Amidoxime Functionalization of Algal/Polyethyleneimine Beads for the Sorption of Sr(II) from Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Materials
2.1.1. SEM and EDX Characterization
2.1.2. Textural Properties
2.1.3. Thermal Degradation—TGA Analysis
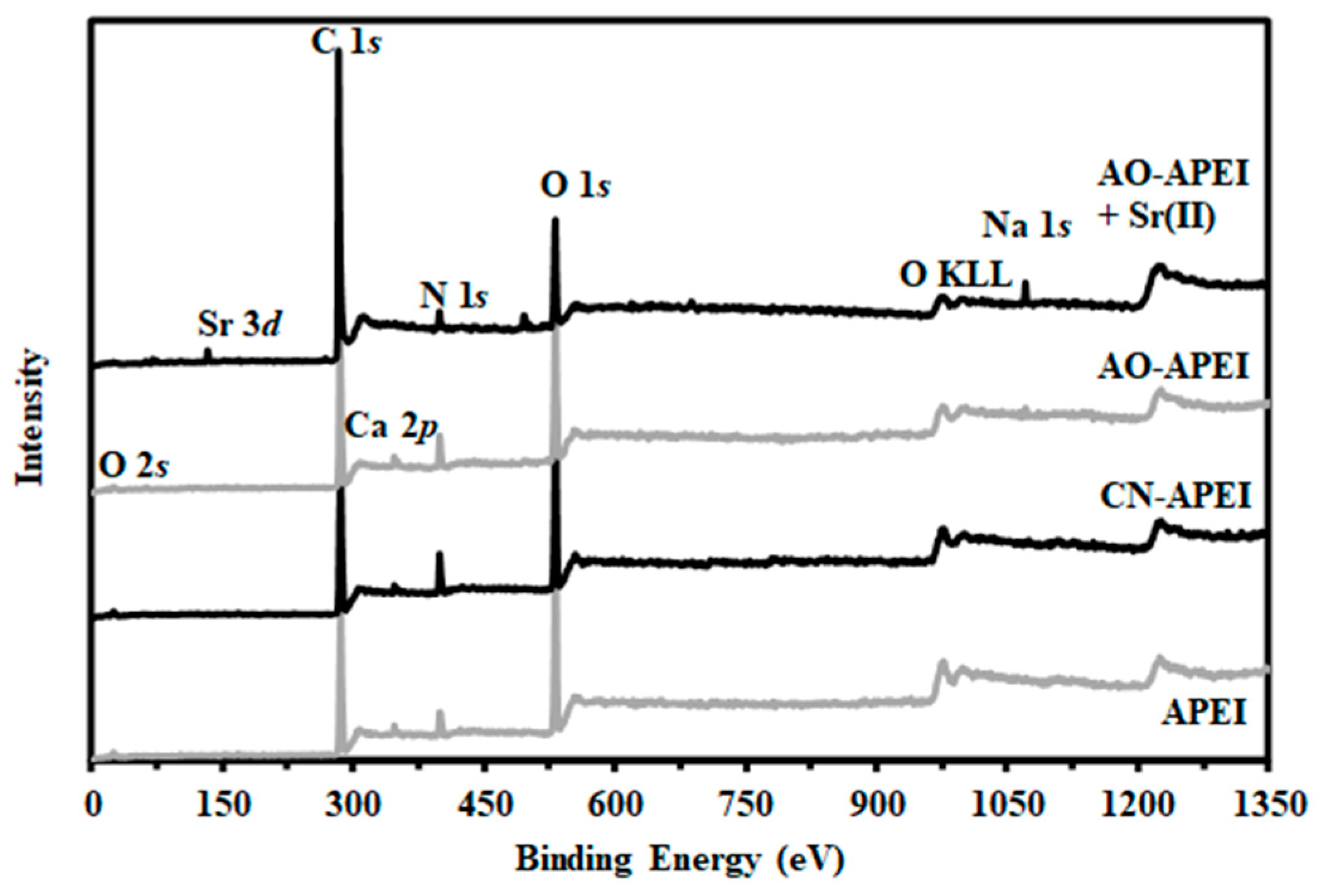
2.1.4. Chemical Characterization of Sorbent—FTIR and XPS Analyses
2.1.5. Determination of pHPZC
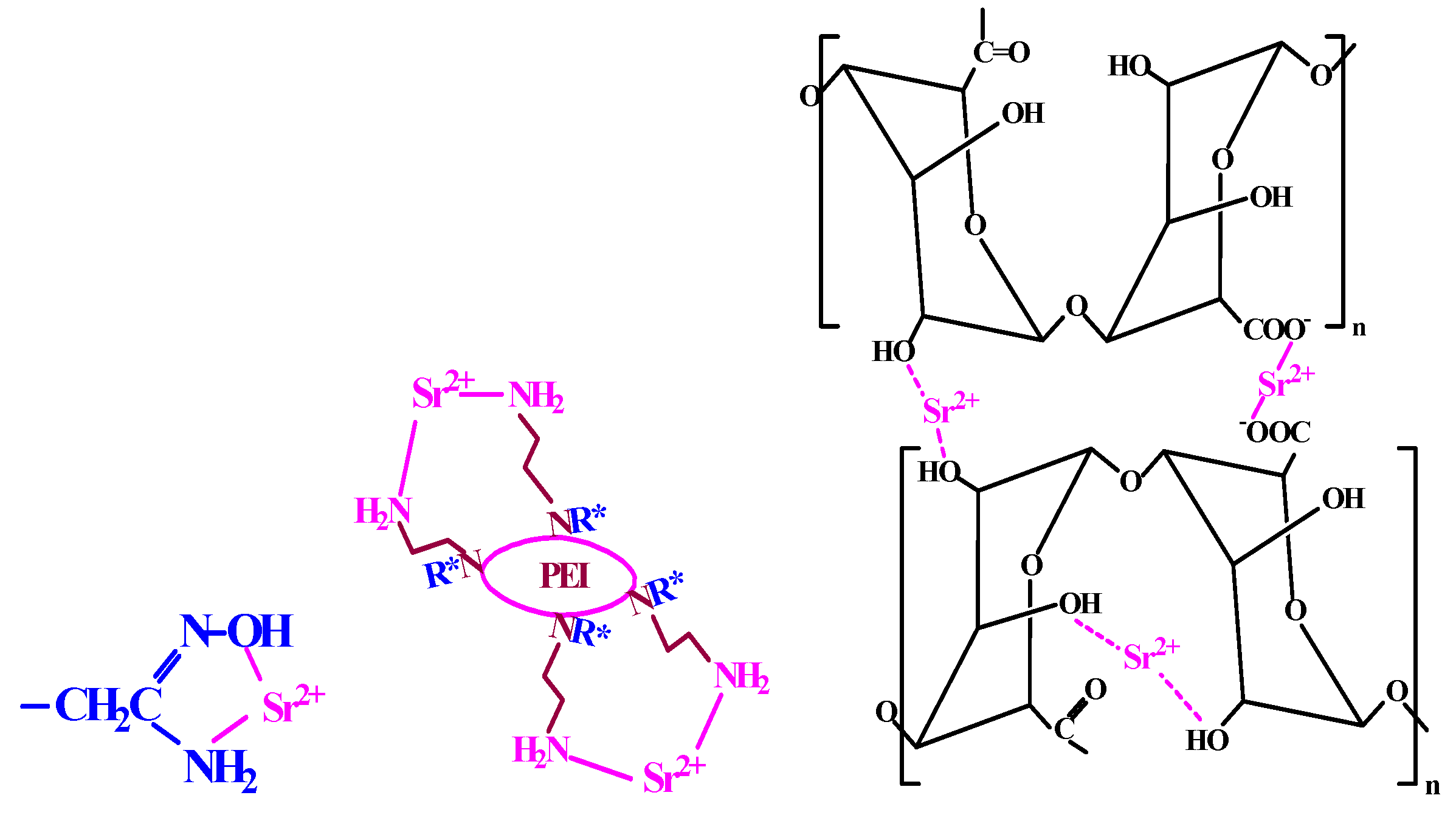
2.1.6. Modes of Interaction Sorbent/Sr(II)
2.2. Sorption Properties
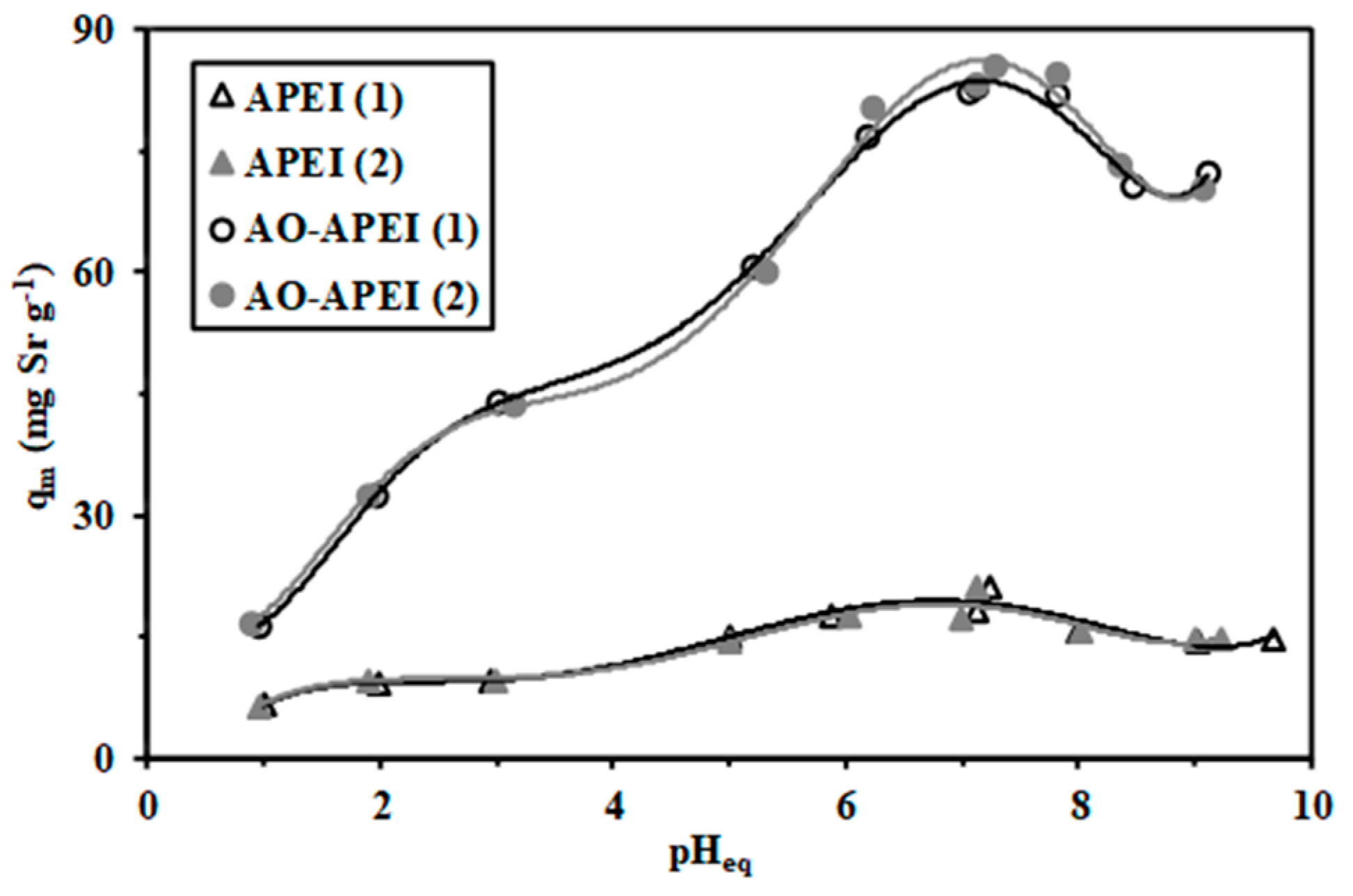
2.2.1. The pH Effect
2.2.2. Uptake Kinetics
2.2.3. Sorption Isotherms
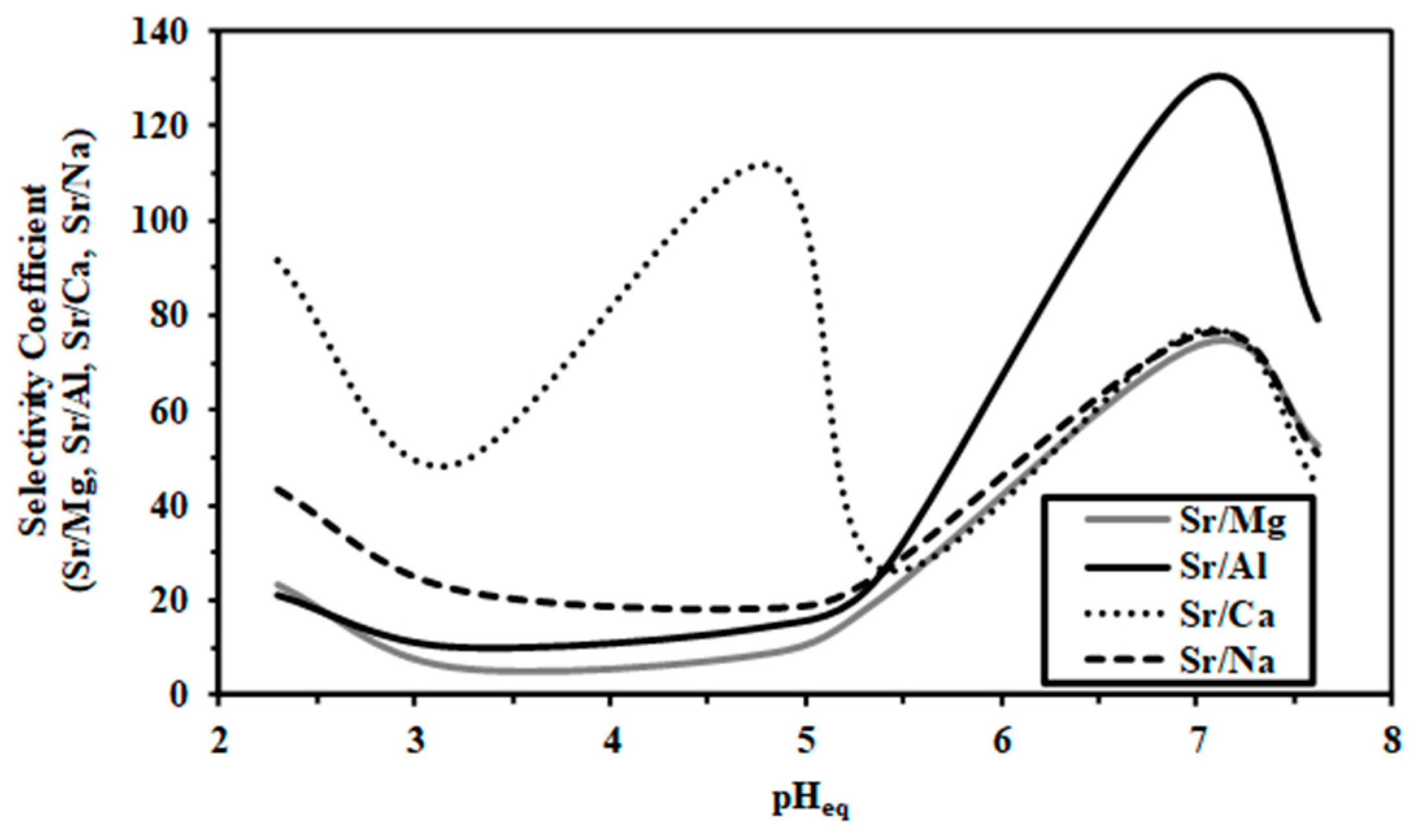
2.2.4. Multicomponent Sorption
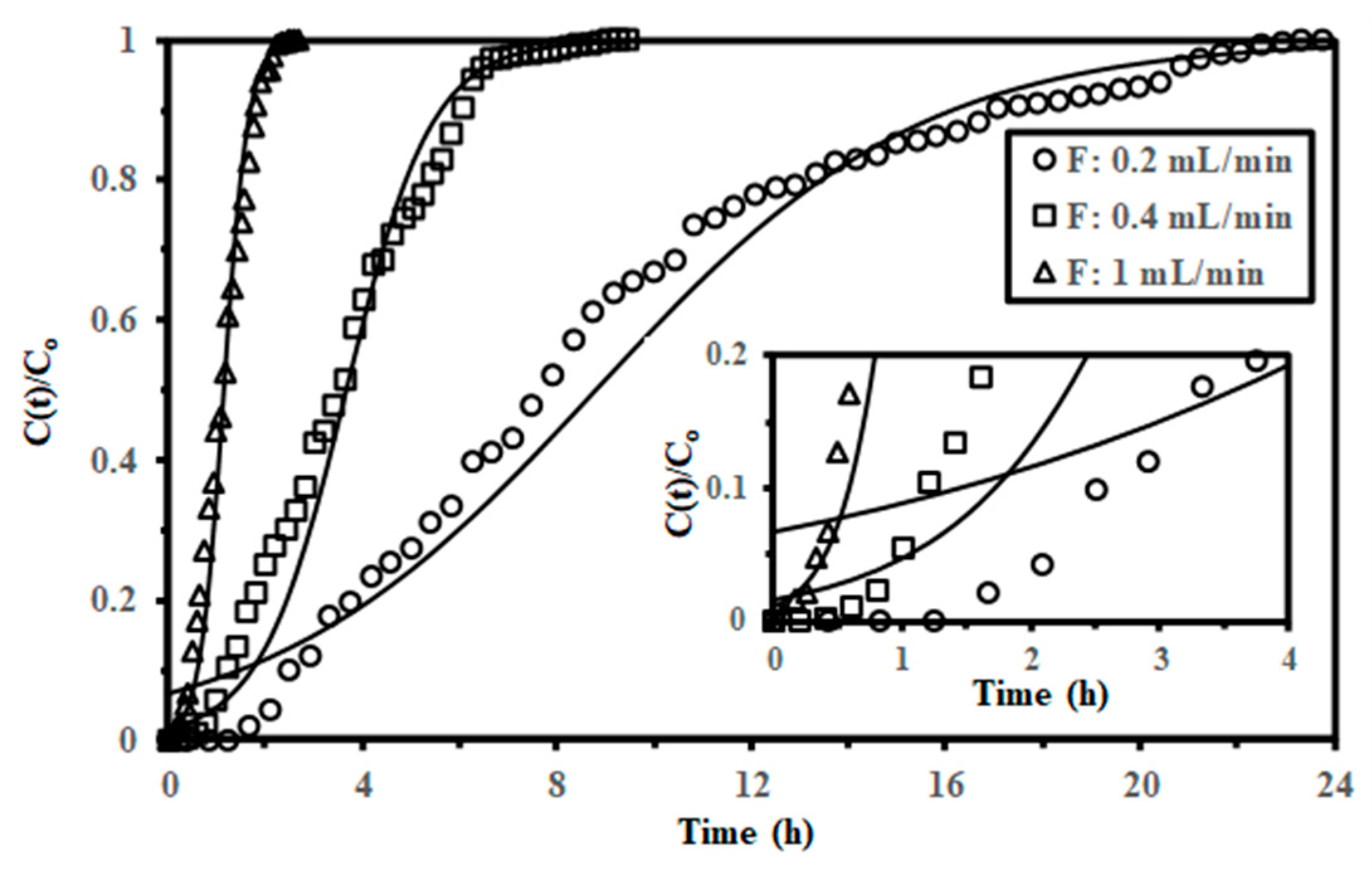
2.2.5. Sorption in Fixed-Bed Columns
2.2.6. Metal Desorption and Sorbent Recycling
2.3. Strontium Removal from Seawater
2.4. Noticeable effect of the Amidoximation Process on the Stability of the Sorbent in Complex Environment
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Algal/PEI Beads
3.3. Functionalization of Algal/PEI Beads (APEI Beads)
3.3.1. Crosslinking of APEI Beads
3.3.2. Nitrilation of CAPEI Beads
3.3.3. Amidoximation of Nitrilated APEI Beads
3.4. Characterization of Materials
3.5. Sorption Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PEI | polyethyleneimine |
| APEI | Algal-polyethyleneimine |
| cAPEI | Crosslinked Algal-polyethyleneimine with poly(ethyleneglycol) diglycidyl ether |
| AO-APEI | Amidoxime- Algal-polyethyleneimine |
| ICP-AES | Inductively coupled plasma atomic emission spectroscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| TGA | Thermal gravimetric analysis or thermogravimetric analysis |
| SEM | Scanning electron microscope |
| SEM-EDX | Scanning electron microscopy-energy dispersive X-ray analysis |
| BET | Brunauer-Emmett-Teller |
| POC | porous organic cages |
| CN-APEI | Nitrile- Algal-polyethyleneimine |
| DrDTG | Derivative- Differential thermogravimetric analysis |
| SD | Sorbent dosage |
| PFORE | Pseudo-first order rate equation |
| PSORE | Pseudo-second order rate equation |
| RIDE | Resistance to intraparticle diffusion equation – Crank equation |
| SC | selectivity coefficient |
| DMF | Di methyl formamide |
| pHPZC | Point of zero charge |
| pH0 | Initial pH of the solution |
| pHeq | Equivalent pH after sorption |
| Kd | Distribution ratio |
| C0 | Initial concentration |
| Ceq | Equilibrium concentration after metal sorption |
| ICPS | Inductively coupled plasma atomic emission spectrometer |
| S | Sorption |
| D | Desorption |
| BEs | Binding energies |
| AF | Atomic Fractions |
References
- Ahmadpour, A.; Zabihi, M.; Tahmasbi, M.; Bastami, T.R. Effect of adsorbents and chemical treatments on the removal of strontium from aqueous solutions. J. Hazard. Mater. 2010, 182, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Shen, J.; Cao, F.; Wang, C.J.; Tang, M.; Zhang, Q.Y.; Wei, S.S. A facile synthesis of hydroxyapatite for effective removal strontium ion. J. Hazard. Mater. 2019, 368, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Strontium in Drinking Water—Guideline Technical Document for Public Consultation; Health Canada: Ottawa, ON, Canada, 2018; p. 65.
- Wu, L.Y.; Zhang, G.H.; Wang, Q.Z.; Hou, L.; Gu, P. Removal of strontium from liquid waste using a hydraulic pellet co-precipitation microfiltration (HPC-MF) process. Desalination 2014, 349, 31–38. [Google Scholar] [CrossRef]
- Alby, D.; Charnay, C.; Heran, M.; Prelot, B.; Zajac, J. Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: Synthesis and shaping, sorption capacity, mechanisms, and selectivity—A review. J. Hazard. Mater. 2018, 344, 511–530. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P. An assessment of strontium sorption onto bentonite buffer material in waste repository. Environ. Sci. Pollut. Res. 2017, 24, 8825–8836. [Google Scholar] [CrossRef]
- Maresova, J.; Pipiska, M.; Rozloznik, M.; Hornik, M.; Remenarova, L.; Augustin, J. Cobalt and strontium sorption by moss biosorbent: Modeling of single and binary metal systems. Desalination 2011, 266, 134–141. [Google Scholar] [CrossRef]
- Sasaki, T.; Okabe, J.; Henmi, M.; Hayashi, H.; Iida, Y. Cesium (Cs) and strontium (Sr) removal as model materials in radioactive water by advanced reverse osmosis membrane. Desalin. Water Treat. 2013, 51, 1672–1677. [Google Scholar] [CrossRef]
- Ghosh, B.; Ghosh, A.K.; Mamtani, V.S.; Bindal, R.C. High-flux thin-film composite polyamide (TFCP) forward osmosis membranes for concentration of simulated cesium- and strontium-bearing effluent solution. Sep. Sci. Technol. 2019, 54, 1542–1552. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Smiciklas, I.D.; Milonjic, S.K.; Pfendt, P.; Raicevic, S. The point of zero charge and sorption of cadmium (II) and strontium (II) ions on synthetic hydroxyapatite. Sep. Purif. Technol. 2000, 18, 185–194. [Google Scholar] [CrossRef]
- Ghalami, Z.; Ghoulipour, V.; Khanchi, A. Highly efficient capturing and adsorption of cesium and strontium ions from aqueous solution by porous organic cage: A combined experimental and theoretical study. Appl. Surf. Sci. 2019, 471, 726–732. [Google Scholar] [CrossRef]
- Basargin, N.N.; Demina, E.V.; Anikin, V.Y.; Kometiani, I.B. Strontium(II) sorption by complexing o-hydroxy-azo-o’-hydroxy functionalized polystyrene polymer sorbents. Russ. J. Inorg. Chem. 2011, 56, 2019–2023. [Google Scholar] [CrossRef]
- Mulani, K.; Patil, V.; Chavan, N.; Donde, K. Adsorptive removal of strontium(II) using macroporous poly(AGE-co-EGDMA) beads modified with resorcin 4 arene. Bull. Mater. Sci. 2019, 42, 82. [Google Scholar] [CrossRef]
- Shevchuk, I.A.; Klimenko, N.A.; Stavskaya, S.S. Sorption of ions of U(VI) and strontium by biosorption based on Bacillus polymyxa IMV 8910 in aqueous systems. J. Water Chem. Technol. 2010, 32, 176–181. [Google Scholar] [CrossRef]
- Qiu, L.; Feng, J.D.; Dai, Y.D.; Chang, S.Q. Biosorption of strontium ions from simulated high-level liquid waste by living Saccharomyces cerevisiae. Environ. Sci. Pollut. Res. 2018, 25, 17194–17206. [Google Scholar] [CrossRef]
- Park, Y.; Shin, W.S.; Choi, S.-J. Removal of cobalt and strontium from groundwater by sorption onto fishbone. J. Radioanal. Nucl. Chem. 2013, 295, 789–799. [Google Scholar] [CrossRef]
- Hu, Y.M.; Guo, X.; Chen, C.; Wang, J.L. Algal sorbent derived from Sargassum horneri for adsorption of cesium and strontium ions: Equilibrium, kinetics, and mass transfer. Appl. Microbiol. Biotechnol. 2019, 103, 2833–2843. [Google Scholar] [CrossRef]
- Hong, H.J.; Park, I.S.; Ryu, T.; Jeong, H.S.; Ryu, J. Demonstration of seawater strontium (Sr(II)) extraction and enrichment by a biosorption technique through continuous column operation. Ind. Eng. Chem. Res. 2018, 57, 12909–12915. [Google Scholar] [CrossRef]
- Kolenchenko, E.A.; Khotimchenko, M.Y.; Khozhaenko, E.V.; Khotimchenko, Y.S. Strontium sorption by pectins isolated from the Sea grasses Zostera marina and Phyllospadix iwatensis. Russ. J. Mar. Biol. 2012, 38, 346–350. [Google Scholar] [CrossRef]
- Song, D.; Park, S.-J.; Kang, H.W.; Park, S.B.; Han, J.-I. Recovery of lithium(I), strontium(II), and lanthanum(III) using Ca-alginate beads. J. Chem. Eng. Data 2013, 58, 2455–2464. [Google Scholar] [CrossRef]
- Yavari, R.; Huang, Y.D.; Mostofizadeh, A. Sorption of strontium ions from aqueous solutions by oxidized multiwall carbon nanotubes. J. Radioanal. Nucl. Chem. 2010, 285, 703–710. [Google Scholar] [CrossRef]
- Aslani, C.K.; Belloni, F.; Cetinkaya, B.; Rondinella, V.V. Sorption studies of strontium on carbon nanotubes using the Box-Behnken design. Radiochim. Acta 2014, 102, 931–940. [Google Scholar] [CrossRef]
- Hayakawa, S.; Matsubara, S.; Sumi, Y.; Yamamoto, S.; Kawamura, N.; Nonami, T. Caesium and strontium adsorption ability of activated bamboo charcoal. Int. J. Nanotechnol. 2018, 15, 683–688. [Google Scholar] [CrossRef]
- Kobayashi, S.; Noda, K.; Shibata, H.; Matsubara, S.; Kawamura, N.; Nonami, T. Rice hull charcoal for adsorption of cesium and strontium in aqueous solution. Mater. Trans. 2019, 60, 458–463. [Google Scholar] [CrossRef]
- Chirkst, D.E.; Litvinova, T.E.; Cheremisina, O.V.; Ivanov, M.V.; Mironenkova, N.A. Isotherm of strontium sorption on clay. Russ. J. Appl. Chem. 2003, 76, 727–730. [Google Scholar] [CrossRef]
- Galambos, M.; Kufcakova, J.; Rajec, P. Sorption of strontium on Slovak bentonites. J. Radioanal. Nucl. Chem. 2009, 281, 347–357. [Google Scholar] [CrossRef]
- Kawamura, T.; Ito, T.; Kim, S.Y. Adsorption and separation behavior of strontium and yttrium using a silica-based CMPO adsorbent. J. Radioanal. Nucl. Chem. 2019, 320, 9–14. [Google Scholar] [CrossRef]
- Ma, J.G.; Zhang, Y.P.; Ouyang, J.X.; Wu, X.K.; Luo, J.Q.; Liu, S.J.; Gong, X. A facile preparation of dicyclohexano-18-crown-6 ether impregnated titanate nanotubes for strontium removal from acidic solution. Solid State Sci. 2019, 90, 49–55. [Google Scholar] [CrossRef]
- Strelko, V.V.; Mardanenko, V.K.; Yatsenko, V.V.; Patrilyak, N. Sorption of cesium and strontium on native vermiculite and vermiculite modified with copper ferrocyanide. Russ. J. Appl. Chem. 1998, 71, 1746–1749. [Google Scholar]
- Voronina, A.V.; Semenishchev, V.S. Mechanism of strontium sorption by the mixed nickel-potassium ferrocyanide based on hydrated titanium dioxide. J. Radioanal. Nucl. Chem. 2016, 307, 577–590. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Alginate and algal-based beads for the sorption of metal cations: Cu(II) and Pb(II). Int. J. Mol. Sci. 2016, 17, 1453. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hamza, M.F.; Vincent, T.; Faur, C.; Guibal, E. Praseodymium sorption on Laminaria digitata algal beads and foams. J. Colloid Interface Sci. 2017, 504, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Algal foams applied in fixed-bed process for lead(II) removal using recirculation or one-pass modes. Mar. Drugs 2017, 15, 315. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Vincent, T.; Roux, J.-C.; Faur, C.; Guibal, E. Innovative conditioning of algal-based sorbents: Macro-porous discs for palladium sorption. Chem. Eng. J. 2017, 325, 521–532. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Faur, C.; Rodriguez-Castellon, E.; Guibal, E. A new method for incorporating polyethyleneimine (PEI) in algal beads: High stability as sorbent for palladium recovery and supported catalyst for nitrophenol hydrogenation. Mater. Chem. Phys. 2019, 221, 144–155. [Google Scholar] [CrossRef]
- Rodriguez-Dorado, R.; Lopez-Iglesias, C.; Garcia-Gonzalez, C.A.; Auriemma, G.; Aquino, R.P.; Del Gaudio, P. Design of aerogels, cryogels and xerogels of alginate: Effect of molecular weight, gelation conditions and drying method on particles’ micromeritics. Molecules 2019, 24, 1049. [Google Scholar] [CrossRef]
- Djelad, A.; Morsli, A.; Robitzer, M.; Bengueddach, A.; di Renzo, F.; Quignard, F. Sorption of Cu(II) ions on chitosan-zeolite X composites: Impact of gelling and drying conditions. Molecules 2016, 21, 109. [Google Scholar] [CrossRef]
- Puspitasari, T.; Kadja, G.T.M.; Radiman, C.L.; Darwis, D.; Mukti, R.R. Two-step preparation of amidoxime-functionalized natural zeolites hybrids for the removal of Pb2+ ions in aqueous environment. Mater. Chem. Phys. 2018, 216, 197–205. [Google Scholar] [CrossRef]
- Hamza, M.F.; Roux, J.-C.; Guibal, E. Uranium and europium sorption on amidoxime-functionalized magnetic chitosan micro-particles. Chem. Eng. J. 2018, 344, 124–137. [Google Scholar] [CrossRef]
- Hamza, M.F.; Aly, M.M.; Abdel-Rahman, A.A.H.; Ramadan, S.; Raslan, H.; Wang, S.; Vincent, T.; Guibal, E. Functionalization of magnetic chitosan particles for the sorption of U(VI), Cu(II) and Zn(II)—Hydrazide derivative of glycine-grafted chitosan. Materials 2017, 10, 539. [Google Scholar] [CrossRef]
- Ojha, N.; Das, N. A statistical approach to optimize the production of polyhydroxyalkanoates from Wickerhamomyces anomalus VIT-NN01 using response surface methodology. Int. J. Biol. Macromol. 2018, 107, 2157–2170. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, T.L.; Hamer, G.K.; Marchessault, R.H.; Fyfe, C.A.; Veregin, R.P. Isodimorphism in bacterial poly(β-hydroxybutyrate-co-β-hydroxyvalerate). Macromolecules 1986, 19, 2871–2876. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Mira, H.I.; Abdel-Rahman, A.A.H.; Guibal, E. Synthesis and adsorption characteristics of grafted hydrazinyl amine magnetite-chitosan for Ni(II) and Pb(II) recovery. Chem. Eng. J. 2019, 362, 310–324. [Google Scholar] [CrossRef]
- Membere, E.; Sails, P. Thermochemical characterization of brown seaweed, Laminaria digitata from UK shores. J. Anal. Appl. Pyrolysis 2018, 131, 42–51. [Google Scholar] [CrossRef]
- Sindhu, R.; Ammu, B.; Binod, P.; Deepthi, S.K.; Ramachandran, K.B.; Soccol, C.R.; Pandey, A. Production and characterization of poly-3-hydroxybutyrate from crude glycerol by Bacillus sphaericus NII 0838 and improving Its thermal properties by blending with other polymers. Braz. Arch. Biol. Technol. 2011, 54, 783–794. [Google Scholar] [CrossRef]
- Lu, S.; Chen, L.; Hamza, M.F.; He, C.; Wang, X.; Wei, Y.; Guibal, E. Amidoxime functionalization of a poly(acrylonitrile)/silica composite for the sorption of Ga(III)—Application to the treatment of Bayer liquor. Chem. Eng. J. 2019, 368, 459–473. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Y.; Na, S.; Yan, F. Selective adsorption of lead(II) from aqueous solution by ion-imprinted PEI-functionalized silica sorbent: Studies on equilibrium isotherm, kinetics, and thermodynamics. Desalin. Water Treat. 2016, 57, 3270–3282. [Google Scholar] [CrossRef]
- Sahiner, N.; Demirci, S.; Sahiner, M.; Al-Lohedan, H. The synthesis of desired functional groups on PEI microgel particles for biomedical and environmental applications. Appl. Surf. Sci. 2015, 354, 380–387. [Google Scholar] [CrossRef]
- Anastasakis, K.; Ross, A.B.; Jones, J.M. Pyrolysis behaviour of the main carbohydrates of brown macro-algae. Fuel 2011, 90, 598–607. [Google Scholar] [CrossRef]
- Rahman, M.L.; Sarjadi, M.S.; Arshad, S.E.; Yusoff, M.M.; Sarkar, S.M.; Musta, B. Kenaf cellulose-based poly(amidoxime) ligand for adsorption of rare earth ions. Rare Met. 2019, 38, 259–269. [Google Scholar] [CrossRef]
- Jurado-Lopez, B.; Vieira, R.S.; Rabelo, R.B.; Beppu, M.M.; Casado, J.; Rodriguez-Castellon, E. Formation of complexes between functionalized chitosan membranes and copper: A study by angle resolved XPS. Mater. Chem. Phys. 2017, 185, 152–161. [Google Scholar] [CrossRef]
- Sun, Y.B.; Wang, X.X.; Ding, C.C.; Cheng, W.C.; Chen, C.L.; Hayat, T.; Alsaedi, A.; Hu, J.; Wang, X.K. Direct synthesis of bacteria-derived carbonaceous nanofibers as a highly efficient material for radionuclides elimination. ACS Sustain. Chem. Eng. 2016, 4, 4608–4616. [Google Scholar] [CrossRef]
- Wang, F.; Li, H.; Liu, Q.; Li, Z.; Li, R.; Zhang, H.; Liu, L.; Emelchenko, G.A.; Wang, J. A graphene oxide/amidoxime hydrogel for enhanced uranium capture. Sci. Rep. 2016, 6, 19367. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.O.; Rossi, A.L.; Archanjo, B.S.; Ospina, R.O.; Mello, A.; Rossi, A.M. Crystalline nano-coatings of fluorine-substituted hydroxyapatite produced by magnetron sputtering with high plasma confinement. Surf. Coat. Technol. 2015, 264, 163–174. [Google Scholar] [CrossRef]
- Yang, K.; Zhong, L.; Guan, R.; Xiao, M.; Han, D.; Wang, S.; Meng, Y. Carbon felt interlayer derived from rice paper and its synergistic encapsulation of polysulfides for lithium-sulfur batteries. Appl. Surf. Sci. 2018, 441, 914–922. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Si, R.; Liao, C.S.; Yan, C.H. Facile alcohothermal synthesis, size-dependent ultraviolet absorption, and enhanced CO conversion activity of ceria nanocrystals. J. Phys. Chem. B 2003, 107, 10159–10167. [Google Scholar] [CrossRef]
- Tas, S.; Kaynan, O.; Ozden-Yenigun, E.; Nijmeijer, K. Polyacrylonitrile (PAN)/crown ether composite nanofibers for the selective adsorption of cations. RSC Adv. 2016, 6, 3608–3616. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Jayaweera, P.M.; Grassian, V.H. XPS study of nitrogen dioxide adsorption on metal oxide particle surfaces under different environmental conditions. Phys. Chem. Chem. Phys. 2009, 11, 8295–8305. [Google Scholar] [CrossRef]
- Haug, A. Dissociation of alginic acid. Acta Chem. Scand. 1961, 15, 950–952. [Google Scholar] [CrossRef]
- Demadis, K.D.; Paspalaki, M.; Theodorou, J. Controlled release of bis(phosphonate) pharmaceuticals from cationic biodegradable polymeric matrices. Ind. Eng. Chem. Res. 2011, 50, 5873–5876. [Google Scholar] [CrossRef]
- Das, S.; Pandey, A.K.; Athawale, A.A.; Manchanda, V.K. Exchanges of uranium(VI) species in amidoxime-functionalized sorbents. J. Phys. Chem. B 2009, 113, 6328–6335. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Jing, Y.; Wang, X.Q.; Yao, Y.; Jia, Y.Z. Preconcentration of uranium(VI) from aqueous solution by amidoxime-functionalized microspheres silica material: Kinetics, isotherm and mechanism study. Chemistryselect 2018, 3, 12346–12356. [Google Scholar] [CrossRef]
- Piechowicz, M.; Chiarizia, R.; Soderholm, L. Insight into selectivity: Uptake studies of radionuclides 90Sr2+, 137Cs+, and 233UO22+ with bis-amidoxime polymers. Dalton Trans. 2018, 47, 5348–5358. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Mahfouz, M.G.; Abdel-Rahman, A.A.H. Adsorption of uranium (VI) ions on hydrazinyl amine and 1,3,4-thiadiazol-2(3 H)-thion chelating resins. J. Dispers. Sci. Technol. 2012, 33, 1544–1551. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Barzegar, S.; Zeidabadi, F. Synthesis and properties of biodegradable hydrogels of kappa-carrageenan grafted acrylic acid-co-2-acrylamido-2-methylpropanesulfonic acid as candidates for drug delivery systems. React. Funct. Polym. 2007, 67, 644–654. [Google Scholar] [CrossRef]
- Rahman, M.L.; Sarkar, S.M.; Farid, E.M.; Arshad, S.E.; Sarjadi, M.S.; Wid, N. Synthesis of tapioca cellulose-based poly(amidoxime) ligand for removal of heavy metal ions. J. Macromol. Sci. Part B Phys. 2018, 57, 83–99. [Google Scholar] [CrossRef]
- Shaaban, A.F.; Mohamed, T.Y.; Fadel, D.A.; Bayomi, N.M. Removal of Ba(II) and Sr(II) ions using modified chitosan beads with pendent amidoxime moieties by batch and fixed bed column methods. Desalin. Water Treat. 2017, 82, 131–145. [Google Scholar] [CrossRef]
- Dragan, E.S.; Loghin, D.F.A.; Cocarta, A.I. Efficient sorption of Cu2+ by composite chelating sorbents based on potato starch-graft-polyamidoxime embedded in chitosan beads. ACS Appl. Mater. Interfaces 2014, 6, 16577–16592. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Divya, L.; Bringle, C.D.; Suchithra, P.S. Removal of copper(II) and zinc(II) from aqueous solutions using a lignocellulosic-based polymeric adsorbent containing amidoxime chelating functional groups. Sep. Sci. Technol. 2010, 45, 2383–2393. [Google Scholar] [CrossRef]
- Marcus, Y. Ion Properties; Marcel Dekker, Inc.: New York, NY, USA, 1997; p. 259. [Google Scholar]
- Hakimifar, A.; Morsali, A. Urea-based metal-organic frameworks as high and fast adsorbent for Hg2+ and Pb2+ removal from water. Inorg. Chem. 2019, 58, 180–187. [Google Scholar] [CrossRef]
- Wang, K.T.; Wang, F.; Chen, F.; Cui, X.M.; Wei, Y.Z.; Shao, L.; Yu, M.H. One-pot preparation of NaA zeolite microspheres for highly selective and continuous removal of Sr(II) from aqueous solution. ACS Sustain. Chem. Eng. 2019, 7, 2459–2470. [Google Scholar] [CrossRef]
- Chu, Y.Y.; Wang, R.; Chen, M.J. Preliminary study on removing Cs+/Sr2+ by activated porous calcium silicate-A by-product from high-alumina fly ash recycling industry. J. Air Waste Manag. Assoc. 2015, 65, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Cakir, P.; Inan, S.; Altas, Y. Investigation of strontium and uranium sorption onto zirconium-antimony oxide/polyacrylonitrile (Zr-Sb oxide/PAN) composite using experimental design. J. Hazard. Mater. 2014, 271, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Kaczvinsky, J.R.; Fritz, J.S.; Walker, D.D.; Ebra, M.A. The effects of reaction conditions on porous chelating polymers designed for the decontamination of nuclear waste. J. Radioanal. Nucl. Chem. Art. 1987, 116, 63–75. [Google Scholar] [CrossRef]
- Gujar, R.B.; Ansari, S.A.; Verboom, W.; Mohapatra, P.K. Multi-podant diglycolamides and room temperature ionic liquid impregnated resins: An excellent combination for extraction chromatography of actinides. J. Chromatogr. A 2016, 1448, 58–66. [Google Scholar] [CrossRef]
- Zhang, A.; Wei, Y.Z.; Kumagai, M.; Koyama, T. Kinetics of the adsorption of strontium(II) by a novel silica-based 4,4′,(5′)-di(tert-butylcyclohexano)-18-crown-6 extraction resin in nitric acid medium. J. Radioanal. Nucl. Chem. 2004, 262, 739–744. [Google Scholar] [CrossRef]
- Li, T.T.; He, F.; Dai, Y.D. Prussian blue analog caged in chitosan surface-decorated carbon nanotubes for removal cesium and strontium. J. Radioanal. Nucl. Chem. 2016, 310, 1139–1145. [Google Scholar] [CrossRef]
- Maranescu, B.; Popa, A.; Lupa, L.; Maranescu, V.; Visa, A. Use of chitosan complex with aminophosphonic groups and cobalt for the removal of Sr2+ ions. Sep. Sci. Technol. 2018, 53, 1058–1064. [Google Scholar] [CrossRef]
- Cheng, R.; Kang, M.; Zhuang, S.T.; Shi, L.; Zheng, X.; Wang, J.L. Adsorption of Sr(II) from water by mercerized bacterial cellulose membrane modified with EDTA. J. Hazard. Mater. 2019, 364, 645–653. [Google Scholar] [CrossRef]
- Chen, Y.W.; Wang, J.L. Removal of radionuclide Sr2+ ions from aqueous solution using synthesized magnetic chitosan beads. Nucl. Eng. Des. 2012, 242, 445–451. [Google Scholar] [CrossRef]
- Hong, H.J.; Ryu, J.; Park, I.S.; Ryu, T.; Chung, K.S.; Kim, B.G. Investigation of the strontium (Sr(II)) adsorption of an alginate microsphere as a low-cost adsorbent for removal and recovery from seawater. J. Environ. Manag. 2016, 165, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Li, K.; Li, M.; Xue, D. Solution-phase electronegativity scale: Insight into the chemical behaviors of metal ions in solution. J. Phys. Chem. A 2012, 116, 4192–4198. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Acids and bases. Science 1966, 151, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tu, Z.; Lu, G.; Duan, X.; Yi, X.; Guo, C.; Dang, Z. Removal of heavy metals from acid mine drainage using chicken eggshells in column mode. J. Environ. Manag. 2017, 188, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Angino, E.E.; Billings, G.K.; Andersen, N. Observed variations in the strontium concentration of sea water. Chem. Geol. 1966, 1, 145–153. [Google Scholar] [CrossRef]
- Kirishima, A.; Sasaki, T.; Sato, N. Solution chemistry study of radioactive Sr on Fukushima Daiichi NPS site. J. Nucl. Sci. Technol. 2015, 52, 152–161. [Google Scholar] [CrossRef]
- Hirose, K. Chemical speciation of trace metals in seawater: A review. Anal. Sci. 2006, 22, 1055–1063. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.A.H.; El-Aassy, I.E.E.; Fadia, Y.A.; Hamza, M.F. Studies on the uptake of rare earth elements on polyacrylamidoxime resins from natural concentrate leachate solutions. J. Dispers. Sci. Technol. 2010, 31, 1128–1135. [Google Scholar]
- Atta, A.M.; Abdel-Rahman, A.A.H.; El Aassy, I.E.; Ahmed, F.Y.; Hamza, M.F. Adsorption properties of uranium (VI) ions on reactive crosslinked acrylamidoxime and acrylic acid copolymer resins. J. Dispers. Sci. Technol. 2011, 32, 84–94. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Guibal, E. Quaternization of algal/PEI beads (a new sorbent): Characterization and application to scandium recovery from aqueous solutions. Chem. Eng. J. 2019, in press. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Model | Parameter | 1st Series | 2nd Series |
|---|---|---|---|
| Experiment | qeq,exp. (mg Sr g−1) | 74.6 | 77.7 |
| PFORE | qeq,1 (mg Sr g−1) | 76.4 | 79.2 |
| k1 × 102 (min−1) | 3.27 | 3.44 | |
| R2 | 0.973 | 0.981 | |
| PSORE | qeq,2 (mg Sr g−1) | 89.2 | 91.8 |
| k2 × 104 (L mg−1 min−1) | 4.30 | 4.53 | |
| R2 | 0.957 | 0.969 | |
| RIDE | De × 109 (m2 min−1) | 6.7 | 7.0 |
| R2 | 0.962 | 0.975 |
| Model | R2 | Parameter | AO-APEI sorbent |
|---|---|---|---|
| Experiment | qm,exp. (mg Sr g−1) | 189.3 | |
| Langmuir | 0.988 | qm,L (mg Sr g−1) | 206.9 |
| bL × 102 (L mg−1) | 2.29 | ||
| Freundlich | 0.971 | KF | 41.2 |
| nF | 3.89 | ||
| Sips | 0.977 | qm,S (mg Sr g−1) | 384.1 |
| bS × 102 (L mg−1) | 8.81 | ||
| nS | 2.48 |
| Sorbent | pH0 | Time (min) | qm (mg Sr g−1) | b×102 (L mg−1) | Reference |
|---|---|---|---|---|---|
| Clay | 3.4 * | 300 | 2.98 | 0.336 | [26] |
| Bentonite | 6 | 120 | 38.6 | − | [27] |
| Zeolite microsphere | 6 | 20 | 109.89 | 15.72 | [73] |
| Activated porous calcium silicate | 7 | 120 | 142.3 | - | [74] |
| Oxidized Multiwall CNTs | 7 | 100 | 6.62 | 0.608 | [22] |
| Moss | 6 | 240 | 13.1 | 5.71 | [7] |
| Zr-Sb oxide/PAN | 4.74 | 270 | 43.67 | 84.0 | [75] |
| Resorcinol-formaldehyde-iminodiacetic acid resin | 9 | 1200 | 188.4 | - | [76] |
| Impregnated resin | 1 M HNO3 | 120 | 4.83 | 5.8 | [77] |
| Extractant impregnated silica/polymer resin | 2 M HNO3 | 360 | 454.5 | 0.013 | [78] |
| Ni-K Ferrocyanide/TiO2 | 7.8 | 180 | 143 | 0.61 | [31] |
| Prussian blue analog on chitosan/CNT | 6 | 240 | 205.1 | 0.46 | [79] |
| Amidoxime chitosan beads | 8.5 | 180 | 153 | - | [68] |
| Aminophosphonic chitosan/Co(II) | 6 | 60 | 3.41 | 89.0 | [80] |
| Saccharomyces cerevisiae | 3–4 ** | 1800 | 37.4 | 3.51 | [16] |
| Bacteria-derived carbonaceous nanofibers | 4.5 | 1440 | 67.11 | 44.7 | [53] |
| EDTA-mercerized bacterial cellulose membrane | 6.0 | 600 | 44.86 | 8.5 | [81] |
| Magnetic chitosan beads | 8.2 | 360 | 11.58 | - | [82] |
| Alginate beads | 6 | 1440 | 111.1 | 9.4 | [83] |
| AO-APEI | 6.0 | 90 | 189 | 2.29 | This work |
| Sorption Efficiency (%) | Desorption Efficiency (%) | |||
|---|---|---|---|---|
| Cycle # | Aver. | St. Dev. | Aver. | St. Dev. |
| 1 | 60.70 | 0.14 | 96.49 | 0.79 |
| 2 | 60.10 | 0.76 | 99.16 | 1.27 |
| 3 | 59.05 | 0.56 | 95.09 | 2.29 |
| 4 | 57.66 | 0.27 | 91.85 | 4.49 |
| 5 | 56.29 | 0.26 | 91.66 | 1.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Salih, K.A.M.; Lu, S.; Hamza, M.F.; Fujita, T.; Vincent, T.; Guibal, E. Amidoxime Functionalization of Algal/Polyethyleneimine Beads for the Sorption of Sr(II) from Aqueous Solutions. Molecules 2019, 24, 3893. https://doi.org/10.3390/molecules24213893
Wei Y, Salih KAM, Lu S, Hamza MF, Fujita T, Vincent T, Guibal E. Amidoxime Functionalization of Algal/Polyethyleneimine Beads for the Sorption of Sr(II) from Aqueous Solutions. Molecules. 2019; 24(21):3893. https://doi.org/10.3390/molecules24213893
Chicago/Turabian StyleWei, Yuezhou, Khalid A. M. Salih, Siming Lu, Mohammed F. Hamza, Toyohisa Fujita, Thierry Vincent, and Eric Guibal. 2019. "Amidoxime Functionalization of Algal/Polyethyleneimine Beads for the Sorption of Sr(II) from Aqueous Solutions" Molecules 24, no. 21: 3893. https://doi.org/10.3390/molecules24213893
APA StyleWei, Y., Salih, K. A. M., Lu, S., Hamza, M. F., Fujita, T., Vincent, T., & Guibal, E. (2019). Amidoxime Functionalization of Algal/Polyethyleneimine Beads for the Sorption of Sr(II) from Aqueous Solutions. Molecules, 24(21), 3893. https://doi.org/10.3390/molecules24213893