A Comparative Characterization of Physicochemical and Antioxidants Properties of Processed Heterotrigona itama Honey from Different Origins and Classification by Chemometrics Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
2.2. Amino Acid Profile
2.3. Antioxidant Properties
2.4. Correlation Coefficients
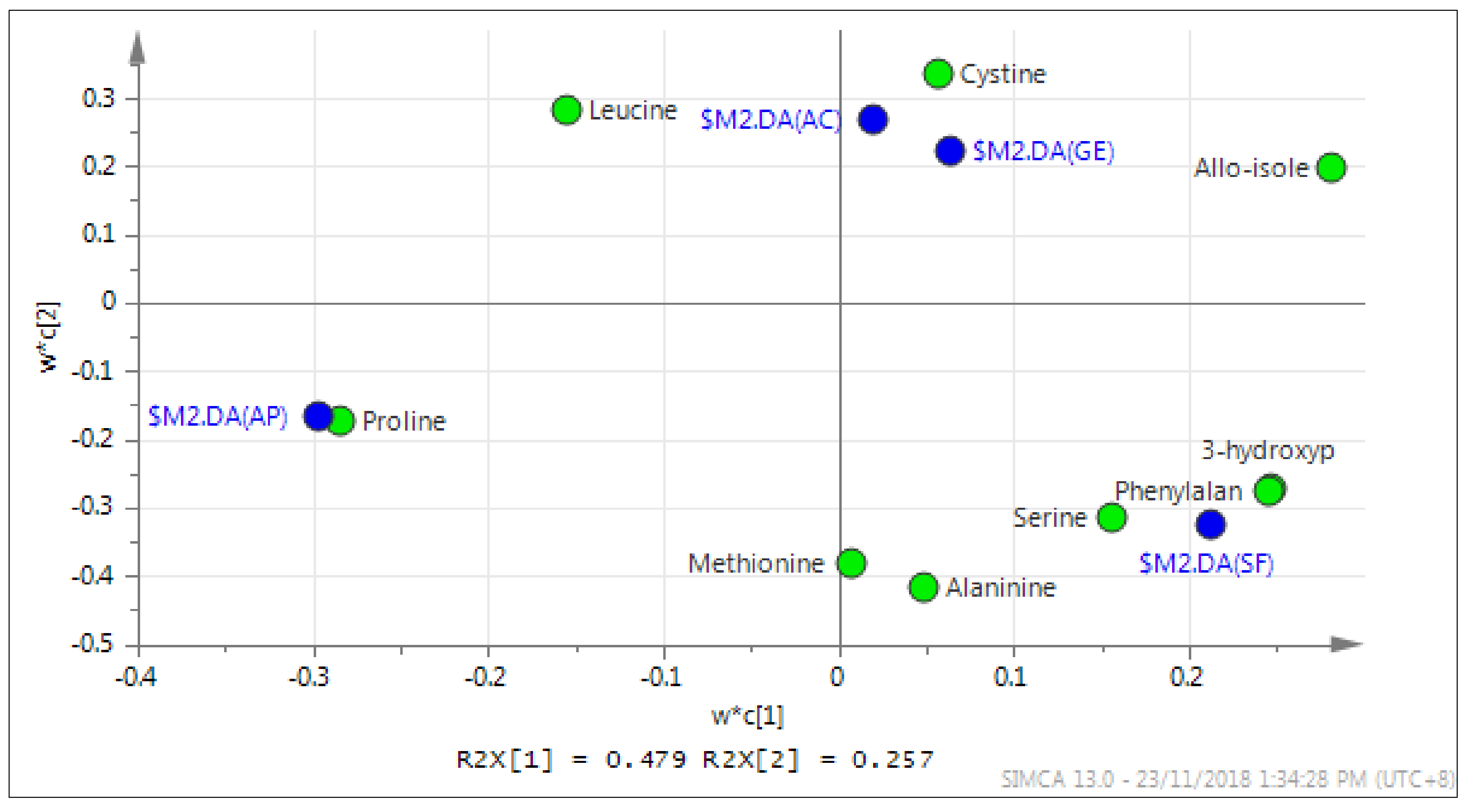
2.5. Chemometric Analysis
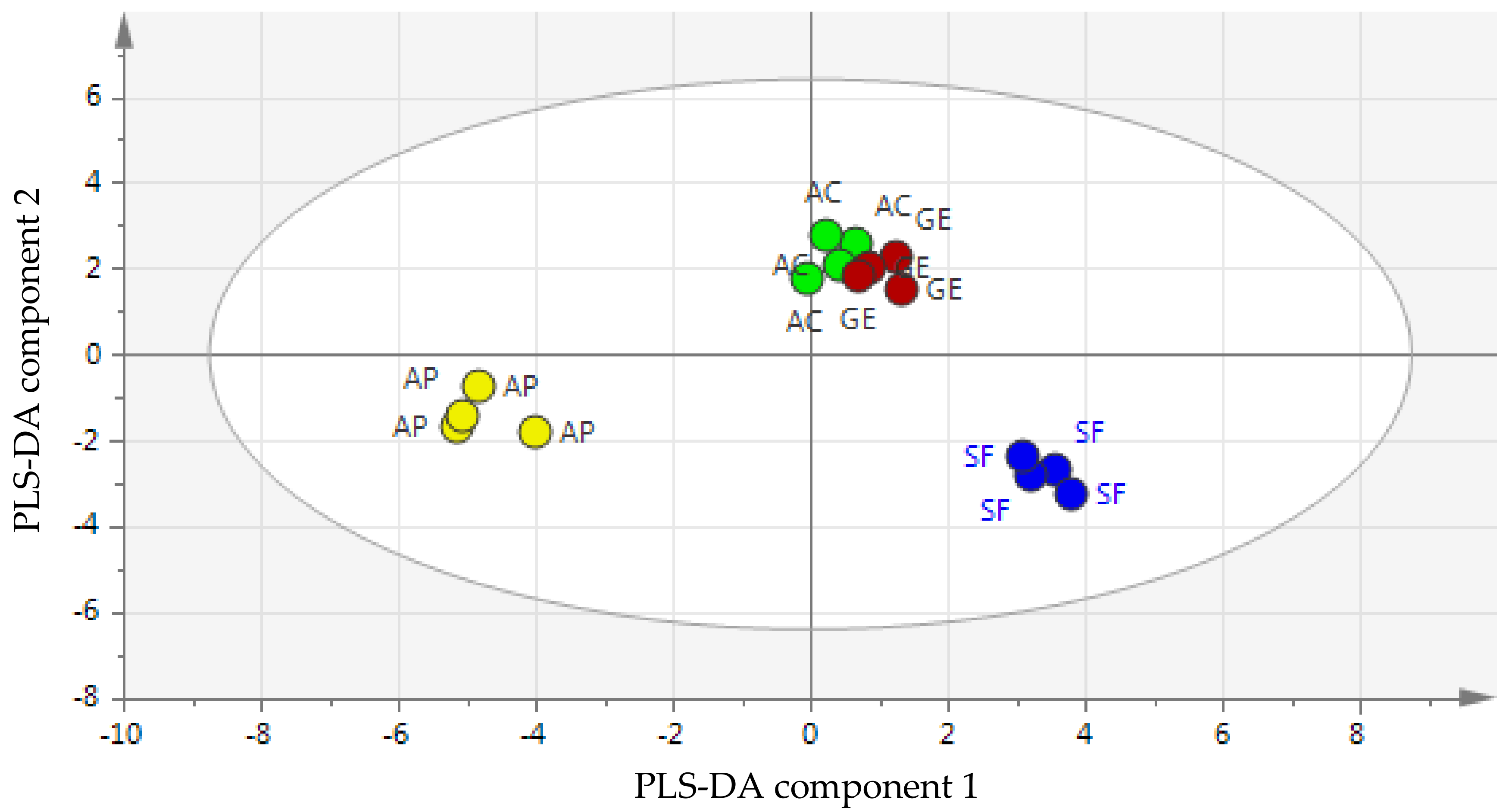
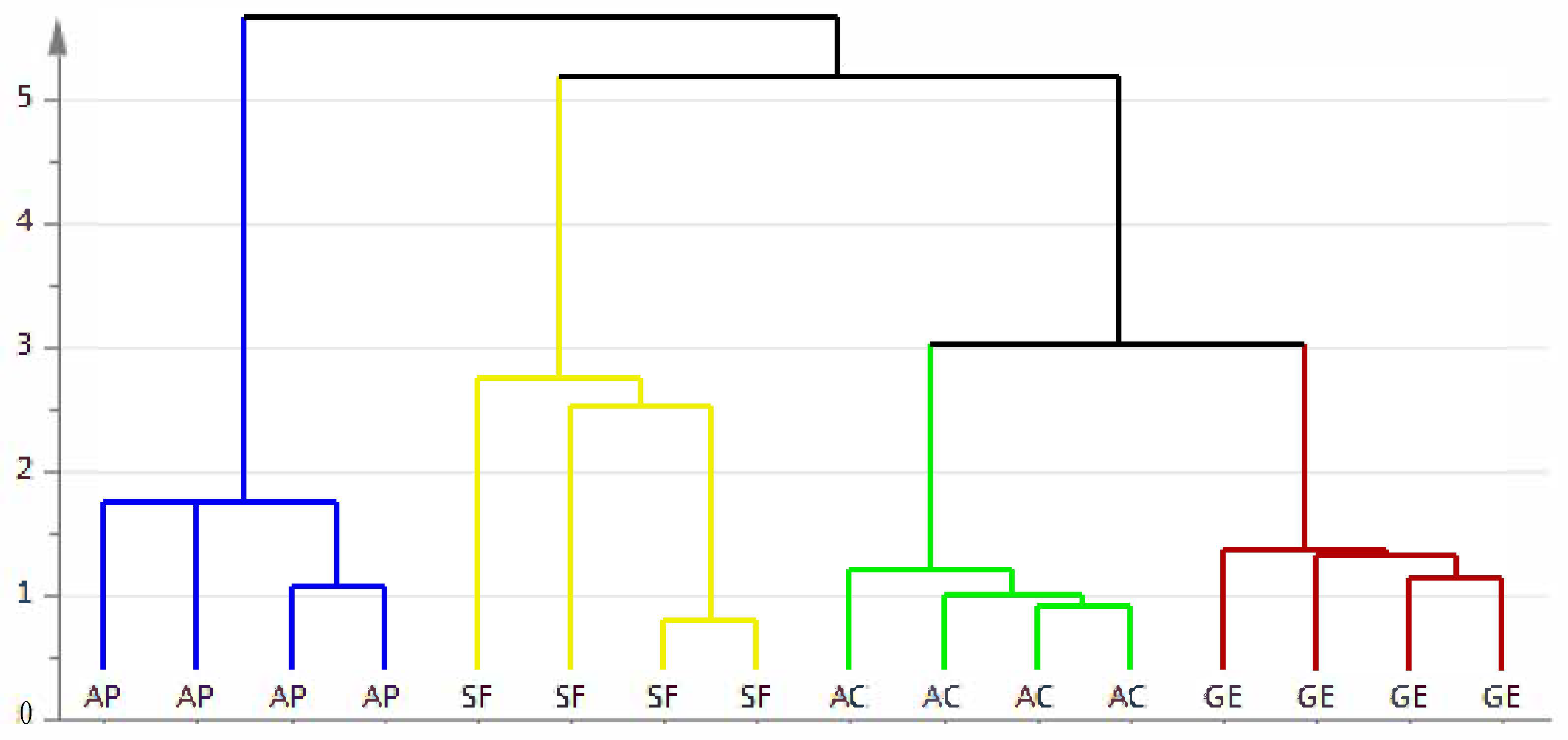
2.5.1. Partial Least Squares-Discriminant Analysis (PLS-DA)
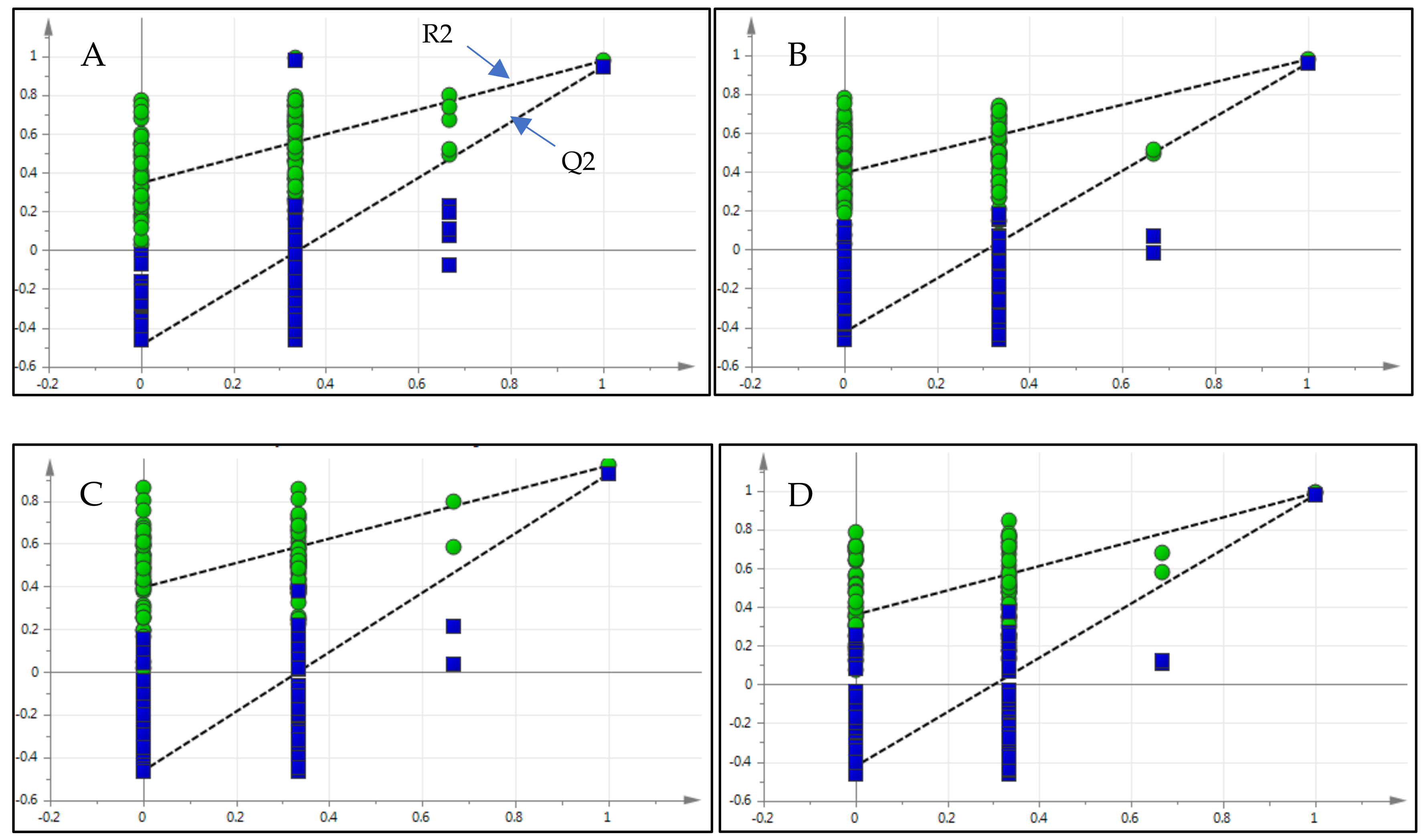
2.5.2. Validation of the PLS-DA Model
2.6. Potential Uses of Stingless Bee Honey
3. Materials and Methods
3.1. Standards and Chemicals
3.2. Geographical and Botanical Description of Honey Samples
3.3. Physicochemical Analyses
3.3.1. Moisture Content
3.3.2. Total Soluble Solid
3.3.3. pH and Free Acidity
3.3.4. Honey Colour Characteristics and Intensity
3.3.5. 5-hydroxymethylfurfural (5-HMF)
3.3.6. Sugar Profile
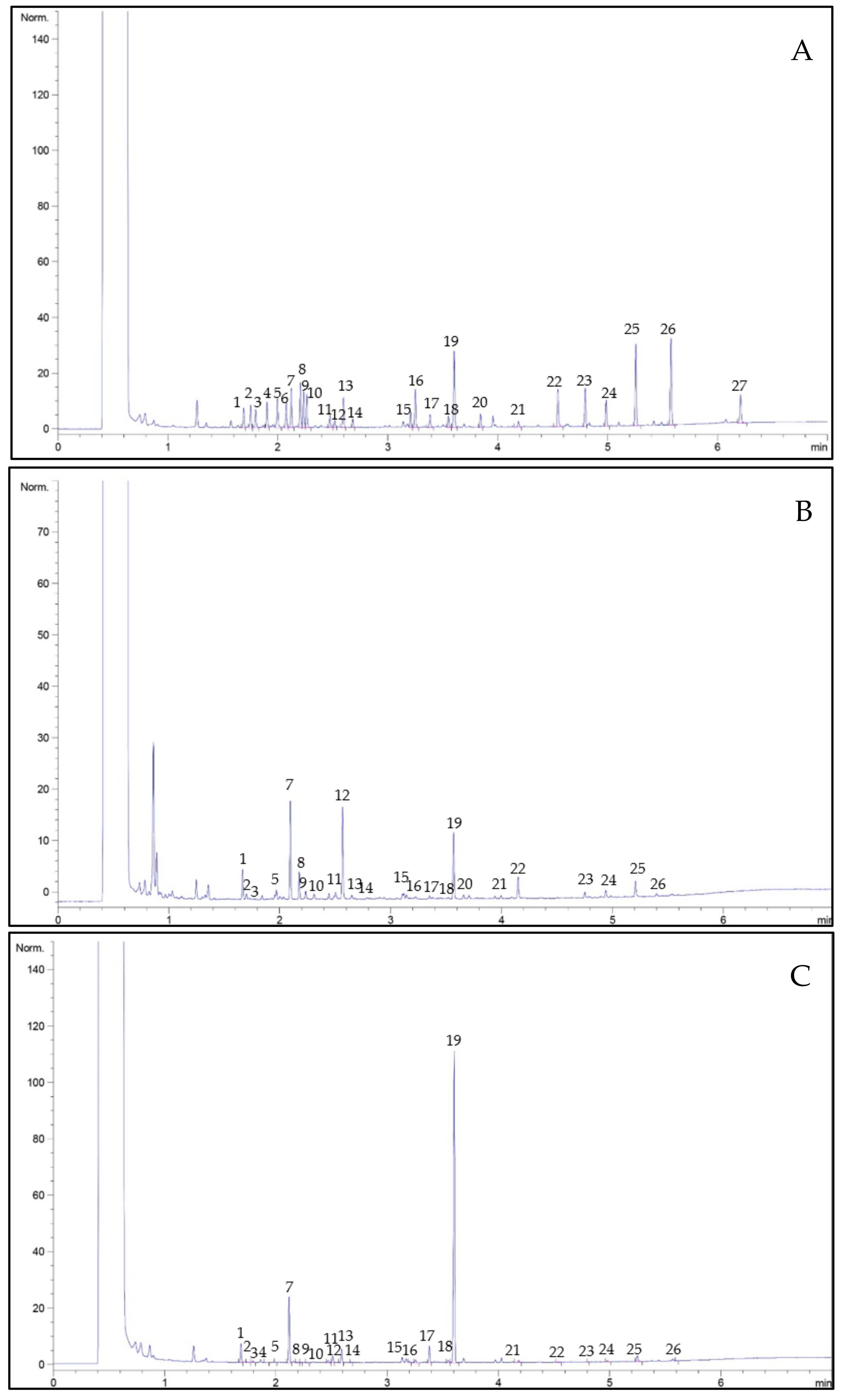
3.3.7. Amino Acid Profile
3.4. Analyses of Antioxidant Properties
3.4.1. Total Phenolic Content (TPC)
3.4.2. Total Flavonoids Content (TFC)
3.4.3. Free Radical Scavenging Activity (DPPH Assay)
3.4.4. Ferric Reducing Antioxidant Power (FRAP Assay)
3.5. Chemometrics Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, J.; Du, X.; Cheng, N.; Chen, L.; Xue, X.; Wu, L.; Cao, W. Identification of monofloral honeys using HPLC–ECD and chemometrics. Food Chem. 2016, 194, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Chuttong, B.; Chanbang, Y.; Sringarm, K.; Burgett, M. Physicochemical profiles of stingless bee (Apidae: Meliponini) honey from South east Asia (Thailand). Food Chem. 2016, 192, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Amin, Z.; Aina, F.; Sabri, S.; Mohammad, S.M.; Ismail, M.; Chan, K.W.; Ismail, N.; Norhaizan, M.E.; Zawawi, N. Therapeutic Properties of Stingless Bee Honey in Comparison with European Bee Honey. Adv. Pharmacol. Sci. 2018, 2018, 6179596. [Google Scholar]
- Fuenmayor, C.A.; Diaz-Moreno, A.C.; Zuluaga-Dominguez, C.M.; Quicazan, M.C. Honey of Colombian stingless bees: Nutritional characteristics and physicochemical quality indicators. In Pot-Honey: A Legacy of Stingless Bee, 1st ed.; Patricia, V., Silvia, R.M.P., David, R., Eds.; Springer: London, UK; New York, NY, USA, 2013. [Google Scholar]
- Kelly, N.; Farisya, M.S.N.; Kumara, T.K.; Marcela, P. Species Diversity and External Nest Characteristics of Stingless Bees in Meliponiculture. Pertanika J. Trop. Agric. Sci. 2014, 37, 293–298. [Google Scholar]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Razali, M.; Zainal, Z.; Maulidiani, M.; Shaari, K.; Zamri, Z.; Mohd Idrus, M.; Khatib, A.; Abas, F.; Ling, Y.S.; Rui, L.L.; et al. Classification of Raw Stingless Bee Honeys by Bee Species Origins Using the NMR-and LC-MS-Based Metabolomics Approach. Molecules 2018, 23, 2160. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Venskutonis, P.R. Floral markers in honey of various botanical and geographic origins: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Kečkeš, J.; Trifković, J.; Andrić, F.; Jovetić, M.; Tešić, Ž.; Milojković-Opsenica, D. Amino acids profile of Serbian unifloral honeys. J. Sci. Food Agric. 2013, 93, 3368–3376. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Tarantilis, P.A.; Harizanis, P.C.; Alissandrakis, E. Botanical discrimination and classification of honey samples applying gas chromatography/mass spectrometry fingerprinting of headspace volatile compounds. Food Chem. 2010, 121, 856–862. [Google Scholar] [CrossRef]
- Wang, J.; Kliks, M.M.; Qu, W.; Jun, S.; Shi, G.; Li, Q.X. Rapid determination of the geographical origin of honey based on protein fingerprinting and barcoding using MALDI TOF MS. J. Agric. Food Chem. 2009, 57, 10081–10088. [Google Scholar] [CrossRef]
- Nozal, M.J.; Bernal, J.L.; Toribio, L.; Alamo, M.; Diego, J.C.; Tapia, J. The use of carbohydrate profiles and chemometrics in the characterization of natural honeys of identical geographical origin. J. Agric. Food Chem. 2005, 53, 3095–3100. [Google Scholar] [CrossRef] [PubMed]
- Vit, P.; Oddo, L.P.; Marano, M.L.; de Mejias, E.S. Venezuelan stingless bee honeys characterized by multivariate analysis of physicochemical properties. Apidologie 1998, 29, 377–389. [Google Scholar] [CrossRef]
- Duarte, A.W.F.; dos Santos Vasconcelos, M.R.; de Menezes, A.P.D.; da Silva, S.C.; Oda-souza, M.; López, A.M.Q. Composition and antioxidant activity of honey from Africanized and stingless bees in Alagoas (Brazil): A multivariate analysis. J. Apic. Res. 2012, 51, 23–35. [Google Scholar] [CrossRef]
- Kek, S.P.; Chin, N.L.; Yusof, Y.A.; Tan, S.W.; Chua, L.S. Classification of entomological origin of honey based on its physicochemical and antioxidant properties. Int. J. Food Prop. 2017, 20, S2723–S2738. [Google Scholar] [CrossRef]
- Kek, S.P.; Chin, N.L.; Tan, S.W.; Yusof, Y.A.; Chua, L.S. Classification of honey from its bee origin via chemical profiles and mineral content. Food Anal. Methods 2017, 10, 19–30. [Google Scholar] [CrossRef]
- Souza, B.A.; Roubik, D.W.; Barth, O.M.; Heard, T.A.; Enríquez, E.; Carvalho, C.; Villas-Boas, J.; Marchini, L.; Locatelli, J.; Persano-Oddo, L.; et al. Composition of stingless bee honey: Setting quality standards. Interciencia 2006, 31, 867–875. [Google Scholar]
- Hermosín, I.; Chicon, R.M.; Cabezudo, M.D. Free amino acid composition and botanical origin of honey. Food Chem. 2003, 83, 263–268. [Google Scholar] [CrossRef]
- Nozal, M.J.; Bernal, J.L.; Toribio, M.L.; Diego, J.C.; Ruiz, A. Rapid and sensitive method for determining free amino acids in honey by gas chromatography with flame ionization or mass spectrometric detection. J. Chromatogr. A 2004, 1047, 137–146. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, L.; Cheng, N.; Xue, X.; Wu, L.; Zheng, J.; Cao, W. Identification of botanical origin of Chinese unifloral honeys by free amino acid profiles and chemometric methods. J. Pharm. Anal. 2017, 7, 317–323. [Google Scholar] [CrossRef]
- Rebane, R.; Herodes, K. Evaluation of the botanical origin of Estonian uni-and polyfloral honeys by amino acid content. J. Agric. Food Chem. 2008, 56, 10716–10720. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Draft Revised Standard for Honey (at Step 10 of the Codex Procedure); Codex Alimentarius Commission, FAO: Rome, Italy, 2001; Volume 25, pp. 19–26. [Google Scholar]
- Malaysian Standard. Kelulut (Stingless Bee) Honey-Specification: MS 2683:2017; Department of Standard Malaysia: Selangor, Malaysia, 2017.
- Keng, C.B.; Haron, H.; Talib, R.A.; Subramaniam, P. Physical Properties, Antioxidant Content and Anti-Oxidative Activities of Malaysian Stingless Kelulut (Trigona spp.) Honey. J. Agric. Sci. 2017, 9, 32–40. [Google Scholar]
- Biluca, F.C.; Braghini, F.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Physicochemical profiles, minerals and bioactive compounds of stingless bee honey (Meliponinae). J. Food Compos. Anal. 2016, 50, 61–69. [Google Scholar] [CrossRef]
- Issaro, N.; Weerakul, T.; Machana, S.; Ornnim, P.; Phanudulkitti, C.; Srijan, T.; Laiwattanaphaisal, J.; Pattarapanich, C. Stingless bee honey II: Qualitative and quantitative studies on honey produced by three stingless bee species collected from a mangosteen garden in Chantaburi province, Thailand. Thai J. Pharm. Sci. 2013, 38, 16–18. [Google Scholar]
- Rodriguez-Malaver, A.J.; Rasmussen, C.; Gutierrez, M.G.; Gil, F.; Nieves, B.; Vit, P. Properties of honey from ten species of Peruvian stingless bees. Nat. Prod. Commun. 2009, 4, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Brenciani, A.; Mazzoni, L.; Gasparrini, M.; González-Paramás, A.M.; Santos-Buelga, C.; Morroni, G.; Simoni, S.; Forbes-Hernandez, T.Y.; et al. Apis mellifera vs Melipona beecheii Cuban polifloral honeys: A comparison based on their physicochemical parameters, chemical composition and biological properties. LWT 2018, 87, 272–279. [Google Scholar] [CrossRef]
- de Almeida-Muradian, L.B.; Stramm, K.M.; Estevinho, L.M. Efficiency of the FT-IR ATR spectrometry for the prediction of the physicochemical characteristics of M elipona subnitida honey and study of the temperature’s effect on those properties. Int. J. Food Sci. Technol. 2014, 49, 188–195. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement. Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef]
- USDA, Agricultural Marketing Services Fruit and Vegetable Division Processed Products Branch. United State Standards for Grades of Extracted Honey; US Department of Agriculture: Washington, DC, USA, 1985.
- Tuksitha, L.; Chen, Y.L.S.; Chen, Y.L.; Wong, K.Y.; Peng, C.C. Antioxidant and antibacterial capacity of stingless bee honey from Borneo (Sarawak). J. Asia Pac. Entomol. 2018, 21, 563–570. [Google Scholar] [CrossRef]
- Oddo, L.P.; Heard, T.A.; Rodríguez-Malaver, A.; Pérez, R.A.; Fernández-Muiño, M.; Sancho, M.T.; Vit, P. Composition and antioxidant activity of Trigona carbonaria honey from Australia. J. Med. Food 2008, 11, 789–794. [Google Scholar] [CrossRef]
- Olaitan, P.B.; Adeleke, O.E.; Iyabo, O.O. Honey: A reservoir for microorganisms and an inhibitory agent for microbes. Afr. Health Sci. 2007, 7, 159–165. [Google Scholar]
- Janiszewska, K.; Aniołowska, M.; Nowakowski, P. Free amino acids content of honeys from Poland. Pol. J. Food Nutr. Sci. 2012, 62, 85–89. [Google Scholar] [CrossRef]
- Qamer, S.; Ehsan, M.; Nadeem, S.; Shakoori, A.R. Free amino acids content of Pakistani unifloral honey produced by Apis mellifera. Pak. J. Zool. 2007, 39, 99–102. [Google Scholar]
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Oliveira, R.G.D.; Jain, S.; Luna, A.C.; Freitas, L.D.S.; Araújo, E.D.D. Screening for quality indicators and phenolic compounds of biotechnological interest in honey samples from six species of stingless bees (Hymenoptera: Apidae). Food Sci. Technol. 2017, 37, 552–557. [Google Scholar] [CrossRef] [Green Version]
- da Silva, G.R.; da Natividade, T.B.; Camara, C.A.; da Silva, E.M.S.; dos Santos, F.D.A.R.; Silva, T.M.S. Identification of sugar, amino acids and minerals from the pollen of Jandaíra stingless bees (Melipona subnitida). Food Nutr. Sci. 2014, 5, 1015–1021. [Google Scholar]
- Ranneh, Y.; Ali, F.; Zarei, M.; Akim, A.M.; Hamid, H.A.; Khazaai, H. Malaysian stingless bee and Tualang honeys: A comparative characterization of total antioxidant capacity and phenolic profile using liquid chromatography-mass spectrometry. LWT 2018, 89, 1–9. [Google Scholar] [CrossRef]
- Idris, Y.M.A.; Mariod, A.A.; Hamad, S.I. Physicochemical properties, phenolic contents and antioxidant activity of Sudanese honey. Int. J. Food Prop. 2011, 14, 450–458. [Google Scholar] [CrossRef]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Baek, Y.; Kim, Y.J.; Baik, M.Y.; Kim, D.O.; Lee, H. Total phenolic contents and antioxidant activities of Korean domestic honey from different floral sources. Food Sci. Biotechnol. 2015, 24, 1453–1457. [Google Scholar] [CrossRef]
- Nweze, J.A.; Okafor, J.I.; Nweze, E.I.; Nweze, J.E. Evaluation of physicochemical and antioxidant properties of two stingless bee honeys: A comparison with Apis mellifera honey from Nsukka, Nigeria. BMC Res. Notes 2017, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Can, Z.; Yildiz, O.; Sahin, H.; Turumtay, E.A.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Imtiaz Shafiq, M.; Khaleeq, A.; Huma, R.; Abdul Qadir, M.; Khalid, A.; Samad, A. Physiochemical, Biochemical, Minerals Content Analysis, and Antioxidant Potential of National and International Honeys in Pakistan. J. Chem. 2016, 2016, 8072305. [Google Scholar] [CrossRef]
- Alzahrani, H.A.; Alsabehi, R.; Boukraâ, L.; Abdellah, F.; Bellik, Y.; Bakhotmah, B.A. Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins. Molecules 2012, 17, 10540–10549. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikström, C. Multi-and Megavariate Data Analysis Basic Principles and Applications; Umetrics Academy: Umeå, Sweden, 2013. [Google Scholar]
- Umetrics, M. User Guide to SIMCA; MKS Umetrics AB: Malmö, Sweden, 2013. [Google Scholar]
- Shin, H.S.; Ustunol, Z. Influence of honey-containing marinades on heterocyclic aromatic amine formation and overall mutagenicity in fried beef steak and chicken breast. J. Food Sci. 2004, 69, FCT147–FCT153. [Google Scholar] [CrossRef]
- Shin, H.S.; Strasburg, G.M.; Ustunol, Z. Influence of different unifloral honeys on heterocyclic aromatic amine formation and overall mutagenicity in fried ground-beef patties. J. Food Sci. 2003, 68, 810–815. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar]
- Jalil, M.A.A.; Kasmuri, A.R.; Hadi, H. Stingless bee honey, the natural wound healer: A review. Skin Pharmacol. Physiol. 2017, 30, 66–75. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey: A l novel antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar] [CrossRef]
- Jamilah, M.S.; Nur-Atiqah, M.H.; Mohamad Hafis, M.; Seriza, M.R. Potential climate change mitigation through carbon stock accumulation by melaleuca cajuputi powell (gelam). Int. J. Agric. For. Plant. 2017, 5, 92–98. [Google Scholar]
- Muthu, N.; Lee, S.Y.; Phua, K.K.; Bhore, S.J. Nutritional, medicinal and toxicological attributes of star-fruits (Averrhoa Carambola L.): A review. Bioinformation 2016, 12, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Khanam, Z.; Sam, K.H.; Zakaria, N.H.B.M.; Ching, C.H.; Bhat, I.U.H. Determination of polyphenolic content, HPLC analyses and DNA cleavage activity of Malaysian Averrhoa carambola L. fruit extracts. J. King Saud Univ. Sci. 2015, 27, 331–337. [Google Scholar] [CrossRef]
- Ismaili, G.; Bakar, B.H.A.; Rahim, K.K.A. Evaluation of Acacia Mangium in Structural Size at Green Condition. J. Civ. Eng. Sci. Technol. 2011, 2, 17–22. [Google Scholar] [CrossRef]
- Silva, T.M.S.; dos Santos, F.P.; Evangelista-Rodrigues, A.; da Silva, E.M.S.; da Silva, G.S.; de Novais, J.S.; Camara, C.A. Phenolic compounds, melissopalynological, physicochemical analysis and antioxidant activity of jandaíra (Melipona subnitida) honey. J. Food Compos. Anal. 2013, 29, 10–18. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Method of Analysis, 19th ed.; AOAC: Washington, DC, USA, 2012. [Google Scholar]
- Colucci, G.; De Vito, V.; Varricchio, E.; De Cunzo, F.; Coccia, E. Identification of Traceability Markers in Italian Unifloral Honeys of different Botanical Origin. J. Nutr. Food Sci. 2016, 6, 462. [Google Scholar]
- de Sousa, J.M.B.; de Souza, E.L.; Marques, G.; de Toledo Benassi, M.; Gullón, B.; Pintado, M.M.; Magnani, M. Sugar profile, physicochemical and sensory aspects of monofloral honeys produced by different stingless bee species in Brazilian semi-arid region. LWT-Food Sci. Technol. 2016, 65, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Bogdanov, S. Harmonised Methods of the International Honey Commission; International Honey Commission: Bern, Switzerland, 2009; Available online: http://www.ihc-platform.net/ihcmethods2009.pdf (accessed on 10 April 2018).
- Gökmen, V.; Acar, J. Simultaneous determination of 5-hydroxymethylfurfural and patulin in apple juice by reversed-phase liquid chromatography. J. Chromatogr. A 1999, 847, 69–74. [Google Scholar] [CrossRef]
- Mustafa, A.; Åman, P.; Andersson, R.; Kamal-Eldin, A. Analysis of free amino acids in cereal products. Food Chem. 2007, 105, 317–324. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Garjanovic, S.Z.; Alvarez-Suarez, J.M.; Novakovic, M.M.; Pastor, F.T.; Pezo, L.; Battino, M.; Suznjevic, D.Z. Comparative analysis of antioxidant activity of honey of different floral sources using recently developed polarographic and various spectrophotometric assays. J. Food Compos. Anal. 2013, 30, 13–18. [Google Scholar] [CrossRef]
- Kamboj, R.; Bera, M.B.; Nanda, V. Evaluation of physicochemical properties, trace metal content and antioxidant activity of Indian honeys. Int. J. Food Sci. Technol. 2013, 48, 578–587. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Khalil, M.I.; Alam, N.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic acid composition and antioxidant properties of Malaysian honeys. J. Food Sci. 2011, 76, C921–C928. [Google Scholar] [CrossRef] [PubMed]
- Javadi, N.; Abas, F.; Hamid, A.A.; Simoh, S.; Shaari, K.; Ismail, I.S.; Mediani, A.; Khatib, A. GC-MS-Based Metabolite Profiling of Cosmos caudatus Leaves Possessing Alpha-Glucosidase Inhibitory Activity. J. Food Sci. 2014, 79, C1130–C1136. [Google Scholar] [CrossRef]
- Murugesu, S.; Ibrahim, Z.; Ahmed, Q.U.; Nik Yusoff, N.I.; Uzir, B.F.; Perumal, V.; Abas, F.; Saari, K.; El-Seedi, H.; Khatib, A. Characterization of α-glucosidase inhibitors from clinacanthus nutans lindau leaves by gas chromatography-mass spectrometry-based metabolomics and molecular docking simulation. Molecules 2018, 23, 2402. [Google Scholar] [CrossRef]

| Parameters | Unit | Botanical Origins of Honey | Apis | ||
|---|---|---|---|---|---|
| Acacia | Starfruit | Gelam | mellifera | ||
| Moisture content * | g/100 g | 21.52 ± 0.66 c | 24.24 ± 0.19 b | 25.49 ± 0.45 a | 14.67 ± 0.11 d |
| Moisture content ** | g/100 g | 13.86 ± 0.38 b | 15.63 ± 0.41 a | 14.24 ± 0.65 b | NA |
| pH | pH | 3.27 ± 0.03 b | 3.00 ± 0.03 d | 3.18 ± 0.03 c | 3.56 ± 0.02 a |
| Free acidity | meq/kg honey | 107.50 ± 6.45 c | 246.25 ± 9.46 a | 176.25 ± 9.46 b | 39.22 ± 1.50 d |
| TSS | °Brix | 74.65 ± 0.39 bc | 73.88 ± 0.34 c | 74.85 ± 0.73 b | 76.40 ± 0.54 a |
| Colour | mm Pfund | 1.25 ± 0.10 d | 36.85 ± 2.00 c | 46.45 ± 2.45 a | 40.70 ± 0.53 b |
| Colour intensity | mAU | 32.25 ± 3.59 d | 122.50 ± 9.57 c | 280.00 ± 18.26 a | 251.00 ± 2.16 b |
| 5-HMF | mg/kg honey | ND b | 0.07 ± 0.06 a | 0.05 ± 0.02 a | ND b |
| Parameters | Botanical Origin of Honey | Apis | ||
|---|---|---|---|---|
| Acacia | Starfruit | Gelam | mellifera | |
| Fructose | 22.05 ± 0.85 c | 15.27 ± 0.51 d | 29.06 ± 1.52 b | 33.99 ± 0.31 a |
| Glucose | 21.17 ± 1.50 c | 17.42 ± 0.82 d | 27.54 ± 1.98 b | 32.24 ± 0.38 a |
| Sucrose | 28.44 ± 0.89 b | 37.32 ± 1.14 a | 17.36 ± 1.09 c | 2.57 ± 0.24 d |
| Maltose | ND b | 0.89 ± 0.43 a | ND b | ND b |
| Total sugar | 71.65 ± 2.04 ab | 70.89 ± 0.80 bc | 73.96 ± 2.74 a | 68.80 ± 0.72 c |
| Amino Acids | Botanical Origin of Honey | |||
|---|---|---|---|---|
| Acacia | Starfruit | Gelam | Apis mellifera | |
| Alanine | 35.04 ± 2.03 c | 46.00 ± 2.06 a | 35.96 ± 2.14 c | 41.34 ± 0.34 b |
| Sarcosine | <LOQ | <LOQ | <LOQ | <LOQ |
| Glycine | <LOQ | <LOQ | <LOQ | <LOQ |
| α-Aminobutyric acid | <LOQ | <LOQ | <LOQ | ND |
| Valine | 1.24 ± 0.77 b | <LOQ b | 5.07 ± 1.65 a | 6.06 ± 1.92 a |
| β-Aminoisobutyric acid | <LOQ | ND | <LOQ | ND |
| Leucine | 24.82 ± 0.64 c | 7.55 ± 0.20 d | 44.50 ± 4.44 a | 32.91 ± 0.26 b |
| Allo-isoleucine | 7.03 ± 1.31 a | 6.19 ± 0.13 a | 6.38 ± 0.03 a | ND b |
| Isoleucine | <LOQ b | <LOQ b | <LOQ b | 7.06 ± 0.84 a |
| Threonine | 4.75 ± 3.67 b | 3.85 ± 1.32 b | 7.07 ± 3.21 ab | 12.08 ± 4.25 a |
| Serine | 13.60 ± 2.46 b | 37.75 ± 5.14 a | 19.07 ± 0.83 b | 19.21 ± 11.45 b |
| Proline | 16.23 ± 5.68 d | 33.01 ± 6.20 c | 48.91 ± 1.44 b | 145.9 ± 3.39 a |
| Asparagine | <LOQ | <LOQ | <LOQ | <LOQ |
| Aspartic acid | <LOQ | <LOQ | <LOQ | <LOQ |
| Methionine | 16.71 ± 0.21 c | 22.62 ± 0.56 a | 19.49 ± 0.19 b | 21.40 ± 2.14 a |
| 3-hydroxyproline | 4.83 ± 3.01 c | 75.53 ± 5.47 a | 29.63 ± 2.28 b | 6.34 ± 0.77 c |
| Glutamic acid | 29.02 ± 4.27 b | 37.29 ± 4.15 a | 29.77 ± 2.31 b | 23.68 ± 0.59 c |
| Phenylalanine | 50.04 ± 4.76 c | 561.10 ± 37.59 a | 237.37 ± 13.73 b | 68.51 ± 0.35 c |
| α-Aminoadipic acid | 4.96 ± 1.00 ab | ND b | ND b | 7.69 ± 5.13 a |
| Glutamine | 42.15 ± 2.73 b | 24.75 ± 4.11 c | 29.32 ± 1.19 c | 134.09 ± 10.81 a |
| Ornithine | 11.26 ± 0.32 ab | 12.15 ± 0.87 a | 11.13 ± 0.50 b | 10.93 ± 0.59 b |
| Lysine | 15.46 ± 1.04 b | 15.17 ± 0.44 b | 14.67 ± 0.04 b | 21.94 ± 0.68 a |
| Histidine | 23.76 ± 4.09 b | 20.27 ± 2.08 bc | 17.82 ± 0.07 c | 30.03 ± 1.00 a |
| Tyrosine | 24.92 ± 0.84 b | 23.31 ± 2.63 b | 19.57 ± 1.97 c | 29.63 ± 0.66 a |
| Tryptophan | 20.32 ± 1.25 a | 20.46 ± 3.16 a | 19.05 ± 0.38 a | 20.58 ± 2.27 a |
| Cysteine | 34.67 ± 2.09 a | ND b | 33.01 ± 2.62 a | ND b |
| TOTAL | 380.82 ± 19.36 c | 947.01 ± 48.42 a | 627.78 ± 21.20 b | 639.47 ± 13.49 b |
| Parameters | Unit | Botanical Origin of Honey | |||
|---|---|---|---|---|---|
| Acacia | Starfruit | Gelam | Apis mellifera | ||
| Total phenolic contents (TPC) | mg GAE/100 g honey | 61.47 ± 3.34 c | 84.10 ± 5.33 b | 114.49 ± 7.31a | 29.05 ± 1.58 d |
| Total flavonoids content (TFC) | mg QAE/100 g honey | 3.63 ± 0.26 c | 11.15 ± 0.55 a | 8.41 ± 0.36 b | 1.57 ± 0.24 d |
| Free radical scavenging activity (IC50) | mg/mL | 58.35 ± 2.45 c | 90.63 ± 5.50 b | 14.29 ± 0.53 d | 202.15 ± 1.60 a |
| Ferric reducing antioxidant power (FRAP) | µmol Fe2SO4.7H2O/100 g honey | 180.59 ± 10.48 b | 263.90 ± 22.1 c | 512.10 ± 47.4 a | 40.22 ± 1.84 d |
| Parameters | TPC | TFC | IC50 | FRAP | Colour Intensity |
|---|---|---|---|---|---|
| TPC | 1.000 | ||||
| TFC | 0.802 | 1.000 | |||
| IC50 | −0.886 | −0.572 | 1.000 | ||
| FRAP | 0.981 * | 0.691 | −0.863 | 1.000 | |
| Colour intensity | 0.165 | −0.010 | 0.182 | 0.303 | 1.000 |
| Sample | Scientific Name | Geographical Origin | Bee Species |
|---|---|---|---|
| Gelam Acacia Starfruit | Meleleuca cajaputi Powell Acacia mangium Averrhoa carambola L | Malacca Johor Pahang | Heterotrigona itama |
| Acacia | Acacia mangium | Johor | Apis mellifera |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamsudin, S.; Selamat, J.; Sanny, M.; A.R., S.B.; Jambari, N.N.; Khatib, A. A Comparative Characterization of Physicochemical and Antioxidants Properties of Processed Heterotrigona itama Honey from Different Origins and Classification by Chemometrics Analysis. Molecules 2019, 24, 3898. https://doi.org/10.3390/molecules24213898
Shamsudin S, Selamat J, Sanny M, A.R. SB, Jambari NN, Khatib A. A Comparative Characterization of Physicochemical and Antioxidants Properties of Processed Heterotrigona itama Honey from Different Origins and Classification by Chemometrics Analysis. Molecules. 2019; 24(21):3898. https://doi.org/10.3390/molecules24213898
Chicago/Turabian StyleShamsudin, Sharina, Jinap Selamat, Maimunah Sanny, Shamsul Bahari A.R., Nuzul Noorahya Jambari, and Alfi Khatib. 2019. "A Comparative Characterization of Physicochemical and Antioxidants Properties of Processed Heterotrigona itama Honey from Different Origins and Classification by Chemometrics Analysis" Molecules 24, no. 21: 3898. https://doi.org/10.3390/molecules24213898
APA StyleShamsudin, S., Selamat, J., Sanny, M., A.R., S. B., Jambari, N. N., & Khatib, A. (2019). A Comparative Characterization of Physicochemical and Antioxidants Properties of Processed Heterotrigona itama Honey from Different Origins and Classification by Chemometrics Analysis. Molecules, 24(21), 3898. https://doi.org/10.3390/molecules24213898