpm, and the A280 values of the supernatants were measured. The bound and free protein concentrations ([Pbound], [Pfree]) were calculated from the standard curve. All assays were performed in triplicate. The equilibrium dissociation constant Kd (µM) and binding capacity Bmax (µmol/g) were determined by fitting the binding isotherms to the following equation: [Pbound] = Bmax × [Pfree]/[Kd + Pfree] by non-linear regression using Origin v7.0 software (OriginLab Corporation, Northampton, MA, USA).
4. Conclusions
Polymers of chitin are synthesized as crystalline fibrils that are hundreds to thousands of monomer units long [23]. Calculations show that the removal of a GlcNAc dimer from chitin crystal comes with a thermodynamic penalty of 8 kcal/mol [24]. Our results demonstrate that a CDA is able to reduce the degree of acetylation for two different types of crystalline chitin (α and β), but only by up to approximately 10%. This prompts us to speculate that the enzymatic action of a CDA is not able to overcome the thermodynamic penalty of removing single polymer chains from the crystal to access all available N-acetyl groups. In nature, chitin is degraded by glycoside hydrolases (GH) and lytic polysaccharide monooxygenases (LPMO) [25,26]. GHs overcome the thermodynamic penalty of decrystallization by strong binding to a single polysaccharide chain through several surface-exposed aromatic amino acids and by this pulling the chain from the crystal into the active site of the enzymes [27,28]. Moreover, the binding affinity of GHs towards the substrate increases with an increasing chain length of the substrate [29,30]. Typical binding free energy of a chitin active GH on six GlcNAc residues is −9 kcal/mol (Kd = 0.2 μM) [28]. LPMOs achieve decrystallization by activating an oxygen species on its copper-active site that is calculated to be strong enough, oxidize a C-H bond of 110 kcal/mol [31]. In contrast, we observe that VcCDA binding free energy to β-chitin of −7.5 kcal/mol (Kd = 5 μM). Such a relative weak binding affinity is also confirmed from obtained Michaelis–Menten constants from various kinetic analyses. SpPgdA is reported to have a Km of 3.8 mM, which translates to a binding free energy of −3.4 kcal/mol, with (GlcNAc)3 as the substrate, while AnCDA9 has a Km of 72 μM, which translates to a binding free energy of −5.9 kcal/mol, with (GlcNAc)5 as the substrate [14,15].
Even though it is not likely that the use of a CDA alone is sufficient to produce chitosan directly from chitin, its action on crystalline chitin may be useful. CDA action introduced NH2-group on the fibril surface, which can serve as a starting point for surface modifications, i.e., through “click chemistry”, thus offering an opportunity for creating new materials [32,33].
Supplementary Materials
The following are available online, Figure S1: MALDI-TOF-MS spectra of N,N-diacetyl chitobiose after incubation with VcCDA for 24 h. Figure S2: Table S1: SDS PAGE GEL analysis of VcCDA, SpPgdA, and AnCDA9.
Author Contributions
Conceptualization: R.A.G.H., V.G.H.E., and M.S. Formal analysis: R.A.G.H., S.G.A., T.R.T., and M.S. Investigation: R.A.G.H., S.G.A., T.R.T., V.G.H.E., and M.S. Supervision: V.G.H.E. and M.S. Writing—original draft: R.A.G.H. Writing—review and editing: V.G.H.E. and M.S.
Funding
This work was supported by Grant 221576 from the Research Council of Norway.
Acknowledgments
We thank Shinsuke Ifuku (Department of Chemistry and Biotechnology, Tottori University) for providing us with α- and β-chitin nanofibers, and Anne Tøndervik (SINTEF Materials and Chemistry, Trondheim, Norway) for purified SpPgdA.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sørlie, M.; Vårum, K.M.; Eijsink, V.G.H. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef] [PubMed]
- Minke, R.; Blackwell, J. The structure of α-chitin. J. Mol. Biol. 1978, 120, 167–181. [Google Scholar] [CrossRef]
- Jang, M.-K.; Kong, B.-G.; Jeong, Y.-I.; Lee, C.H.; Nah, J.-W. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitins and chitosans as immunoadjuvants and non-allergenic drug carriers. Mar. Drugs 2010, 8, 292–312. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.J. The effect of chitin size, shape, source and purification method on immune recognition. Molecules 2014, 19, 4433–4451. [Google Scholar] [CrossRef]
- Vårum, K.M.; Ottoy, M.H.; Smidsrød, O. Acid hydrolysis of chitosans. Carbohyd. Polym. 2001, 46, 89–98. [Google Scholar] [CrossRef]
- Vårum, K.M.; Anthonsen, M.W.; Grasdalen, H.; Smidsrød, O. 13c-nmr. Studies of the acetylation sequences in partially n-deacetylated chitins (chitosans). Carbohydr. Res. 1991, 217, 19–27. [Google Scholar] [CrossRef]
- Vårum, K.M.; Ottøy, M.H.; Smidsrød, O. Water-solubility of partially n-acetylated chitosans as a function of ph-effect of chemical-composition and depolymerization. Carbohydr. Polym. 1994, 25, 65–70. [Google Scholar] [CrossRef]
- Sannan, T.; Kurita, K.; Iwakura, Y. Studies on chitin: 2. Effect of deacetylation on solubility. Makromol. Chem. 1976, 177, 3589–3600. [Google Scholar] [CrossRef]
- Cai, J.; Yang, J.; Du, Y.; Fan, L.; Qiu, Y.; Li, J.; Kennedy, J.F. Enzymatic preparation of chitosan from the waste aspergillus niger mycelium of citric acid production plant. Carbohydr. Polym. 2006, 64, 151–157. [Google Scholar] [CrossRef]
- Lombard, V.; Ramulu, H.G.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (cazy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed]
- Andrés, E.; Albesa-Jové, D.; Biarnés, X.; Moerschbacher, B.M.; Guerin, M.E.; Planas, A. Structural basis of chitin oligosaccharide deacetylation. Angew. Chem. Int. Ed. Engl. 2014, 53, 6882–6887. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Wang, L.X.; Wang, X.S.; Roseman, S. The chitin catabolic cascade in the marine bacterium vibrio cholerae: Characterization of a unique chitin oligosaccharide deacetylase. Glycobiology 2007, 17, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Gay, L.M.; Tuveng, T.R.; Agger, J.W.; Westereng, B.; Mathiesen, G.; Horn, S.J.; Vaaje-Kolstad, G.; van Aalten, D.M.F.; Eijsink, V.G.H. Structure and function of a broadspecificity chitin deacetylase from aspergillus nidulans fgsc a4. Sci. Rep. 2017, 7, 12. [Google Scholar]
- Blair, D.E.; Schüttelkopf, A.W.; MacRae, J.I.; van Aalten, D.M.F. Structure and metal-dependent mechanism of peptidoglycan deacetylase, a streptococcal virulence factor. Proc. Natl. Acad. Sci. USA 2005, 102, 15429–15434. [Google Scholar] [CrossRef] [PubMed]
- Einbu, A.; Vårum, K.M. Characterization of chitin and its hydrolysis to glcnac and glcn. Biomacromolecules 2008, 9, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef]
- Ifuku, S.; Saimoto, H. Chitin nanofibers: Preparations, modifications, and applications. Nanoscale 2012, 4, 3308–3318. [Google Scholar] [CrossRef]
- Savitsky, P.; Bray, J.; Cooper, C.D.O.; Marsden, B.D.; Mahajan, P.; Burgess-Brown, N.A.; Gileadi, O. High-throughput production of human proteins for crystallization: The sgc experience. J. Struct. Biol. 2010, 172, 3–13. [Google Scholar] [CrossRef]
- Aslanidis, C.; de Jong, P.J. Ligation-independent cloning of pcr products (lic-pcr). Nucleic Acids Res. 1990, 18, 6069–6074. [Google Scholar] [CrossRef]
- Cederkvist, F.H.; Parmer, M.P.; Vårum, K.M.; Eijsink, V.G.H.; Sørlie, M. Inhibition of a family 18 chitinase by chitooligosaccharides. Carbohyd. Polym. 2008, 74, 41–49. [Google Scholar] [CrossRef]
- Zakariassen, H.; Klemetsen, L.; Sakuda, S.; Vaaje-Kolstad, G.; Vårum, K.M.; Sørlie, M.; Eijsink, V.G.H. Effect of enzyme processivity on the efficacy of a competitive chitinase inhibitor. Carbohyd. Polym. 2010, 82, 779–785. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. 2003, 43, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Beckham, G.T.; Crowley, M.F. Examination of the α-chitin structure and decrystallization thermodynamics at the nanoscale. J. Phys. Chem. B 2011, 115, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Monreal, J.; Reese, E.T. Chitinase of Serratia marcescens. Can. J. Microbiol. 1969, 15, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Chylenski, P.; Bissaro, B.; Sørlie, M.; Røhr, Å.K.; Várnai, A.; Horn, S.J.; Eijsink, V.G.H. Lytic polysaccharide monooxygenases in enzymatic processing of lignocellulosic biomass. ACS Catal. 2019, 9, 4970–4991. [Google Scholar] [CrossRef]
- Beckham, G.T.; Ståhlberg, J.; Knott, B.C.; Himmel, M.E.; Crowley, M.F.; Sandgren, M.; Sørlie, M.; Payne, C.M. Towards a molecular-level theory of carbohydrate processivity in glycoside hydrolases. Curr. Opin. Struct. Biol. 2014, 27, 96–106. [Google Scholar] [CrossRef]
- Hamre, A.G.; Jana, S.; Holen, M.M.; Mathiesen, G.; Väljamäe, P.; Payne, C.M.; Sørlie, M. Thermodynamic relationships with processivity in Serratia marcescens family 18 chitinases. J. Phys. Chem. B 2015, 119, 9601–9613. [Google Scholar] [CrossRef]
- Norberg, A.L.; Karlsen, V.; Hoell, I.A.; Bakke, I.; Eijsink, V.G.H.; Sørlie, M. Determination of substrate binding energies in individual subsites of a family 18 chitinase. FEBS Lett. 2010, 584, 4581–4585. [Google Scholar] [CrossRef]
- Zolotnitsky, G.; Cogan, U.; Adir, N.; Solomon, V.; Shoham, G.; Shoham, Y. Mapping glycoside hydrolase substrate subsites by isothermal titration calorimetry. Proc. Natl. Acad. Sci. USA 2004, 101, 11275–11280. [Google Scholar] [CrossRef]
- Hedegård, E.D.; Ryde, U. Targeting the reactive intermediate in polysaccharide monooxygenases. J. Biol. Inorg. Chem. 2017, 22, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Kritchenkov, A.S.; Skorik, Y.A. Click reactions in chitosan chemistry. Russ. Chem. Bull. 2017, 66, 769–781. [Google Scholar] [CrossRef]
- Peng, P.; Cao, X.; Peng, F.; Bian, J.; Xu, F.; Sun, R. Binding cellulose and chitosan via click chemistry: Synthesis, characterization, and formation of some hollow tubes. Polym. Chem. 2012, 50, 5201–5210. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).