Anti-Cancer and Ototoxicity Characteristics of the Curcuminoids, CLEFMA and EF24, in Combination with Cisplatin
Abstract
:1. Introduction
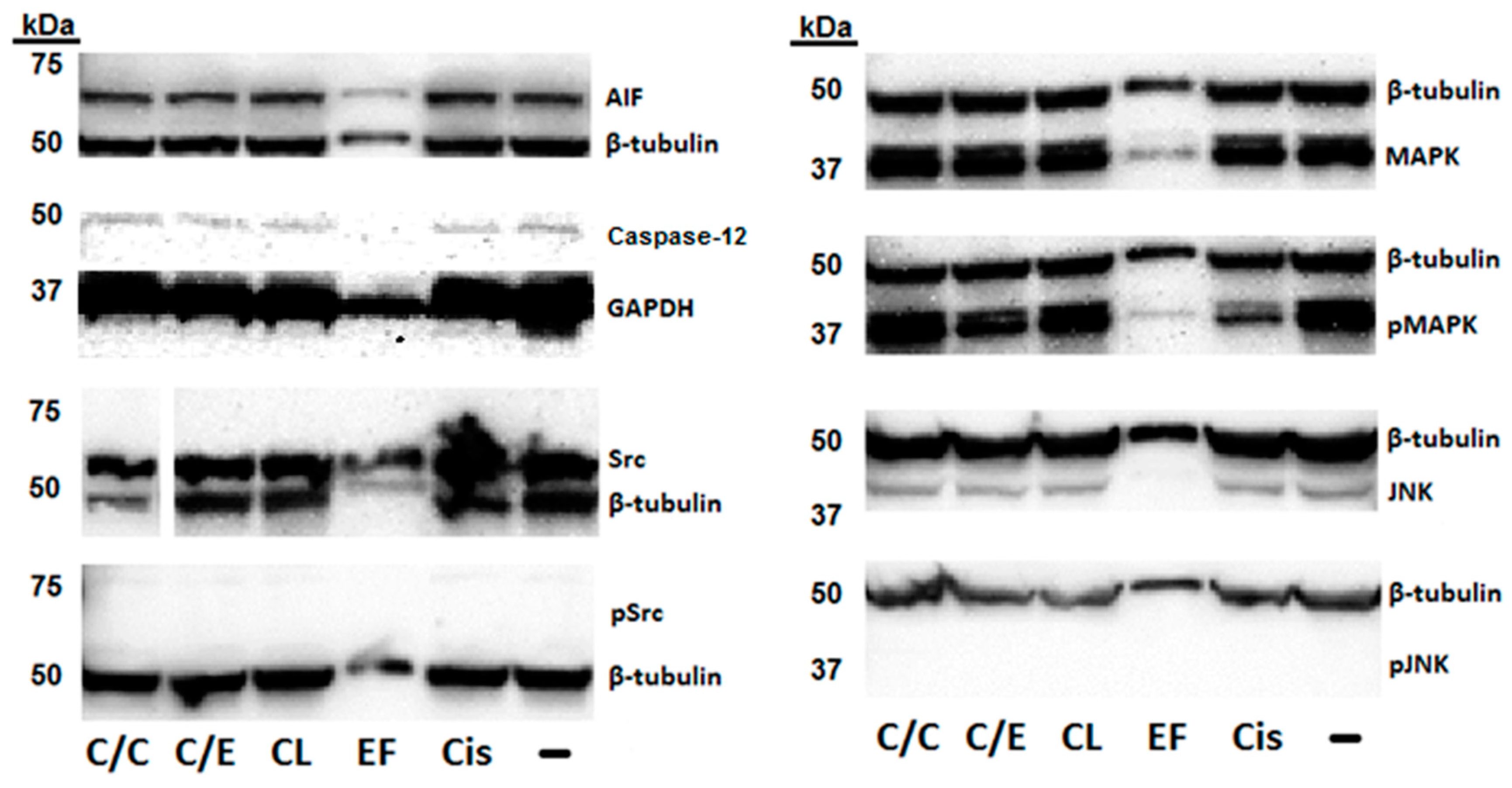
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cellular Viability Assay
4.3. Reactive Oxygen Species Assay
4.4. Caspase Luminescence Assays
4.5. Western Blot Assay
4.6. Cell Migration Assay
4.7. Zebrafish Maintenance
4.8. Auditory Evoked Potential
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cepeda, V.; Fuertes, M.; Castilla, J.; Alonso, C.; Quevedo, C.; Perez, J. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med. Chem. 2007, 7, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Evangelopoulos, A.; Schizas, N.; Kazazis, C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 2015, 35, 645–651. [Google Scholar] [PubMed]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.H.; Kumar, A.P.; Sethi, G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef]
- Hill, G.W.; Morest, D.K.; Parham, K. Cisplatin-induced ototoxicity: Effect of intratympanic dexamethasone injections. Otol. Neurotol. 2008, 29, 1005–1011. [Google Scholar] [CrossRef]
- Tian, C.J.; Kim, Y.J.; Kim, S.W.; Lim, H.J.; Kim, Y.S.; Choung, Y.-H. A combination of cilostazol and Ginkgo biloba extract protects against cisplatin-induced Cochleo-vestibular dysfunction by inhibiting the mitochondrial apoptotic and ERK pathways. Cell Death Dis. 2013, 4, e509. [Google Scholar] [CrossRef]
- Salehi, P.; Akinpelu, O.V.; Waissbluth, S.; Peleva, E.; Meehan, B.; Rak, J.; Daniel, S.J. Attenuation of cisplatin ototoxicity by otoprotective effects of nanoencapsulated curcumin and dexamethasone in a guinea pig model. Otol. Neurotol. 2014, 35, 1. [Google Scholar] [CrossRef]
- Karasawa, T.; Steyger, P.S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 2015, 237, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Cai, J.; Li, X.; Li, H.; Li, J.; Bai, X.; Liu, W.; Han, Y.; Xu, L.; Zhang, D.; et al. Allicin protects against cisplatin-induced vestibular dysfunction by inhibiting the apoptotic pathway. Eur. J. Pharmacol. 2017, 805, 108–117. [Google Scholar] [CrossRef]
- Tuorkey, M.J. Curcumin a potent cancer preventive agent: Mechanisms of cancer cell killing. Interv. Med. Appl. Sci. 2014, 6, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trujillo, J.; Chirino, Y.I.; Molina-Jijón, E.; Andérica-Romero, A.C.; Tapia, E.; Pedraza-Chaverri, J. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Boil. 2013, 1, 448–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fetoni, A.R.; Paciello, F.; Mezzogori, D.; Rolesi, R.; Eramo, S.L.M.; Paludetti, G.; Troiani, D. Molecular targets for anticancer redox chemotherapy and cisplatin-induced ototoxicity: The role of curcumin on pSTAT3 and Nrf-2 signalling. Br. J. Cancer 2015, 113, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Baharuddin, P.; Satar, N.; Fakiruddin, K.S.; Zakaria, N.; Lim, M.N.; Yusoff, N.M.; Zakaria, Z.; Yahaya, B.H. Curcumin improves the efficacy of cisplatin by targeting cancer stem-like cells through p21 and cyclin D1-mediated tumour cell inhibition in non-small cell lung cancer cell lines. Oncol. Rep. 2016, 35, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.-H.; Dicato, M.; Diederich, M. Hybrid curcumin compounds: A new strategy for cancer treatment. Molecules 2014, 19, 20839–20863. [Google Scholar] [CrossRef]
- Tomren, M.A.; Másson, M.; Loftsson, T.; Tønnesen, H.H. Studies on curcumin and curcuminoids XXXI. Symmetric and asymmetric curcuminoids: Stability, activity and complexation with cyclodextrin. Int. J. Pharm. 2007, 338, 27–34. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. C 2017, 77, 1316–1326. [Google Scholar] [CrossRef]
- Adams, B.K.; Ferstl, E.M.; Davis, M.C.; Herold, M.; Kurtkaya, S.; Camalier, R.F.; Hollingshead, M.G.; Kaur, G.; Sausville, E.A.; Rickles, F.R.; et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorganic Med. Chem. 2004, 12, 3871–3883. [Google Scholar] [CrossRef]
- Sahoo, K.; Dozmorov, M.G.; Anant, S.; Awasthi, V. The curcuminoid CLEFMA selectively induces cell death in H441 lung adenocarcinoma cells via oxidative stress. Invest. New Drugs 2012, 30, 558–567. [Google Scholar] [CrossRef]
- Lagisetty, P.; Vilekar, P.; Sahoo, K.; Anant, S.; Awasthi, V. CLEFMA-an anti-proliferative curcuminoid from structure-activity relationship studies on 3,5-bis(benzylidene)-4-piperidones. Bioorg. Med. Chem. 2010, 18, 6109–6120. [Google Scholar] [CrossRef]
- Agashe, H.; Sahoo, K.; Lagisetty, P.; Awasthi, V. Cyclodextrin-mediated entrapment of curcuminoid 4-[3,5-bis(2-chlorobenzylidene-4-oxo-piperidine-1-yl)-4-oxo-2-butenoic acid] or CLEFMA in liposomes for treatment of xenograft lung tumor in rats. Colloids Surfaces B: Biointerfaces 2011, 84, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, V.R.; Sahoo, K.; Roberts, P.R.; Awasthi, V. Pharmacologic suppression of inflammation by a diphenyldifluoroketone, EF24, in a rat model of fixed-volume hemorrhage improves survival. J. Pharmacol. Exp. Ther. 2013, 347, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sidell, N.; Mancini, A.; Huang, R.-P.; Wang, S.; Horowitz, I.R.; Liotta, D.C.; Taylor, R.N.; Wieser, F. Multiple anticancer activities of EF24, a novel curcumin analog, on human ovarian carcinoma cells. Reprod. Sci. 2010, 17, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Skoupa, N.; Dolezel, P.; Ruzickova, E.; Mlejnek, P. Apoptosis induced by the curcumin analogue EF-24 is neither mediated by oxidative stress-related mechanisms nor affected by expression of main drug transporters ABCB1 and ABCG2 in human leukemia cells. Int. J. Mol. Sci. 2017, 18, 2289. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, W.; Hu, G.; Sun, H.; Kong, Q. Bioactivities of EF24, a novel curcumin analog: A review. Front. Oncol. 2018, 8, 614. [Google Scholar] [CrossRef]
- Monroe, J.D.; Hruska, H.L.; Ruggles, H.K.; Williams, K.M.; Smith, M.E. Anti-cancer characteristics and ototoxicity of platinum(II) amine complexes with only one leaving ligand. PLoS ONE 2018, 13, e0192505. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Dugbartey, G.J.; Peppone, L.J.; De Graaf, I.A. An integrative view of cisplatin-induced renal and cardiac toxicities: Molecular mechanisms, current treatment challenges and potential protective measures. Toxicol. 2016, 371, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Kasinski, A.L.; Du, Y.; Thomas, S.L.; Zhao, J.; Sun, S.-Y.; Khuri, F.R.; Wang, C.-Y.; Shoji, M.; Sun, A.; Snyder, J.P.; et al. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol. Pharmacol. 2008, 74, 654–661. [Google Scholar] [CrossRef]
- Onen, H.I.; Yilmaz, A.; Alp, E.; Celik, A.; Demiroz, S.M.; Konac, E. EF24 and RAD001 potentiates the anticancer effect of platinum-based agents in human malignant pleural mesothelioma (MSTO-211H) cells and protects nonmalignant mesothelial (MET-5A) cells. Hum. Exp. Toxicol. 2015, 34, 117–126. [Google Scholar] [CrossRef]
- Kuhar, M.; Sen, S.; Singh, N. Role of mitochondria in quercetin-enhanced chemotherapeutic response in human non-small cell lung carcinoma H-520 cells. Anticancer. Res. 2006, 26, 1297–1303. [Google Scholar] [PubMed]
- Yang, X.; Fraser, M.; Abedini, M.R.; Bai, T.; Tsang, B.K. Regulation of apoptosis-inducing factor-mediated, cisplatin-induced apoptosis by Akt. Br. J. Cancer 2008, 98, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Urbano, A.; Lakshmanan, U.; Choo, P.H.; Kwan, J.C.; Ng, P.Y.; Guo, K.; Dhakshinamoorthy, S.; Porter, A. AIF suppresses chemical stress-induced apoptosis and maintains the transformed state of tumor cells. EMBO J. 2005, 24, 2815–2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, A.J.; Walker, S.A.; Krank, J.J.; Wilkinson, A.S.; Johnson, K.M.; Lewis, E.M.; Wilkinson, J.C. AIF promotes a JNK1-mediated cadherin switch independently of respiratory chain stabilization. J. Boil. Chem. 2018, 293, 14707–14722. [Google Scholar] [CrossRef] [Green Version]
- Pearson, G. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar]
- Achkar, I.W.; Abdulrahman, N.; Al-Sulaiti, H.; Joseph, J.M.; Uddin, S.; Mraiche, F. Cisplatin based therapy: The role of the mitogen activated protein kinase signaling pathway. J. Transl. Med. 2018, 16, 96. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Hewage, S.R.K.M.; Ryu, Y.S.; Oh, M.C.; Kwon, T.K.; Chae, S.; Hyun, J.W. Fisetin induces apoptosis and endoplasmic reticulum stress in human non-small cell lung cancer through inhibition of the MAPK signaling pathway. Tumor Boil. 2016, 37, 9615–9624. [Google Scholar] [CrossRef]
- Kim, L.C.; Song, L.; Haura, E.B. Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 2009, 6, 587–595. [Google Scholar] [CrossRef]
- Azijli, K.; Weyhenmeyer, B.; Peters, G.J.; De Jong, S.; Kruyt, F.A.E. Non-canonical kinase signaling by the death ligand TRAIL in cancer cells: Discord in the death receptor family. Cell Death Differ. 2013, 20, 858–868. [Google Scholar] [CrossRef]
- Selvi, S.K.; Vinoth, A.; Varadharajan, T.; Weng, C.F.; Padma, V.V. Neferine augments therapeutic efficacy of cisplatin through ROS- mediated non-canonical autophagy in human lung adenocarcinoma (A549 cells). Food Chem. Toxicol. 2017, 103, 28–40. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Xu, K.; Lu, G.; Ying, Z.; Wu, L.; Zhan, J.; Fang, R.; Wu, Y.; Zhou, J. Curcumin induces apoptosis in human lung adenocarcinoma A549 cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Oncol. Rep. 2010, 23, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Duan, X.; Cai, H.; Wang, L.; Li, M.; Qu, J.; Li, W.; Wang, Y.; Wang, J. Curcumin inhibits the invasion of lung cancer cells by modulating the PKCα/Nox-2/ROS/ATF-2/MMP-9 signaling pathway. Oncol. Rep. 2015, 34, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Chen, Q.; Yao, S.; Bai, E.; Fu, W.; Wang, L.; Wang, J.; Du, X.; Wei, T.; Xu, H.; et al. Synthesis and anti-tumor activity of EF24 analogues as IKKβ inhibitors. Eur. J. Med. Chem. 2018, 144, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS ONE 2011, 6, 26012. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Amin, A.R.; Chen, Z.G.; Shin, D.M. New perspectives of curcumin in cancer prevention. Cancer Prev. Res. 2013, 6, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Rad. Res. 2010, 44, 1–31. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, N.-Y.; Suh, Y.-A.; Lee, C. Involvement of ROS in curcumin-induced autophagic cell death. Korean J. Physiol. Pharmacol. 2011, 15, 1–7. [Google Scholar] [CrossRef]
- Gersey, Z.C.; Rodriguez, G.A.; Barbarite, E.; Sanchez, A.; Walters, W.M.; Ohaeto, K.C.; Komotar, R.J.; Graham, R.M. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer 2017, 17, 99. [Google Scholar] [CrossRef]
- Mukherjea, D.; Rybak, L.P. Pharmacogenomics of cisplatin-induced ototoxicity. Pharmacogenomics 2011, 12, 1039–1050. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Chen, G.G.; Lu, Y.-N.; Liu, Y.; Wu, K.-F.; Gong, X.-L.; Gou, Z.-P.; Li, M.-Y.; Liang, N.-C. Ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid inhibits growth of human lung cancer A549 cells by arresting cell cycle and triggering apoptosis. Chin. J. Cancer Res. 2012, 24, 109–115. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Shi, J.; Zhang, K.; Xue, J.; Li, J.; Yang, J.; Chen, J.; Wei, J.; Ren, H.; Liu, X. Sox2 inhibits Wnt-β-catenin signaling and metastatic potency of cisplatin-resistant lung adenocarcinoma cells. Mol. Med. Rep. 2017, 15, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Park, J.-A.; Zhang, D.; Cho, S.-H.; Yi, H.; Cho, S.-M.; Chang, B.-J.; Kim, J.-S.; Shim, J.-H.; El-Aty, A.M.A.; et al. Sustainability of CD24 expression, cell proliferation and migration, cisplatin-resistance, and caspase-3 expression during mesenchymal-epithelial transition induced by the removal of TGF-β1 in A549 lung cancer cells. Oncol. Lett. 2017, 14, 2410–2416. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xu, Z.; Wu, G.; Chen, X.; Huang, Y.; Wang, Y.; Jiang, W.; Ke, B. Up-regulation of miR-146a increases the sensitivity of non-small cell lung cancer to DDP by downregulating cyclin J. BMC Cancer 2017, 17, 138. [Google Scholar] [CrossRef]
- Tung, C.-L.; Jian, Y.-J.; Chen, J.-C.; Wang, T.-J.; Chen, W.-C.; Zheng, H.-Y.; Chang, P.-Y.; Liao, K.-S.; Lin, Y.-W. Curcumin downregulates p38 MAPK-dependent X-ray repair cross-complement group 1 (XRCC1) expression to enhance cisplatin-induced cytotoxicity in human lung cancer cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 657–666. [Google Scholar] [CrossRef]
- Jiao, D.; Wang, J.; Lu, W.; Tang, X.; Chen, J.; Mou, H.; Chen, Q.-Y. Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways in lung cancer. Mol. Ther. - Oncolytics 2016, 3, 16018. [Google Scholar] [CrossRef] [Green Version]
- Zhan, J.; Jiao, D.; Wang, Y.; Song, J.; Wu, J.; Wu, L.; Chen, Q.; Ma, S. Integrated microRNA and gene expression profiling reveals the crucial miRNAs in curcumin anti-lung cancer cell invasion. Thorac. Cancer 2017, 8, 461–470. [Google Scholar] [CrossRef]
- He, B.; Wei, W.; Liu, J.; Xu, Y.; Zhao, G. Synergistic anticancer effect of curcumin and chemotherapy regimen FP in human gastric cancer MGC-803 cells. Oncol. Lett. 2017, 14, 3387–3394. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Yadav, A.; Hideg, K.; Kuppusamy, P.; Teknos, T.N.; Kumar, P. A novel curcumin analog (H-4073) enhances the therapeutic efficacy of cisplatin treatment in head and neck cancer. PLoS ONE 2014, 9, e93208. [Google Scholar] [CrossRef]
- Xiong, F.; Jiang, M.; Huang, Z.; Chen, M.; Chen, K.; Zhou, J.; Yin, L.; Tang, Y.; Wang, M.; Ye, L.; et al. A novel herbal formula induces cell cycle arrest and apoptosis in association with suppressing the PI3K/AKT pathway in human lung cancer A549 cells. Integr. Cancer 2014, 13, 152–160. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, F.; Wang, X.; Tsai, Y.; Chuang, K.-H.; Keng, P.C.; Lee, S.O.; Chen, Y. A FASN-TGF-β1-FASN regulatory loop contributes to high EMT/metastatic potential of cisplatin-resistant non-small cell lung cancer. Oncotarget 2016, 7, 55543–55554. [Google Scholar] [PubMed]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-Induced Oxidative Stress and Toxicity. J. Toxicol. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schacht, J.; Talaska, A.E.; Rybak, L.P. Cisplatin and aminoglycoside antibiotics: Hearing loss and its prevention. Anat. Rec. Adv. Integr. Anat. Evol. Boil. 2012, 295, 1837–1850. [Google Scholar] [CrossRef] [PubMed]
- Waissbluth, S.; Daniel, S.J. Cisplatin-induced ototoxicity: Transporters playing a role in cisplatin toxicity. Hear. Res. 2013, 299, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Paken, J.; Govender, C.D.; Pillay, M.; Sewram, V. Cisplatin-associated ototoxicity: A Review for the health professional. J. Toxicol. 2016, 1809394. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Eramo, S.L.M.; Paciello, F.; Rolesi, R.; Podda, M.V.; Troiani, D.; Paludetti, G.; Eramo, S.L.M. Curcuma longa (curcumin) decreases in vivo cisplatin-induced ototoxicity through heme oxygenase-1 induction. Otol. Neurotol. 2014, 35, e169–e177. [Google Scholar] [CrossRef]
- Fujisawa, S.; Atsumi, T.; Ishihara, M.; Kadoma, Y. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer. Res. 2004, 24, 563–569. [Google Scholar]
- Tharakan, B.; Hunter, F.A.; Smythe, W.R.; Childs, E.W. Curcumin inhibits reactive oxygen species formation and vascular hyperpermeability following haemorrhagic shock. Clin. Exp. Pharmacol. Physiol. 2010, 37, 939–944. [Google Scholar] [CrossRef]
- Fischer, S.J.; Benson, L.M.; Fauq, A.; Naylor, S.; Windebank, A.J. Cisplatin and dimethyl sulfoxide react to form an adducted compound with reduced cytotoxicity and neurotoxicity. NeuroToxicology 2008, 29, 444–452. [Google Scholar] [CrossRef]
- Hall, M.D.; Telma, K.A.; Chang, K.-E.; Lee, T.D.; Madigan, J.P.; Lloyd, J.R.; Goldlust, I.S.; Hoeschele, J.D.; Gottesman, M.M. Say no to DMSO: Dimethylsulfoxide inactivates cisplatin, carboplatin, and other platinum complexes. Cancer Res. 2014, 74, 3913–3922. [Google Scholar] [CrossRef]
- Park, G.Y.; Wilson, J.J.; Song, Y.; Lippard, S.J. Phenanthriplatin, a monofunctional DNA-binding platinum anticancer drug candidate with unusual potency and cellular activity profile. Proc. Natl. Acad. Sci. USA 2012, 109, 11987–11992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanmartín-Suárez, C.; Soto-Otero, R.; Sánchez-Sellero, I.; Méndez-Álvarez, E. Antioxidant properties of dimethyl sulfoxide and its viability as a solvent in the evaluation of neuroprotective antioxidants. J. Pharmacol. Toxicol. Methods 2011, 63, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.H.; Kong, S.M.; Tiong, Y.L.; Maah, M.J.; Sukram, N.; Ahmad, M.; Khoo, A.S.B. Selective anticancer copper(ii)-mixed ligand complexes: Targeting of ROS and proteasomes. Metallomics 2014, 6, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish; University of Oregon: Eugene, OR, USA, 1994. [Google Scholar]
- Uribe, P.M.; Sun, H.; Wang, K.; Asuncion, J.D.; Wang, Q.; Chen, C.W.; Steyger, P.; Smith, M.E.; Matsui, J.I. Aminoglycoside-induced hair cell death of inner ear organs causes functional deficits in adult zebrafish (Danio rerio). PLoS ONE 2013, 8, e58755. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


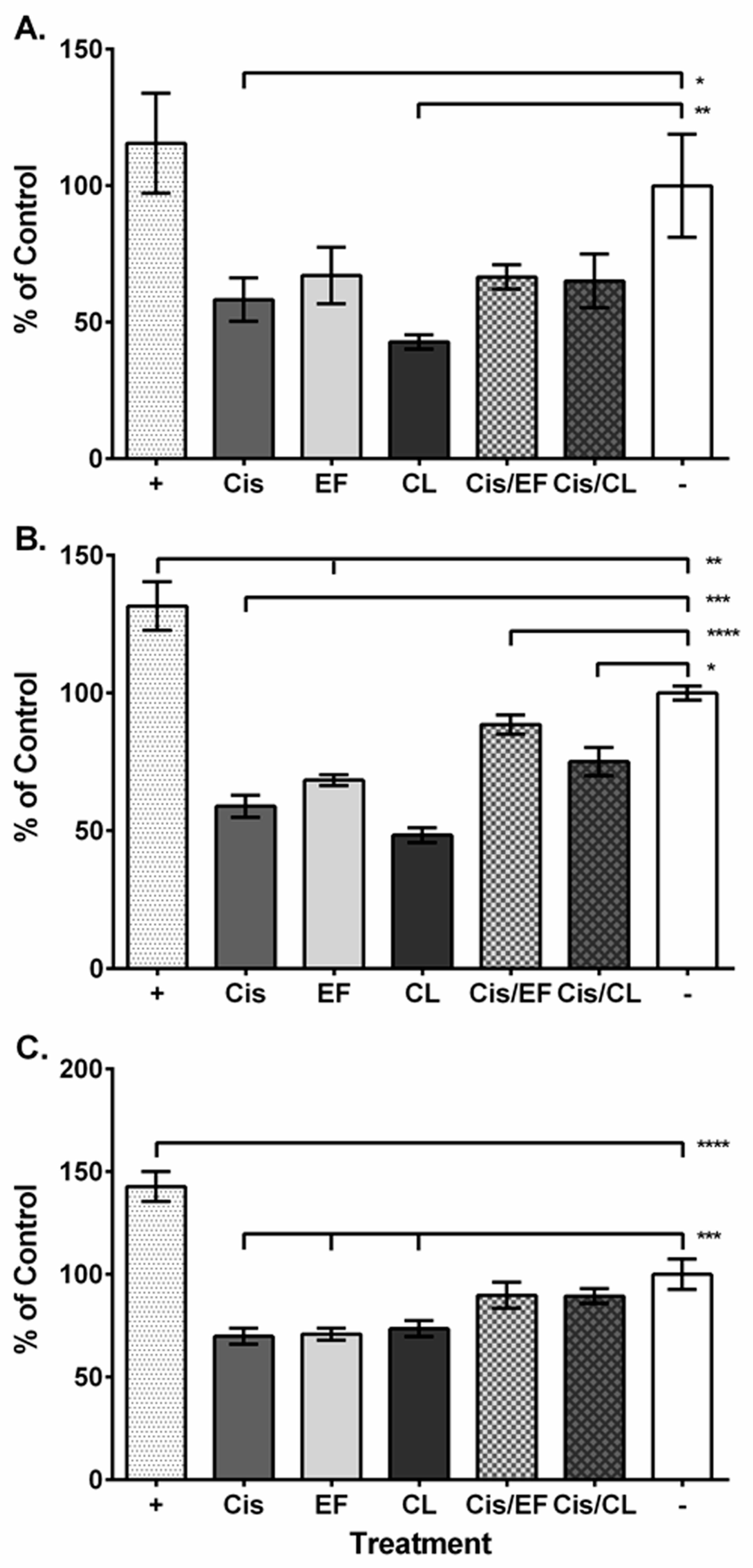



| Treatment | IC50 (µM) |
|---|---|
| Cisplatin (24 h) | 10.91 ± 0.19 |
| Cisplatin (48 h) | 7.49 ± 0.16 |
| CLEFMA (0–24 h) | 13.82 ± 0.18 |
| CLEFMA (24–48 h) | 16.05 ± 0.15 |
| EF24 (0–24 h) | 1.74 ± 0.28 |
| EF24 (24–48 h) | 2.47 ± 0.14 |
| Cisplatin (48 h) + CLEFMA (24–48 h) | 2.19 ± 0.17 |
| Cisplatin (48 h) + EF24 (24–48 h) | 2.94 ± 0.09 |
| Freq (Hz) | Cisplatin | EF24 | CLEFMA | Cisplatin/EF24 | Cisplatin/CLEFMA | ||||
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | Vehicle | Cisplatin | Vehicle | Cisplatin | Vehicle | Cisplatin | Vehicle | Cisplatin | |
| 100 | ns | ns | ns | ns | ns | ns | ns | ** | ** |
| 250 | ns | ns | ns | ns | ns | ns | ns | ** | ** |
| 400 | ns | * | ns | ** | ns | ns | ns | * | ns |
| 600 | ns | ns | ** | ns | * | ns | ns | ns | ** |
| 800 | **** | ns | **** | ns | **** | ns | **** | ** | **** |
| 1000 | **** | **** | ns | * | ns | ns | **** | ns | **** |
| 1500 | **** | **** | ns | ** | ns | * | ns | ** | ns |
| 3000 | ns | ns | ns | ns | ns | ns | ns | ns | ** |
| Freq (Hz) | Cisplatin | EF24 | CLEFMA | Cisplatin/EF24 | Cisplatin/CLEFMA | Cisplatin/DMSO | |
|---|---|---|---|---|---|---|---|
| DMSO | DMSO | DMSO | DMSO | DMSO | DMSO | Cisplatin | |
| 100 | ns | ns | ns | ns | ns | ns | ** |
| 250 | ns | ns | ns | ** | ns | ns | * |
| 400 | ns | ns | ns | ns | ns | ns | ns |
| 600 | ns | ns | ns | ns | ns | ns | ** |
| 800 | **** | * | ns | ns | ns | ns | **** |
| 1000 | **** | ** | ns | ns | ns | ns | **** |
| 1500 | ns | ns | ns | ns | ns | ns | ** |
| 3000 | ns | ns | ns | ns | ns | ns | ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monroe, J.D.; Hodzic, D.; Millay, M.H.; Patty, B.G.; Smith, M.E. Anti-Cancer and Ototoxicity Characteristics of the Curcuminoids, CLEFMA and EF24, in Combination with Cisplatin. Molecules 2019, 24, 3889. https://doi.org/10.3390/molecules24213889
Monroe JD, Hodzic D, Millay MH, Patty BG, Smith ME. Anti-Cancer and Ototoxicity Characteristics of the Curcuminoids, CLEFMA and EF24, in Combination with Cisplatin. Molecules. 2019; 24(21):3889. https://doi.org/10.3390/molecules24213889
Chicago/Turabian StyleMonroe, Jerry D., Denis Hodzic, Matthew H. Millay, Blaine G. Patty, and Michael E. Smith. 2019. "Anti-Cancer and Ototoxicity Characteristics of the Curcuminoids, CLEFMA and EF24, in Combination with Cisplatin" Molecules 24, no. 21: 3889. https://doi.org/10.3390/molecules24213889
APA StyleMonroe, J. D., Hodzic, D., Millay, M. H., Patty, B. G., & Smith, M. E. (2019). Anti-Cancer and Ototoxicity Characteristics of the Curcuminoids, CLEFMA and EF24, in Combination with Cisplatin. Molecules, 24(21), 3889. https://doi.org/10.3390/molecules24213889







