Synthesis and Characterization of N-Methyl Fatty Hydroxamic Acids from Ketapang Seed Oil Catalyzed by Lipase
Abstract
:1. Introduction
2. Results and Discussion
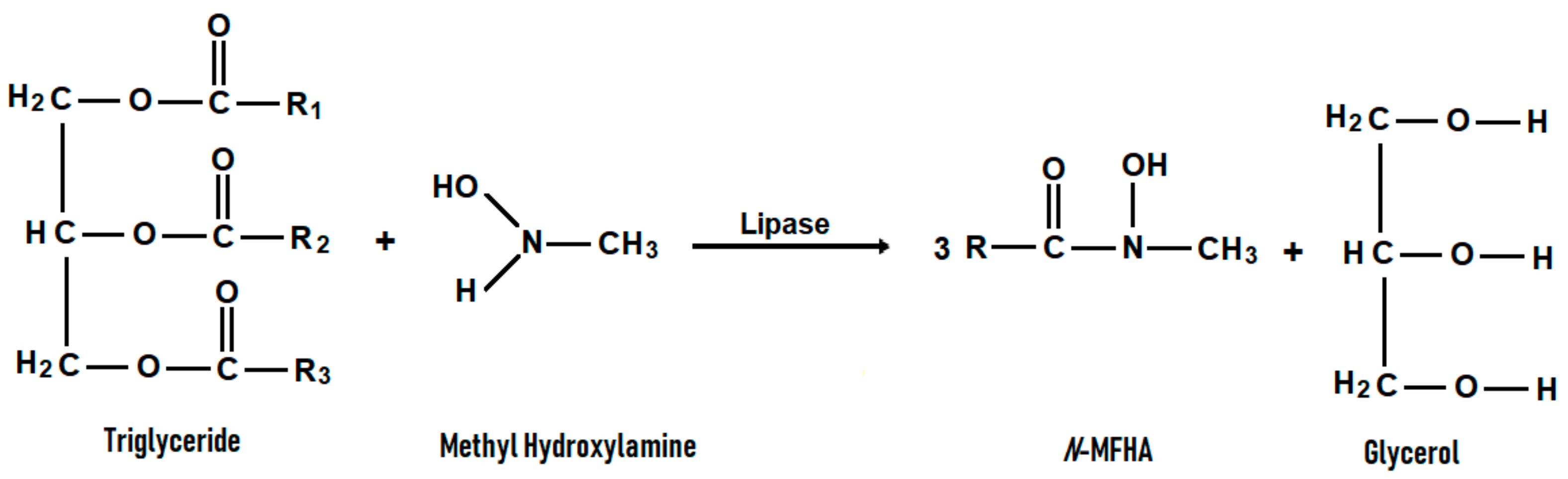
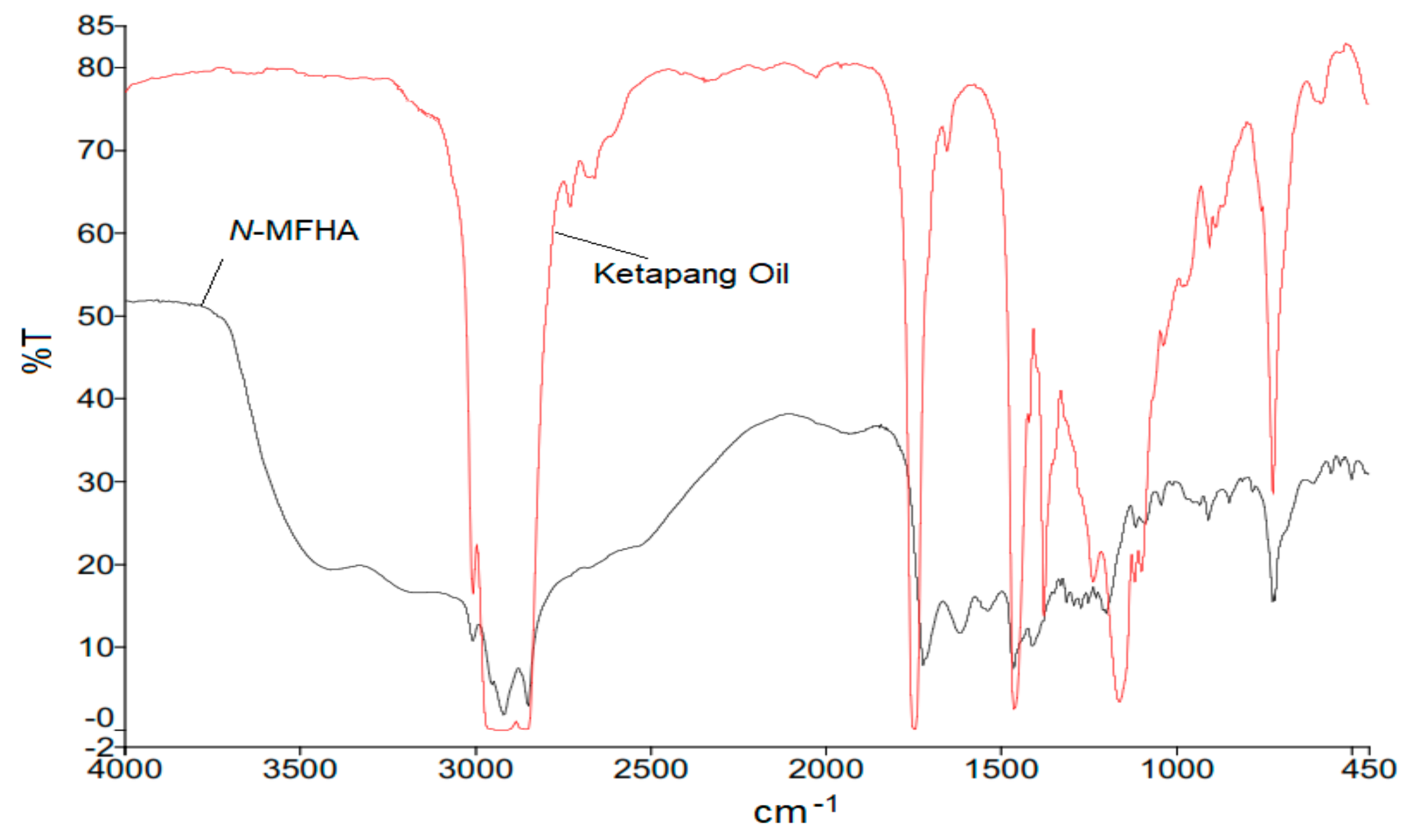
2.1. Identification of the Product
2.2. Optimization of the Reaction Conditions
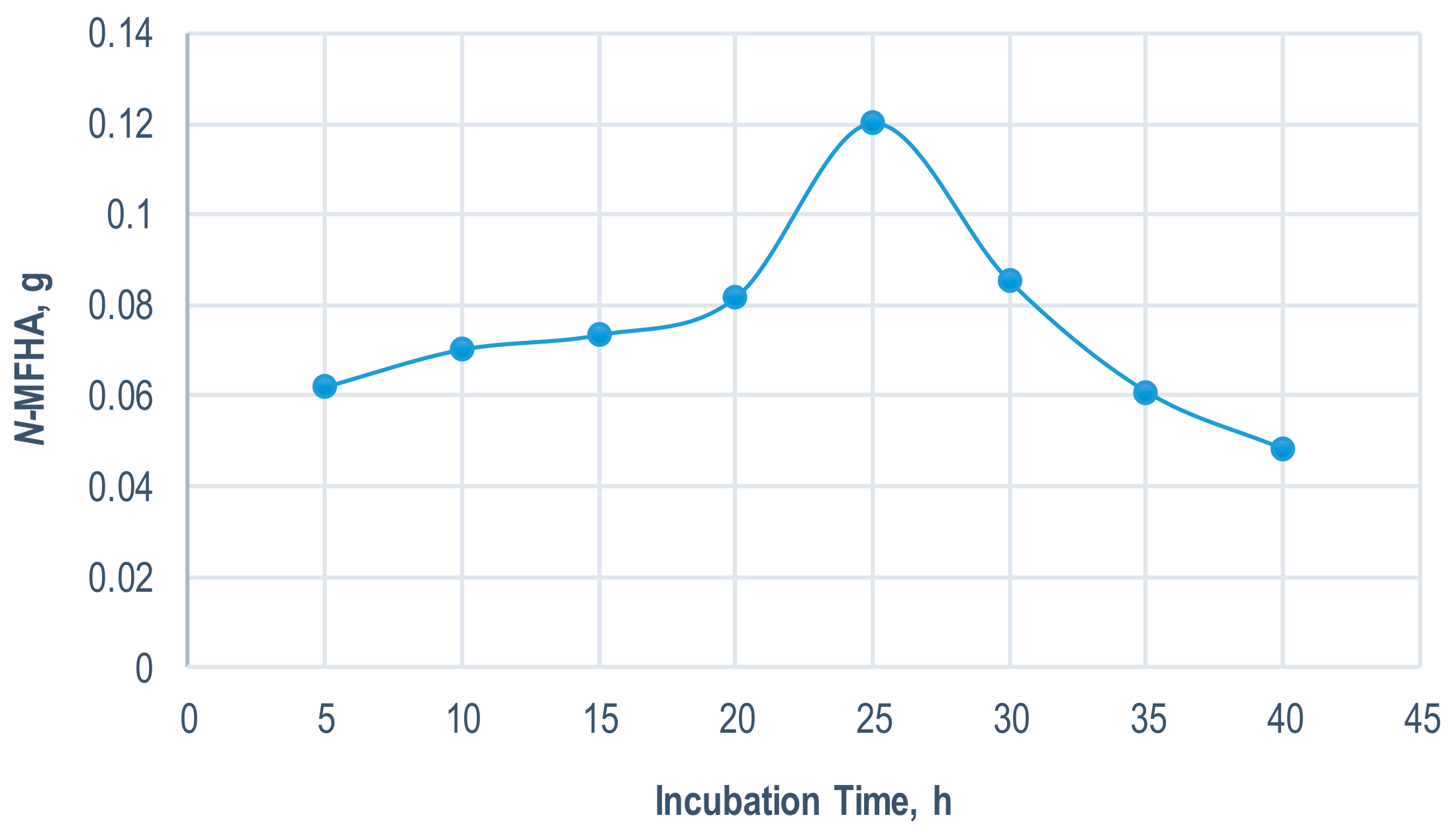
2.2.1. Effect of Incubation Time
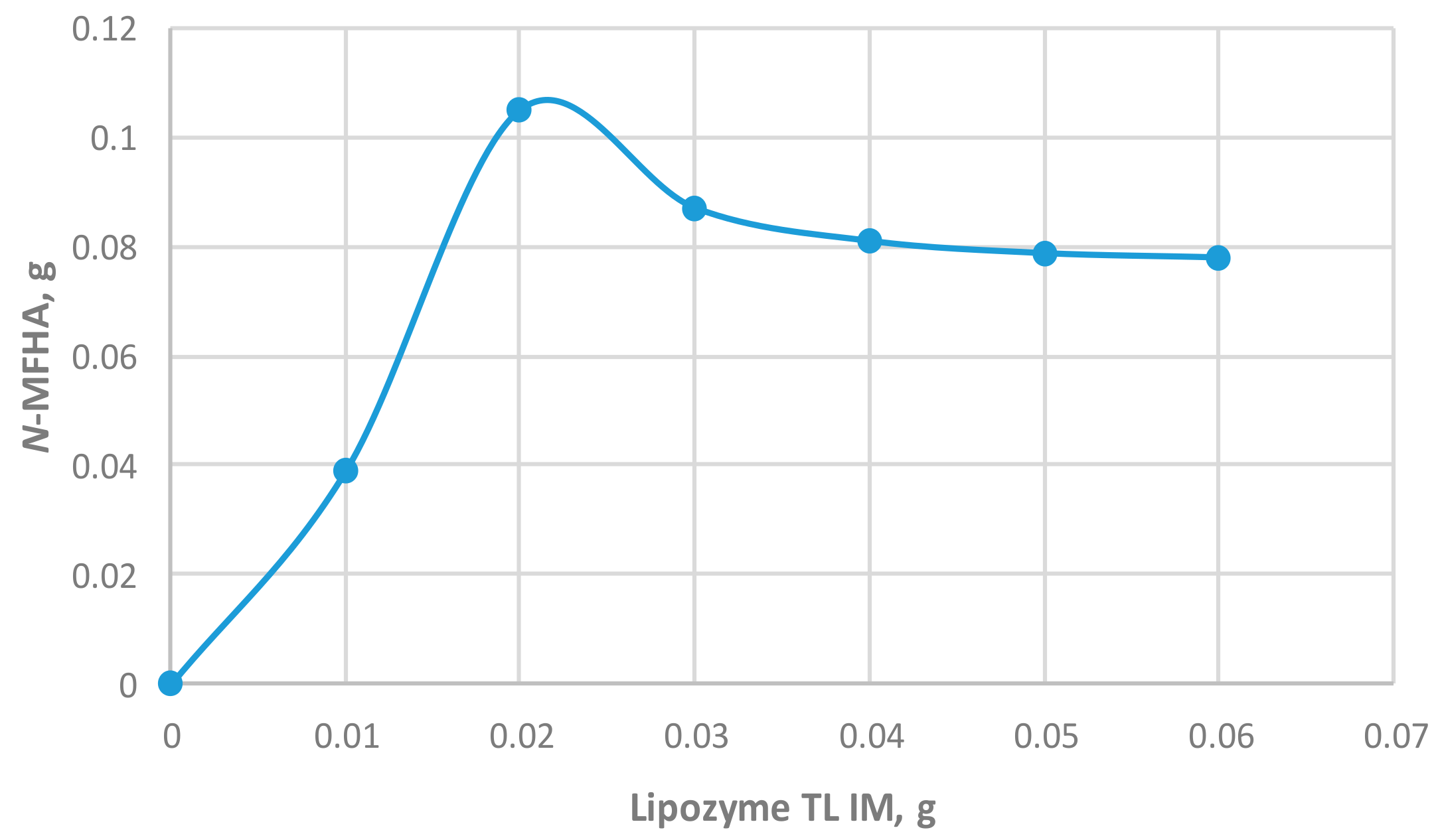
2.2.2. Effect of Amount of Immobilized Lipase
2.2.3. Effect of Temperature
2.3. Synthesis Using the Optimum Reaction Conditions
3. Material and Methods
3.1. Material
3.2. Extraction of Ketapang Seed Oil
3.3. Synthesis of N-MFHA
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kakkar, R. Theoretical Studies on Hydroxamic Acids. In Hydroxamic Acids: A Unique Family of Chemicals with Multiple Biological Activities; Gupta, S.P., Ed.; Springer Heidelberg: New York, NY, USA, 2013; pp. 19–53. [Google Scholar]
- Xu, H.; Zhong, H.; Tang, Q.; Wang, S.; Zhao, G.; Liu, G. A novel collector 2-ethyl-2-hexenoic hydroxamic acid: Flotation performance and adsorption mechanism to ilmenite. Appl. Surf. Sci. 2015, 353, 882–889. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Yang, J.K.; Choi, H.R.; Park, K.C.; Lee, Y.S.; Kim, Y.B. Synthesis and dual biological effects of hydroxycinnamoyl phenylalanyl/prolyl hydroxamic acid derivatives as tyrosinase inhibitor and antioxidant. Bioorg. Med. Chem. Lett. 2013, 23, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Giannini, G.; Battistuzzi, G.; Vignola, D. Hydroxamic Acid Based Histone Deacetylase Inhibitors With Confirmed Activity Against The Malaria Parasite. Bioorg. Med. Chem. 2015, 25, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.S.T.; Gao, A.; Yang, W.; Sheng, L.; Zang, Y.; Li, J.; Liu, H. Design, Synthesis and Biological Evaluation of Thienopyrimidine Hydroxamic Acid Based Derivatives as Structurally Novel Histone Deacetylase (HDAC) Inhibitors. Eur. J. Med. Chem. 2017, 128, 293–299. [Google Scholar] [CrossRef] [PubMed]
- De Vreese, R.; D’hooghe, M. Synthesis and Applications of Benzohydroxamic Acid-Based Histone Deacetylase Inhibitors. Eur. J. Med. Chem. 2017, 135, 174–195. [Google Scholar] [CrossRef]
- Luckhurst, C.A.; Maillard, M.C.; Aziz, O.; Beaumont, V.; Bürli, R.W.; Breccia, P.; Raphy, G.; Haughan, A.F.; Lamers, M.; Leonard, P.; et al. Development and characterization of a CNS-penetrant benzhydryl hydroxamic acid class IIa histone deacetylase inhibitor. Bioorg. Med. Chem. Lett. 2019, 29, 83–88. [Google Scholar] [CrossRef]
- Stranix, B.R.; Wu, J.J.; Milot, G.; Beaulieu, F.; Bouchard, J.E.; Gouveia, K.; Forte, A.; Garde, S.; Wang, Z.; Mouscadet, J.F.; et al. Pyridoxine Hydroxamic Acids As Novel Hiv-Integrase Inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 1233–1236. [Google Scholar] [CrossRef]
- Nam, N.H.; Huong, T.L.; Dung, D.T.M.; Dung, P.T.P.; Oanh, D.T.K.; Quyen, D.; Thao, L.T.; Park, S.H.; Kim, K.R.; Han, B.W.; et al. Novel Isatin-Based Hydroxamic Acids as Histone Deacetylase Inhibitors and Antitumor Agents. Eur. J. Med. Chem. 2013, 70, 477–486. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Wu, J.; Zhou, Q.; Wu, S. Synthesis and Biological Activity of Salinomycin-Hydroxamic Acid Conjugates. Bioorg. Med. Chem. Lett. 2017, 27, 1624–1626. [Google Scholar] [CrossRef]
- Zhang, W.; Pranolo, Y.; Urbani, M.; Cheng, C.Y. Extraction and Separation of Nickel and Cobalt with Hydroxamic Acids LIX®1104, LIX®1104SM and The Mixture of LIX®1104 and Versatic 10. Hydrometallurgy 2012, 119, 67–72. [Google Scholar] [CrossRef]
- Majewski, M.W.; Cho, S.; Miller, P.A.; Franzblau, S.G.; Miller, M.J. Syntheses and evaluation of substituted aromatic hydroxamates and hydroxamic acids that target mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 2015, 25, 4933–4936. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.; Qiu, L.; Xie, J.; Geraghty, R.J.; Chen, L. Design and synthesis of an activity-based protein profiling probe derived from cinnamic hydroxamic acid. Bioorg. Med. Chem. 2016, 24, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Phillips, O.A.; D’Silva, R.; Bahta, T.O.; Sharaf, L.H.; Udo, E.E.; Benov, L.; Walters, D.E.; Sharaf, L.H. Synthesis and Biological Evaluation of Novel 5-(hydroxamic acid) Methyl Oxazolidinone Derivatives. Eur. J. Med. Chem. 2015, 106, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Pacco, A.; Absillis, G.; Binnemans, K.; Parac-Vogt, T.N. Copper (II) 15-Metallacrown-5 Lanthanide (III) Complexes Derived from l-serine and l-threonine Hydroxamic Acids. J. Alloys Compd. 2008, 451, 38–41. [Google Scholar] [CrossRef]
- Isha, A.; Suhendra, D.; Yusof, N.A.; Ahmad, M.; Wan Yunus, W.M.Z.; Zainal, Z. Optical Fiber Chemical Sensor for Trace Vanadium(V) Determination Based On Newly Synthesized Palm Based Fatty Hydroxamic Acid Immobilized in Polyvinyl Chloride Membrane. Spectrochim. Acta A 2007, 67, 1398–1402. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Sivapathan, S.; Saratale, G.D.; Banu, J.R.; Kim, D. Hydroxamic acid mediated heterogeneous Fenton-like catalysts for the efficient removal of Acid Red 88, textile wastewater and their phytotoxicity studies. Ecotoxicol. Environ. Saf. 2019, 167, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, E.R.; Suhendra, D.; Hidayat, I.; Kurniawati, L. Optimization of Alkyldiethanolamides Synthesis from Terminalia catappa L. Kernel Oil through Enzymatic Reaction. J. Oleo Sci. 2018, 67, 949–955. [Google Scholar] [CrossRef]
- Servat, F.; Montet, D.; Pina, M.; Gazly, P.; Arnaud, A.; Ledon, H.; Marcau, L.; Graillie, J. Synthesis of Fatty Hydroxamic Acids Catalyzed by The Lipase of Mucor Meihei. JOACS 1990, 67, 646–649. [Google Scholar]
- Suhendra, D.; Haron, M.J.; Silong, S.; Basri, M.; Wan Yunus, W.M.Z. Enzymatic Synthesis of Fatty Hydroxamic Acids from Palm Oil. J. Oleo Sci. 2005, 54, 33–38. [Google Scholar] [CrossRef]
- Suhendra, D.; Gunawan, E.R.; Yuanita, E.; Nazili, M. Optimization of Lipase-catalyzed Synthesis of Fatty Hydroxamic acids from Terminalia catappa L. Kernel oil. Orient. J. Chem. 2018, 34, 2370–2377. [Google Scholar] [CrossRef]
- Salwanee, S.; Wan Aida, W.M.; Mamot, S.; Maskat, M.Y.; Ibrahim, S. Effects of Enzyme Concentration, Temperature, pH and Time on the Degree of Hydrolysis of Protein Extract from Viscera of Tuna (Euthynnus affinis) by Using Alcalase. Sains Malaysiana 2013, 42, 279–287. [Google Scholar]
- Gunawan, E.R.; Suhendra, D. Four-Factor Response Surface Optimization of the Enzymatic Synthesis of Wax Ester from Palm Kernel Oil. Indones. J. Chem. 2008, 8, 83–90. [Google Scholar] [CrossRef]
- Jamil, N.; Che Man, R.; Suhaimi, S.; Shaarani, S.M.; Arshad, Z.I.M.; Mudalip, S.K.A.; Sulaiman, S.Z. Effect of enzyme concentration and temperature on the immobilization of cyclodextrin glucanotransferase (CGTase) on hollow fiber membrane. Mater. Today Proc. 2018, 5, 22036–22042. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Parameters | Condition |
|---|---|
| Immobilized lipase | Lipozyme TL IM |
| Solvent | Hexane |
| Ratio of oil (g) to N-methylhydroxylamine (mmol) | 1:5 |
| Temperature | 40 °C |
| Incubation time | 25 h |
| Ratio of lipozyme TL IM (g) to oil (g) | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhendra, D.; Ryantin Gunawan, E.; Hajidi, H. Synthesis and Characterization of N-Methyl Fatty Hydroxamic Acids from Ketapang Seed Oil Catalyzed by Lipase. Molecules 2019, 24, 3895. https://doi.org/10.3390/molecules24213895
Suhendra D, Ryantin Gunawan E, Hajidi H. Synthesis and Characterization of N-Methyl Fatty Hydroxamic Acids from Ketapang Seed Oil Catalyzed by Lipase. Molecules. 2019; 24(21):3895. https://doi.org/10.3390/molecules24213895
Chicago/Turabian StyleSuhendra, Dedy, Erin Ryantin Gunawan, and Hajidi Hajidi. 2019. "Synthesis and Characterization of N-Methyl Fatty Hydroxamic Acids from Ketapang Seed Oil Catalyzed by Lipase" Molecules 24, no. 21: 3895. https://doi.org/10.3390/molecules24213895





