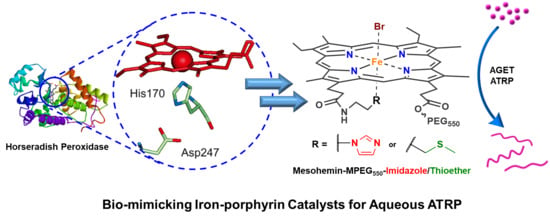
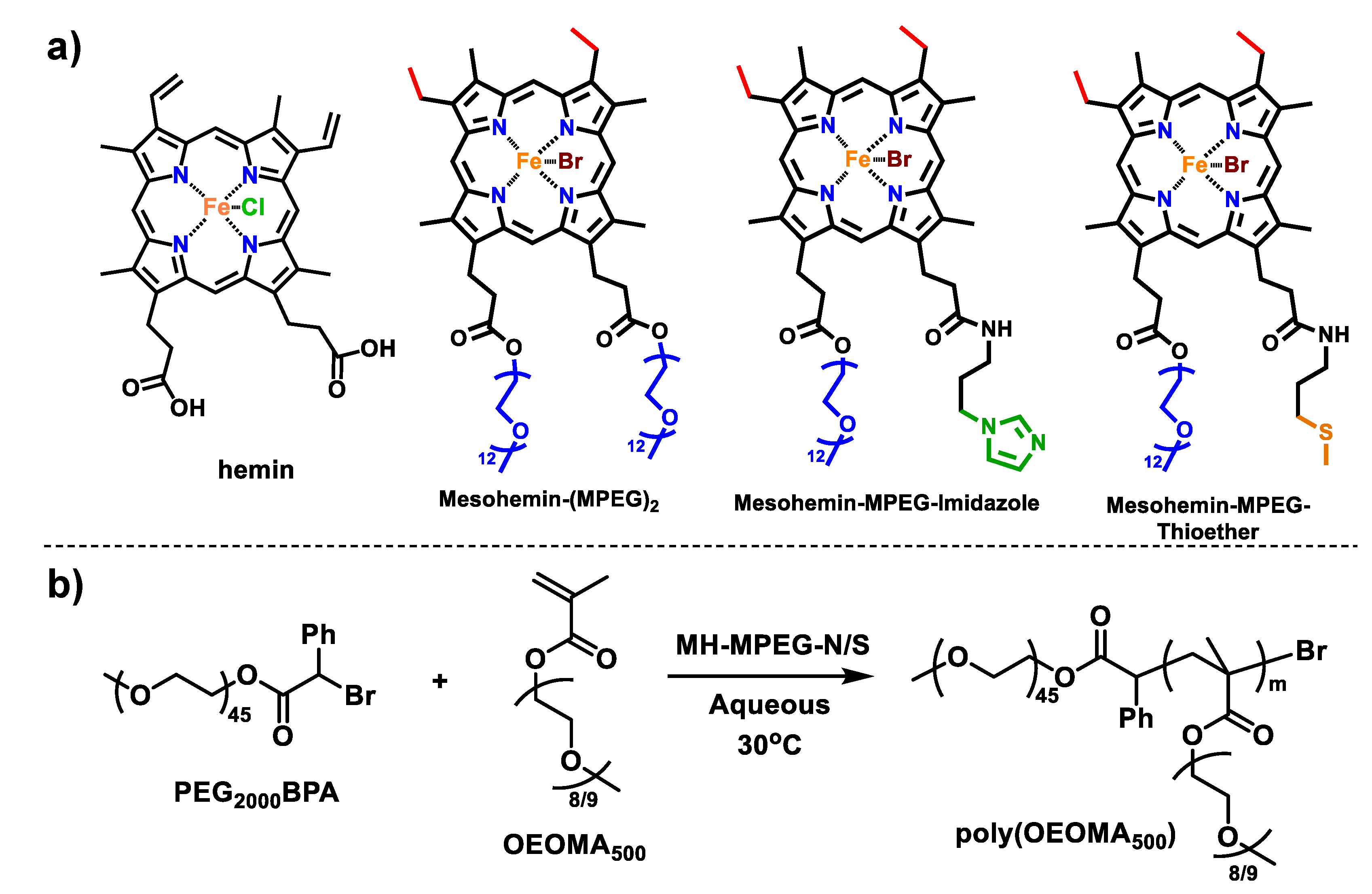
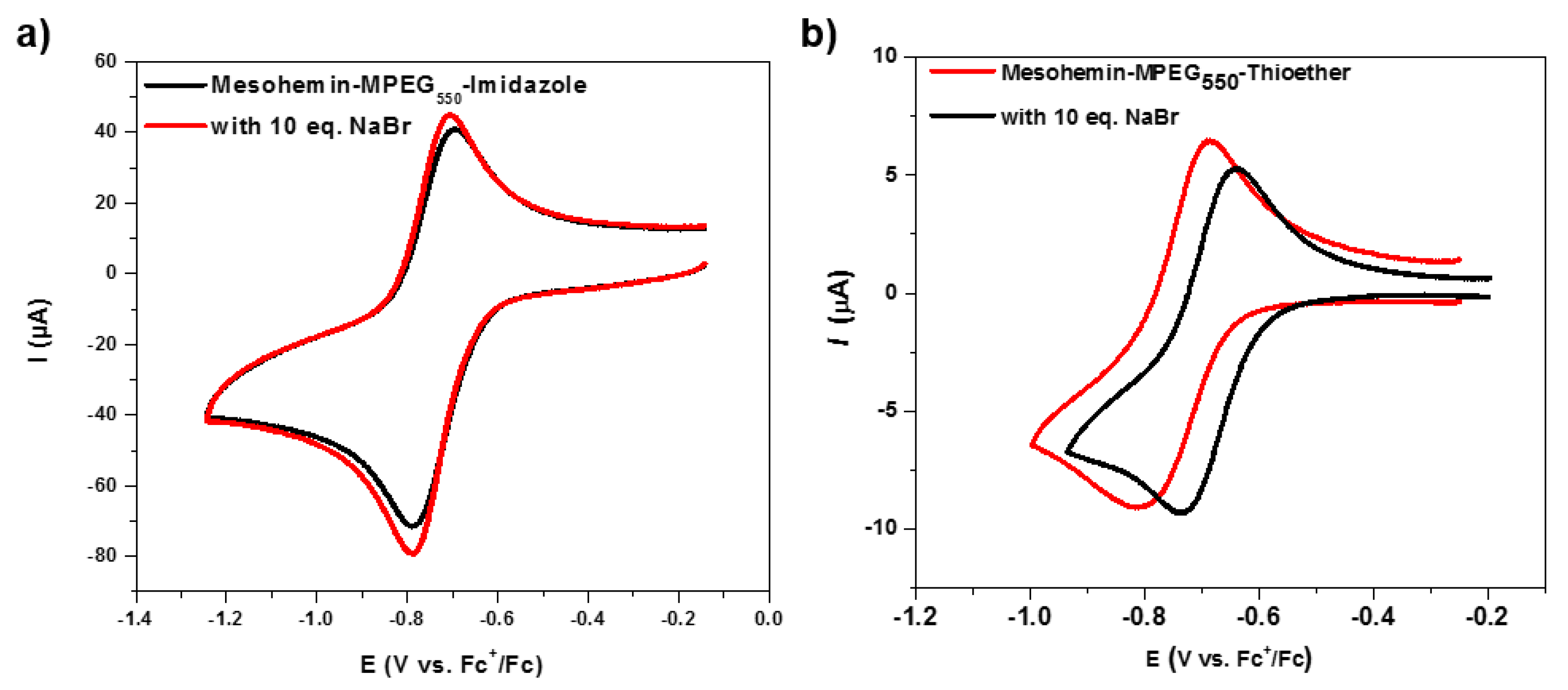
Axially Ligated Mesohemins as Bio-Mimicking Catalysts for Atom Transfer Radical Polymerization
Abstract
:Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 2001, 101, 3689–3745. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Tsarevsky, N.V. Nanostructured functional materials prepared by atom transfer radical polymerization. Nat. Chem. 2009, 1, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Xia, J.H. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Ribelli, T.G.; Lorandi, F.; Fantin, M.; Matyjaszewski, K. Atom Transfer Radical Polymerization: Billion Times More Active Catalysts and New Initiation Systems. Macromol. Rapid Commun. 2019, 40, 1800616. [Google Scholar] [CrossRef]
- di Lena, F.; Matyjaszewski, K. Transition metal catalysts for controlled radical polymerization. Prog. Polym. Sci. 2010, 35, 959–1021. [Google Scholar] [CrossRef]
- He, W.W.; Zhang, L.F.; Miao, J.; Cheng, Z.P.; Zhu, X.L. Facile Iron-Mediated AGET ATRP for Water-Soluble Poly(ethylene glycol) Monomethyl Ether Methacrylate in Water. Macromol. Rapid Commun. 2012, 33, 1067–1073. [Google Scholar] [CrossRef]
- Schroeder, H.; Yalalov, D.; Buback, M.; Matyjaszewski, K. Activation-Deactivation Equilibrium Associated With Iron-Mediated Atom-Transfer Radical Polymerization up to High Pressure. Macromol. Chem. Phys. 2012, 213, 2019–2026. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.Z.; Parker, B.; Matyjaszewski, K. ATRP of MMA with ppm Levels of Iron Catalyst. Macromolecules 2011, 44, 4022–4025. [Google Scholar] [CrossRef]
- Allan, L.E.N.; Perry, M.R.; Shaver, M.P. Organometallic mediated radical polymerization. Prog. Polym. Sci. 2012, 37, 127–156. [Google Scholar] [CrossRef] [Green Version]
- Poli, R.; Shaver, M.P. ATRP/OMRP/CCT Interplay in Styrene Polymerization Mediated by Iron(II) Complexes: A DFT Study of the alpha-Diimine System. Chem. Eur. J. 2014, 20, 17530–17540. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.A.; Klok, H.A. Peptide/protein-polymer conjugates: Synthetic strategies and design concepts. Chem. Commun. 2008, 2591–2611. [Google Scholar] [CrossRef] [PubMed]
- Heredia, K.L.; Bontempo, D.; Ly, T.; Byers, J.T.; Halstenberg, S.; Maynard, H.D. In situ preparation of protein—“Smart” polymer conjugates with retention of bioactivity. J. Am. Chem. Soc. 2005, 127, 16955–16960. [Google Scholar] [CrossRef]
- Peeler, J.C.; Woodman, B.F.; Averick, S.; Miyake-Stoner, S.J.; Stokes, A.L.; Hess, K.R.; Matyjaszewski, K.; Mehl, R.A. Genetically Encoded Initiator for Polymer Growth from Proteins. J. Am. Chem. Soc. 2010, 132, 13575–13577. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Armes, S.P. Facile atom transfer radical polymerization of methoxy-capped oligo(ethylene glycol) methacrylate in aqueous media at ambient temperature. Macromolecules 2000, 33, 6640–6647. [Google Scholar] [CrossRef]
- Fantin, M.; Isse, A.A.; Matyjaszewski, K.; Gennaro, A. ATRP in Water: Kinetic Analysis of Active and Super-Active Catalysts for Enhanced Polymerization Control. Macromolecules 2017, 50, 2696–2705. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Magenau, A.J.D.; Averick, S.E.; Simakova, A.; He, H.K.; Matyjaszewski, K. ICAR ATRP with ppm Cu Catalyst in Water. Macromolecules 2012, 45, 4461–4468. [Google Scholar] [CrossRef]
- Mougin, N.C.; van Rijn, P.; Park, H.; Muller, A.H.E.; Boker, A. Hybrid Capsules via Self-Assembly of Thermoresponsive and Interfacially Active Bionanoparticle-Polymer Conjugates. Adv. Funct. Mater. 2011, 21, 2470–2476. [Google Scholar] [CrossRef]
- Simakova, A.; Averick, S.E.; Konkolewicz, D.; Matyjaszewski, K. Aqueous ARGET ATRP. Macromolecules 2012, 45, 6371–6379. [Google Scholar] [CrossRef]
- Bortolamei, N.; Isse, A.A.; Magenau, A.J.D.; Gennaro, A.; Matyjaszewski, K. Controlled Aqueous Atom Transfer Radical Polymerization with Electrochemical Generation of the Active Catalyst. Angew. Chem. Int. Ed. 2011, 50, 11391–11394. [Google Scholar] [CrossRef] [PubMed]
- Tsarevsky, N.V.; Pintauer, T.; Matyjaszewski, K. Deactivation efficiency and degree of control over polymerization in ATRP in protic solvents. Macromolecules 2004, 37, 9768–9778. [Google Scholar] [CrossRef]
- Zhang, Q.; Wilson, P.; Li, Z.D.; McHale, R.; Godfrey, J.; Anastasaki, A.; Waldron, C.; Haddleton, D.M. Aqueous Copper-Mediated Living Polymerization: Exploiting Rapid Disproportionation of CuBr with Me6TREN. J. Am. Chem. Soc. 2013, 135, 7355–7363. [Google Scholar] [CrossRef]
- Fantin, M.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Understanding the Fundamentals of Aqueous ATRP and Defining Conditions for Better Control. Macromolecules 2015, 48, 6862–6875. [Google Scholar] [CrossRef]
- Averick, S.; Simakova, A.; Park, S.; Konkolewicz, D.; Magenau, A.J.D.; Mehl, R.A.; Matyjaszewski, K. ATRP under Biologically Relevant Conditions: Grafting from a Protein. ACS Macro Lett. 2012, 1, 6–10. [Google Scholar] [CrossRef]
- Russell, A.J.; Baker, S.L.; Colina, C.M.; Figg, C.A.; Kaar, J.L.; Matyjaszewski, K.; Simakova, A.; Sumerlin, B.S. Next generation protein-polymer conjugates. AIChE J. 2018, 64, 3230–3245. [Google Scholar] [CrossRef]
- Sigg, S.J.; Seidi, F.; Renggli, K.; Silva, T.B.; Kali, G.; Bruns, N. Horseradish Peroxidase as a Catalyst for Atom Transfer Radical Polymerization. Macromol. Rapid Commun. 2011, 32, 1710–1715. [Google Scholar] [CrossRef]
- Silva, T.B.; Spulber, M.; Kocik, M.K.; Seidi, F.; Charan, H.; Rother, M.; Sigg, S.J.; Renggli, K.; Kali, G.; Bruns, N. Hemoglobin and Red Blood Cells Catalyze Atom Transfer Radical Polymerization. Biomacromolecules 2013, 14, 2703–2712. [Google Scholar] [CrossRef]
- Ng, Y.H.; di Lena, F.; Chai, C.L.L. Metalloenzymatic radical polymerization using alkyl halides as initiators. Polym. Chem. 2011, 2, 589–594. [Google Scholar] [CrossRef]
- Ng, Y.H.; di Lena, F.; Chai, C.L.L. PolyPEGA with predetermined molecular weights from enzyme-mediated radical polymerization in water. Chem. Commun. 2011, 47, 6464–6466. [Google Scholar] [CrossRef]
- Polizzi, K.M.; Bommarius, A.S.; Broering, J.M.; Chaparro-Riggers, J.F. Stability of biocatalysts. Curr. Opin. Chem. Biol. 2007, 11, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Simakova, A.; Mackenzie, M.; Averick, S.E.; Park, S.; Matyjaszewski, K. Bioinspired Iron-Based Catalyst for Atom Transfer Radical Polymerization. Angew. Chem. Int. Ed. 2013, 52, 12148–12151. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.Y.; Simakova, A.; Fantin, M.; Wang, Y.; Matyjaszewski, K. Direct ATRP of Methacrylic Acid with Iron-Porphyrin Based Catalysts. ACS Macro Lett. 2018, 7, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Marques, H.M.; Munro, O.Q.; Munro, T.; de Wet, M.; Vashi, P.R. Coordination of N-donor ligands by the monomeric ferric porphyrin N-acetylmicroperoxidase-8. Inorg. Chem. 1999, 38, 2312–2319. [Google Scholar] [CrossRef]
- Tezcan, F.A.; Winkler, J.R.; Gray, H.B. Effects of ligation and folding on reduction potentials of heme proteins. J. Am. Chem. Soc. 1998, 120, 13383–13388. [Google Scholar] [CrossRef]
- Chang, C.K.; Dolphin, D. Ferrous Porphyrin Mercaptide Complexes—Models for Reduced Cytochrome-P-450. J. Am. Chem. Soc. 1975, 97, 5948–5950. [Google Scholar] [CrossRef]
- Fantin, M.; Isse, A.A.; Venzo, A.; Gennaro, A.; Matyjaszewski, K. Atom Transfer Radical Polymerization of Methacrylic Acid: A Won Challenge. J. Am. Chem. Soc. 2016, 138, 7216–7219. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Entry | M/I/RA/Cat | Catalyst | Conv./%, (Time, h) | Mn,th × 10−3 [b] | Mn,GPC × 10−3 [c] | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 | 216/1/1/1 | MH-(MPEG)2 | 60 (6) | 61 | 62 | 1.19 |
| 2 | 216/1/1/1 | MH-MPEG-N | 76 (2.5) | 84 | 76 | 1.27 |
| 3 | 216/1/1/1 | MH-MPEG-S | 25 (3.5) | 27 | 40 | 1.28 |
| 4 | 227:1:0.3 × 2:1 | MH-MPEG-N | 75 (5) | 83 | 108 | 1.16 |
| 5 | 227:1:0.3 × 2:1 | MH-MPEG-S | 41 (5) | 43 | 43 | 1.07 |
| 6 | 216/1/1/1 | MH-MPEG2 + imidazole | 33 (2) | 37 | 78 | 1.91 |
| 7 | 216:1:0.3 × 2:0.1 | MH-(MPEG)2 | 70 (5) | 72 | 98 | 1.17 |
| 8 | 216:1:0.3 × 2:0.1 | MH-MPEG-N | 60 (5) | 69 | 190 | 1.42 |
| 9 | 216/1/0.3 × 2:0.1 | MH-MPEG-S | 61 (8.7) | 61 | 57 | 1.18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; Simakova, A.; Park, S.; Wang, Y.; Fantin, M.; Matyjaszewski, K. Axially Ligated Mesohemins as Bio-Mimicking Catalysts for Atom Transfer Radical Polymerization. Molecules 2019, 24, 3969. https://doi.org/10.3390/molecules24213969
Fu L, Simakova A, Park S, Wang Y, Fantin M, Matyjaszewski K. Axially Ligated Mesohemins as Bio-Mimicking Catalysts for Atom Transfer Radical Polymerization. Molecules. 2019; 24(21):3969. https://doi.org/10.3390/molecules24213969
Chicago/Turabian StyleFu, Liye, Antonina Simakova, Sangwoo Park, Yi Wang, Marco Fantin, and Krzysztof Matyjaszewski. 2019. "Axially Ligated Mesohemins as Bio-Mimicking Catalysts for Atom Transfer Radical Polymerization" Molecules 24, no. 21: 3969. https://doi.org/10.3390/molecules24213969
APA StyleFu, L., Simakova, A., Park, S., Wang, Y., Fantin, M., & Matyjaszewski, K. (2019). Axially Ligated Mesohemins as Bio-Mimicking Catalysts for Atom Transfer Radical Polymerization. Molecules, 24(21), 3969. https://doi.org/10.3390/molecules24213969