In Vivo and In Vitro Study of Immunostimulation by Leuconostoc lactis-Produced Gluco-Oligosaccharides
Abstract
:1. Introduction
2. Results and Discussion
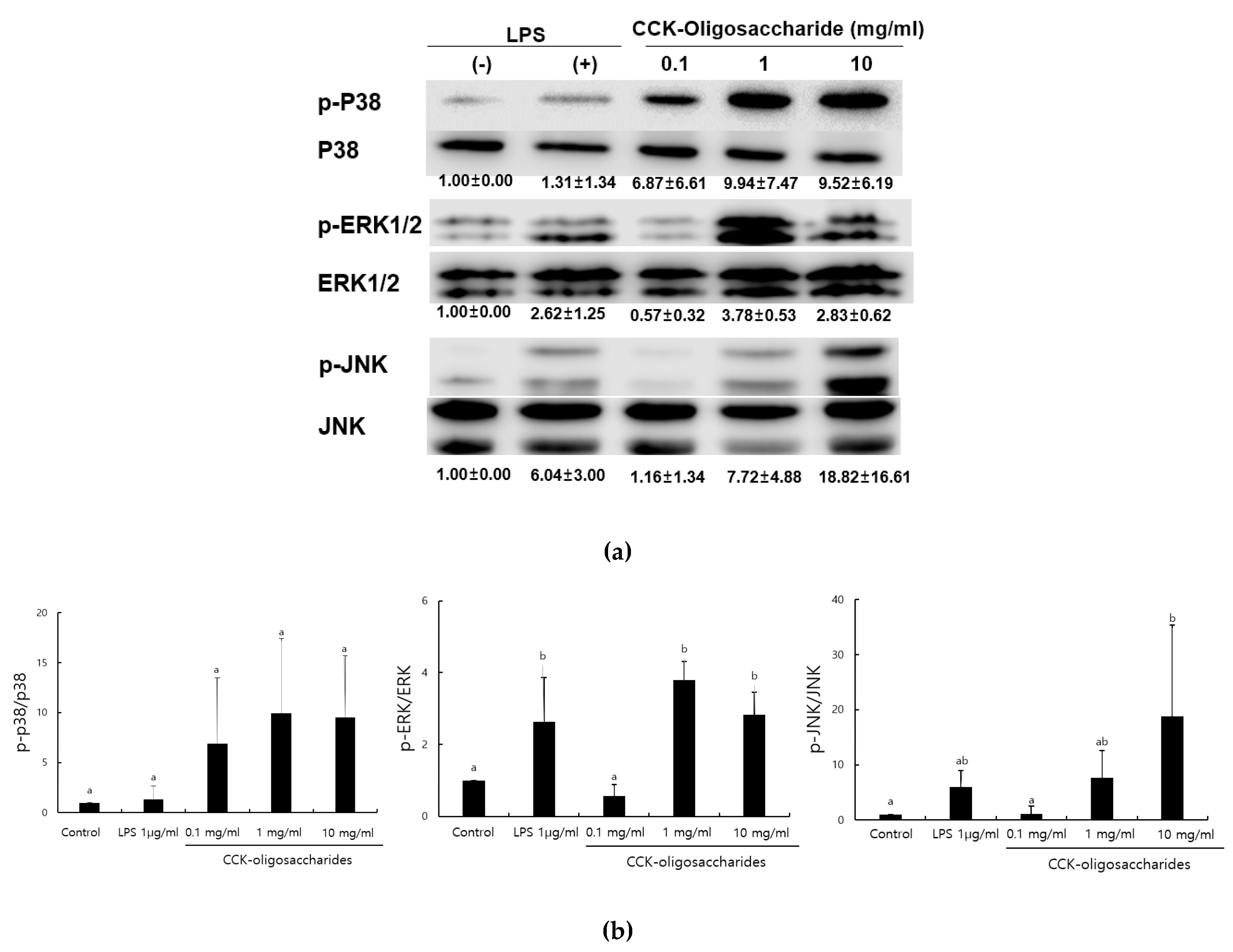
2.1. Effect of CCK-Oligosaccharides on MAPK Signaling Pathway of RAW264.7 Cells
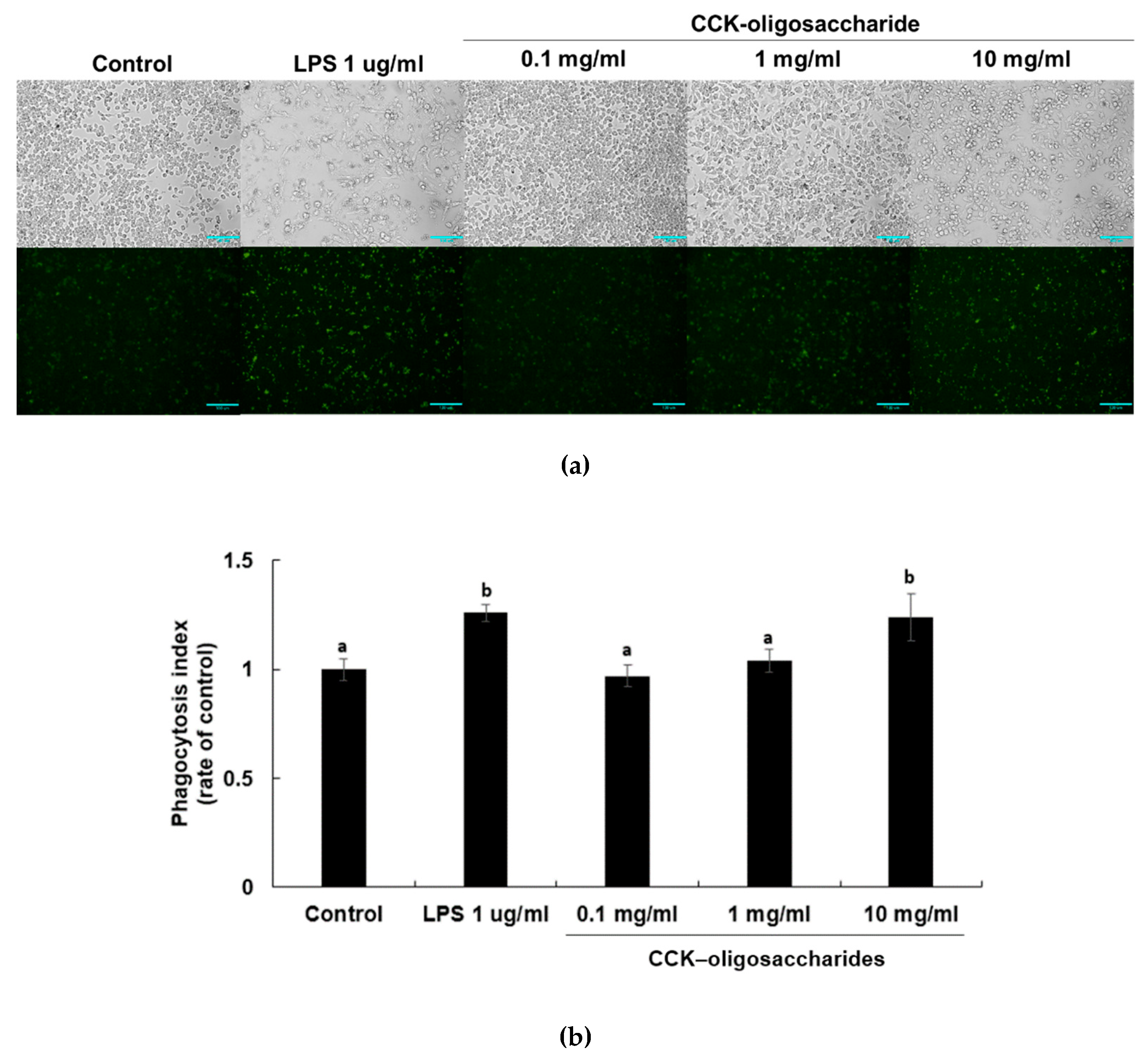
2.2. Effect of CCK-Oligosaccharides on Phagocytic Activity of RAW264.7 Cells
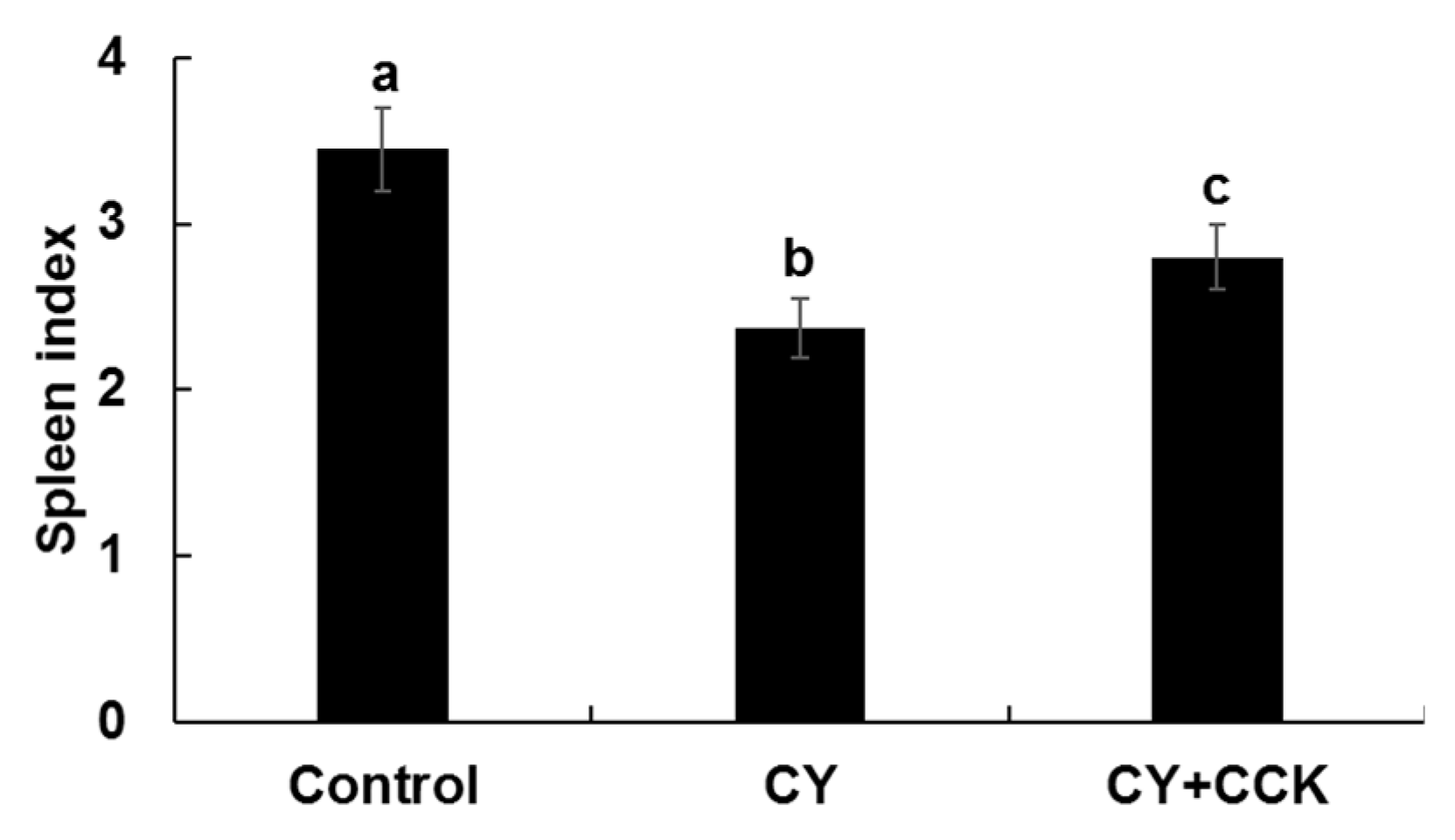
2.3. Effect of CCK-Oligosaccharides on Spleen Indices of CY-Induced Mice
2.4. Effect of CCK-Oligosaccharides on mRNA Expression of Peritoneal Macrophages
3. Materials and Methods
3.1. Preparation of CCK-Oligosaccharides
3.2. Cell Culture
3.3. Western Blot
3.4. Phagocytosis Assay
3.5. Animal Experiments
3.5.1. Animals
3.5.2. Induction of Immunosuppressed Murine Model and Treatment Protocols
3.5.3. Calculation of Spleen Indices
3.5.4. Isolation of Mouse Peritoneal Macrophages
3.5.5. RNA Extraction and cDNA Synthesis
3.5.6. Real-Time PCR
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Argüello Morales, M.A.; Remaud–Simeon, M.; Willemot, R.M.; Vignon, M.R.; Monsan, P. Novel oligosaccharides synthesized from sucrose donor and cellobiose acceptor by alternansucrase. Carbohydr. Res. 2001, 331, 403–411. [Google Scholar] [CrossRef]
- Lee, S.; Hanh, N.T.T.; Cho, J.Y.; Kim, J.Y.; Moon, Y.H.; Yeom, S.C.; Kim, G.J.; Kim, D. Glucooligosaccharide production by Leuconostoc mesenteroides fermentation with efficient pH control, using a calcium hydroxide–sucrose solution. Biotechnol. Bioproc. E. 2016, 21, 39–45. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Seo, Y.S.; Cho, J.Y.; Lee, S.; Kim, G.J.; Yoon, J.W.; Ahn, S.H.; Hwang, K.H.; Park, J.S.; Jang, T.S.; et al. Synthesis of oligosaccharide–containing orange juice using glucansucrase. Biotechnol. Bioproc. E. 2015, 20, 447–452. [Google Scholar] [CrossRef]
- Salim, А.S.; Bivolarski, V.P.; Vasileva, T.A.; Iliev, I.N. Enzymatic synthesis of fructo–oligosaccharides by recombinant levansucrase from Leuconostoc mesenteroides Lm17. Bulg. Chem. Commun. 2017, 49, 259–264. [Google Scholar]
- Lee, S.; Park, Y.S. Oligosaccharide production by Leuconostoc lactis CCK940 which has glucansucrase activity. Food Eng. Prog. 2017, 21, 383–390. [Google Scholar] [CrossRef]
- Lee, S.; Park, G.G.; Jang, J.K.; Park, Y.S. Optimization of oligosaccharide production from Leuconostoc lactis using a response surface methodology and the immunostimulating effects of these oligosaccharides on macrophage cells. Molecules 2018, 23, 2118. [Google Scholar] [CrossRef]
- Xu, X.; Wu, X.; Wang, Q.; Cai, N.; Zhang, H.; Jiang, Z.; Wan, M.; Oda, T. Immunomodulatory effects of alginate oligosaccharides on murine macrophage RAW264.7 cells and their structure–activity relationships. J. Agric. Food Chem. 2014, 62, 3168–3176. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Kanneganti, T.D.; Lamkanfi, M.; Amer, A.O. Innate immune pathways in host defense. Mediators Inflamm. 2012, 708972. [Google Scholar] [CrossRef]
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C.A. Innate immunity: Impact on the adaptive immune response. Curr. Opin. Immunol. 1997, 9, 4–9. [Google Scholar] [CrossRef]
- Chen, H.H.; Chen, Y.K.; Chang, H.C.; Lin, S.Y. Immunomodulatory effects of xylooligosaccharides. Food Sci. Technol. Res. 2012, 18, 195–199. [Google Scholar] [CrossRef]
- Kim, H.M.; Hong, S.H.; Yoo, S.J.; Baek, K.S.; Jeon, Y.J.; Choung, S.Y. Differential effects of chitooligosaccharides on serum cytokine levels in aged subjects. J. Med. Food 2006, 9, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N.; Cooper, P.D. Carbohydrate–based immune adjuvants. Expert Rev. Vaccines 2011, 10, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Vulevic, J.; Drakoularakou, A.; Yaqoob, P.; Tzortzis, G.; Gibson, G.R. Modulation of the fecal microflora profile and immune function by a novel trans–galactooligosaccharide mixture (B–GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 2008, 88, 1438–1446. [Google Scholar] [CrossRef]
- Xu, X.; Bi, D.; Wu, X.; Wang, Q.; Wei, G.; Chi, L.; Jiang, Z.; Oda, T.; Wan, M. Unsaturated guluronate oligosaccharide enhances the antibacterial activities of macrophages. FASEB J. 2014, 8, 2645–2654. [Google Scholar] [CrossRef]
- Xiao, J.H.; Liang, Z.Q.; Liu, A.Y.; Chen, D.X.; Xiao, Y.; Liu, J.W.; Wan, W.H. Immunosuppressive activity of polysaccharides from Cordyceps gunnii mycelia in mice in vivo/vitro. J. Food Agric. Environ. 2004, 2, 69–73. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Chen, J.; Tang, Y.; Dou, J.; Yu, J.; Xi, T.; Zhou, C. A polysaccharide from Strongylocentrotus nudus eggs protects against myelosuppression and immunosuppression in cyclophosphamide–treated mice. Int. Immunopharmacol. 2011, 11, 1946–1953. [Google Scholar] [CrossRef]
- Chen, J.R.; Yang, Z.Q.; Hu, T.J.; Yan, Z.T.; Niu, T.X.; Wang, L.; Cui, D.A.; Wang, M. Immunomodulatory activity in vitro and in vivo of polysaccharide from Potentilla anserina. Fitoterapia 2010, 81, 1117–1124. [Google Scholar] [CrossRef]
- Mei, Y.; Chen, H.; Zhang, J.; Zhang, X.; Liang, Y. Protective effect of chitooligosaccharides against cyclophosphamide–induced immunosuppression in mice. Int. J. Biol. Macromol. 2013, 62, 330–335. [Google Scholar] [CrossRef]
- Rao, K.M. MAP kinase activation in macrophages. J. Leukoc. Biol. 2001, 69, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Schorey, J.S.; Cooper, A.M. Macrophage signalling upon mycobacterial infection: The MAP kinases lead the way. Cell. Microbiol. 2003, 5, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.L.; Meng, L.Z.; Chen, X.Q.; Wang, L.Y.; Wu, D.T.; Zhao, J.; Li, S.P. Structural elucidation, chain conformation and immuno–modulatory activity of glucogalactomannan from cultured Cordyceps sinensis fungus UM01. J. Funct. Foods 2016, 25, 174–185. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, C.; Peng, G.; Liu, Y.; Zhang, X.; Meng, Z. Immune–enhancing effects of a polysaccharide PRG1–1 from Russula griseocarnosa on RAW264.7 macrophage cells via the MAPK and NF–κB signalling pathways. Food Agr. Immunol. 2018, 29, 833–844. [Google Scholar] [CrossRef]
- Aderem, A.; Underhill, D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef]
- Navegantes, K.C.; de Souza Gomes, R.; Pereira, P.A.T.; Czaikoski, P.G.; Azevedo, C.H.M.; Monteiro, M.C. Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Transl. Med. 2017, 15, 36. [Google Scholar] [CrossRef]
- Jaumouillé, V.; Grinstein, S. Receptor mobility, the cytoskeleton, and particle binding during phagocytosis. Curr. Opin. Cell Biol. 2011, 23, 22–29. [Google Scholar] [CrossRef]
- Kusner, D.J.; Hall, C.F.; Jackson, S. Fc gamma receptor–mediated activation of phospholipase D regulates macrophage phagocytosis of IgG–opsonized particles. J. Immunol. 1999, 162, 2266–2274. [Google Scholar]
- Rajagopalan, S.; Meng, X.P.; Ramasamy, S.; Harrison, D.G.; Galis, Z.S. Reactive oxygen species produced by macrophage–derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J. Clin. Invest. 1996, 98, 2572–2579. [Google Scholar] [CrossRef]
- Razali, F.N.; Ismail, A.; Abidin, N.Z.; Shuib, A.S. Stimulatory effects of polysaccharide fraction from Solanum nigrum on RAW 264.7 murine macrophage cells. PLoS ONE 2014, 9, 108988. [Google Scholar] [CrossRef]
- Maeda, R.; Ida, T.; Ihara, H.; Sakamoto, T. Immunostimulatory activity of polysaccharides isolated from Caulerpa lentillifera on macrophage cells. Biosci. Biotechnol. Biochem. 2012, 76, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhang, X.; Li, B.; Liu, Z.; Wang, M.; Liu, S. Anti–tumour and immunomodulatory activities of oligosaccharides isolated from Panax ginseng C.A. Meyer. Int. J. Biol. Macromol. 2014, 65, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, T.; Zheng, R. Antioxidant activities of Sophora subprosrate polysaccharide in immunosuppressed mice. Int. Immunopharmacol. 2007, 7, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nie, W.; Fan, S.; Zhang, J.; Wang, Y.; Lu, J.; Jin, L. A polysaccharide from Sargassum fusiforme protects against immunosuppression in cyclophosphamide–treated mice. Carbohyd. Polym. 2012, 90, 1114–1119. [Google Scholar] [CrossRef]
- Wang, H.; Actor, J.K.; Indrigo, J.; Olsen, M.; Dasgupta, A. Asian and Siberian ginseng as a potential modulator of immune function: An in vitro cytokine study using mouse macrophages. Clin. Chim. Acta 2003, 327, 123–128. [Google Scholar] [CrossRef]
- Peters, M.; Meyer Zum Büschenfelde, K.H.; Rose–John, S. The function of the soluble IL–6 receptor in vivo. Immunol. Lett. 1996, 54, 177–184. [Google Scholar] [CrossRef]
- Sobota, R.M.; Müller, P.J.; Khouri, C.; Ullrich, A.; Poli, V.; Noguchi, T.; Heinrich, P.C.; Schaper, F. SHPS–1/SIRP1α contributes to interleukin–6 signalling. Cell. Signal. 2008, 20, 1385–1391. [Google Scholar] [CrossRef]
- Marshall, B.G.; Chambers, M.A.; Wangoo, A.; Shaw, R.J.; Young, D.B. Production of tumor necrosis factor and nitric oxide by macrophages infected with live and dead mycobacteria and their suppression by an interleukin–10–secreting recombinant. Infect. Immun. 1997, 65, 1931–1935. [Google Scholar]
- Stober, C.B.; Lange, U.G.; Roberts, M.T.M.; Alcami, A.; Blackwell, J.M. IL–10 from regulatory T cells determines vaccine efficacy in murine Leishmania major infection. J. Immunol. 2005, 175, 2517–2524. [Google Scholar] [CrossRef]
- Villalta, S.A.; Rinaldi, C.; Deng, B.; Liu, G.; Fedor, B.; Tidball, J.G. Interleukin–10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum. Mol. Genet. 2011, 20, 790–805. [Google Scholar] [CrossRef]
- Filippi, C.; von Herrath, M. IL–10 and the resolution of infections. J. Pathol. 2008, 214, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Marincola, F.; Riccardo Rossi, C.; Nitti, D.; Lise, M. The multifaceted relationship between IL–10 and adaptive immunity: Putting together the pieces of a puzzle. Cytokine Growth Factor Rev. 2004, 15, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Baud, V.; Karin, M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001, 11, 372–377. [Google Scholar] [CrossRef]
- Na, Y.S.; Kim, W.J.; Kim, S.M.; Park, J.K.; Lee, S.M.; Kim, S.O.; Synytsya, A.; Park, Y.I. Purification, characterization and immunostimulating activity of water–soluble polysaccharide isolated from Capsosiphon fulvescens. Int. Immunopharmacol. 2010, 10, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Maresz, K.; Lee, P.S.; Wu, J.C.; Ho, C.T.; Popko, J.; Mehta, D.S.; Stohs, S.J.; Badmaev, V. Inhibition of TNF–α, IL–1α, and IL–1β by pretreatment of human monocyte–derived macrophages with menaquinone–7 and cell activation with TLR agonists in vitro. J. Med. Food 2016, 19, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhen, H.; Wang, N.; Yan, Q.; Jing, H.; Jiang, Z. Curdlan oligosaccharides having higher immunostimulatory activity than curdlan in mice treated with cyclophosphamide. Carbohyd. Polym. 2019, 207, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Wang, G.; You, L.; Zhang, L.; Ren, H.; Hu, W.; Qiang, Q.; Wang, X.; Ji, L.; Gu, Z.; et al. Polysaccharide from wheat bran induces cytokine expression via the toll–like receptor 4–mediated p38 MAPK signaling pathway and prevents cyclophosphamide–induced immunosuppression in mice. Food Nutr. Res. 2017, 61, 1344523. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, Q.; Kan, X.; Peng, L.; Xu, X.; Fang, Y.; Yang, J. Immunomodulatory activity of a novel polysaccharide from Lonicera japonica in immunosuppressed mice induced by cyclophosphamide. PLoS ONE 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Monmai, C.; Park, S.H.; You, S.; Park, W.J. Immuno–enhancement effect of polysaccharide extracted from Stichopus japonicus on cyclophosphamide–induced immunosuppression mice. Food Sci. Biotechnol. 2018, 27, 565–573. [Google Scholar] [CrossRef]
- Kim, D.; Robyt, J.F. Production, selection, and characteristics of mutants of Leuconostoc mesenteroides B–742 constitutive for dextransucrases. Enzyme Microb. Tech. 1995, 17, 689–695. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds CCK-oligosaccharides are available from the authors. |

| Gene | Sequence | |
|---|---|---|
| GAPDH | Forward | 5′―ATC CCA TCA CCA TCT TCC AG―3′ |
| Reverse | 5′―CCT GCT TCA CCA CCT TCT TG―3′ | |
| IL-6 | Forward | 5′―CAA GAG ACT TCC ATC CAG TTG C―3′ |
| Reverse | 5′―TTG CCG AGT TCT CAA AGT GAC―3′ | |
| IL-10 | Forward | 5′―TGC TAT GCT GCC TGC TCT TA―3′ |
| Reverse | 5′―GGC AAC CCA AGT AAC CCA TA―3′ | |
| TNF-α | Forward | 5′―ATG AGC ACA GAA AGC ATG ATC CG―3′ |
| Reverse | 5′―CCA AAG TAG ACC TGC CCG GAC TC―3′ | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Song, I.H.; Park, Y.-S. In Vivo and In Vitro Study of Immunostimulation by Leuconostoc lactis-Produced Gluco-Oligosaccharides. Molecules 2019, 24, 3994. https://doi.org/10.3390/molecules24213994
Lee S, Song IH, Park Y-S. In Vivo and In Vitro Study of Immunostimulation by Leuconostoc lactis-Produced Gluco-Oligosaccharides. Molecules. 2019; 24(21):3994. https://doi.org/10.3390/molecules24213994
Chicago/Turabian StyleLee, Sulhee, In Ho Song, and Young-Seo Park. 2019. "In Vivo and In Vitro Study of Immunostimulation by Leuconostoc lactis-Produced Gluco-Oligosaccharides" Molecules 24, no. 21: 3994. https://doi.org/10.3390/molecules24213994
APA StyleLee, S., Song, I. H., & Park, Y.-S. (2019). In Vivo and In Vitro Study of Immunostimulation by Leuconostoc lactis-Produced Gluco-Oligosaccharides. Molecules, 24(21), 3994. https://doi.org/10.3390/molecules24213994





