Brief Story on Prostaglandins, Inhibitors of their Synthesis, Hematopoiesis, and Acute Radiation Syndrome
Abstract
1. Introduction
2. Hematological Effects of Prostaglandin E2 (PGE2)
3. Concise Overview of Acute Radiation Syndrome
4. Prostaglandins Act Radioprotectively
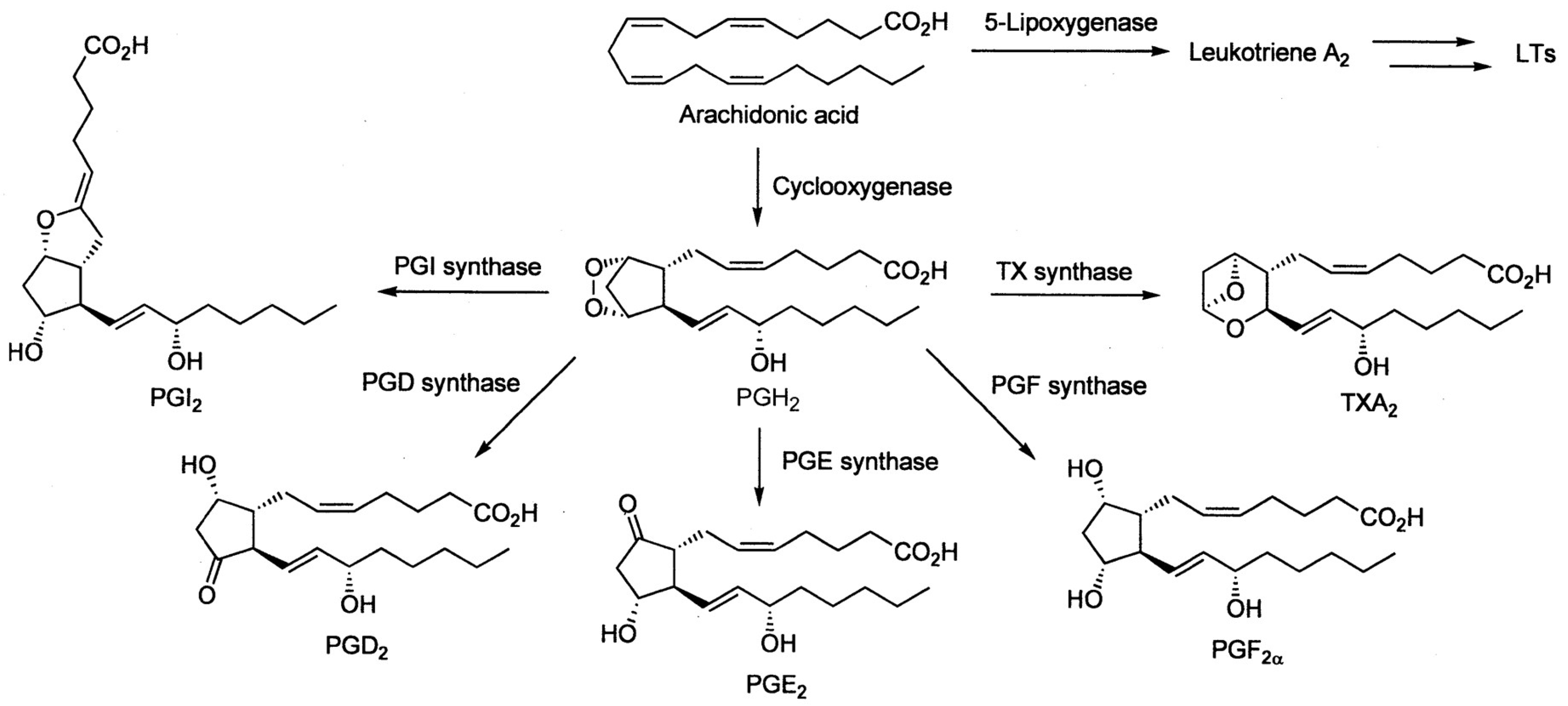
5. Cyclooxygenases Carry out Prostaglandin Synthesis, Their Inhibition Can Be Selective
6. Effects of Non-Selective COX Inhibitors in Sublethally and Lethally Irradiated Experimental Animals
7. Effects of Selective COX-2 Inhibitors in Sublethally and Lethally Irradiated Experimental Animals
8. Summarization and Considerations on Hematological and Radiation-Modulating Effects of Selective COX-2 Inhibitor Meloxicam
9. Considerations Concerning Connecting Pharmacological Interventions with Prostaglandins and Inhibitors of Their Synthesis into One Treatment Scheme
10. Supplementary Note on COX-2-Deficient Mice, Hematopoiesis, and Myelosuppression
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pelus, L.M.; Hoggatt, J. Pleiotropic effects of prostaglandin E2 in hematopoiesis; prostaglandin E2 and other eicosanoids regulate hematopoietic stem and progenitor cell function. Prostaglandins Other Lipid Mediat. 2011, 96, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Pospíšil, M.; Hoferová, Z.; Weiterová, L.; Komůrková, D. Stimulatory action of cyclooxygenase inhibitors on hematopoiesis: A review. Molecules 2012, 17, 5615–5625. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Hoferová, Z.; Falk, M. Pharmacological modulation of radiation damage. Does it exist a chance for other substances than hematopoietic growth factors and cytokines? Int. J. Mol. Sci. 2017, 18, 1385. [Google Scholar] [CrossRef]
- Moulder, J.E. Post-irradiation approaches to treatment of radiation injuries in the context of radiological terrorism and radiation accidents: A review. Int. J. Radiat. Biol. 2004, 80, 546–555. [Google Scholar] [CrossRef]
- Pellmar, T.C.; Rockwell, S. Priority list of reserach areas for radiological nuclear threat countermeasures. Radiat. Res. 2005, 163, 115–123. [Google Scholar] [CrossRef]
- Dainiak, N. Medical management of acute radiation syndrome and associated infections in a high-casualty incident. J. Radiat. Res. 2018, 59, ii54–ii64. [Google Scholar] [CrossRef]
- Fehér, O.; Gidáli, J. Prostaglandin E2 as stimulator of haemopoietic stem cell proliferation. Nature 1974, 247, 550–551. [Google Scholar] [CrossRef]
- Fontagné, J.; Adolphe, M.; Semichon, M.; Zizina, L.; Lechat, P. Effect of in vivo treatment with indomethacin on mouse granulocyte-macrophage colony-forming cells in culture (CFUc). Possible role of prostaglandins. Exp. Hematol. 1980, 8, 1157–1164. [Google Scholar]
- Hanson, W.R.; Thomas, C. 16,16-dimethyl prostaglandin E2 increases survival of murine intestinal stem-cells when given before photo radiation. Radiat. Res. 1983, 96, 393–398. [Google Scholar] [CrossRef]
- Kozubík, A.; Pospíšil, M.; Netíková, J. The stimulatory effect of single-dose pre-irradiation administration of indomethacin and diclofenac on hematopoietic recovery in the spleen of gamma-irradiated mice. Stud. Biophys. 1989, 131, 93–101. [Google Scholar]
- Pelus, L.M.; Broxmeyer, H.E.; Kurland, J.I.; Moore, M.A. Regulation of macrophage and granulocyte proliferation. Specificities of prostaglandin E and lactoferrin. J. Exp. Med. 1979, 150, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Pelus, L.M.; Broxmeyer, H.E.; Moore, M.A. Regulation of human myelopoiesis by prostaglandin E and lactoferrin. Cell Tissue Kinet. 1981, 14, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Kurland, J.I.; Moore, M.A.S. Modulation of hemopoiesis by prostaglandins. Exp. Hematol. 1977, 5, 357–373. [Google Scholar] [PubMed]
- Kurland, J.I.; Broxmeyer, H.E.; Pelus, L.M.; Bockman, R.S.; Moore, M.A.S. Role of monocyte-macrophage-derived factor and prostaglandin E in the positive and negative feedback control of myeloid stem cell proliferation. Blood 1978, 52, 388–407. [Google Scholar] [CrossRef] [PubMed]
- Kurland, J.I.; Bockman, R.S.; Broxmeyer, H.E.; Moore, M.A.S. Limitation of excessive myelopoiesis by intrinsic modulation of macrophage-derived prostaglandin-E. Science 1978, 199, 552–555. [Google Scholar] [CrossRef]
- Gentile, P.; Byer, D.; Pelus, L.M. In vivo modulation of murine myelopoiesis following intravenous administration of prostaglandin E2. Blood 1983, 62, 1100–1107. [Google Scholar] [CrossRef]
- Pelus, L.M.; Ottmann, O.G.; Nocka, K.H. Synergistic inhibition of human bone marrow granulocyte-macrophage progenitor cells by prostaglandin E and recombinant interferon-alpha, -beta, and -gamma and an effect mediated by tumor necrosis factor. J. Immunol. 1988, 140, 479–484. [Google Scholar]
- Dukes, P.P.; Shore, N.A.; Hammond, D.; Ortega, J.A.; Datta, M.C. Enhancement of erythropoiesis by prostaglandins. J. Lab. Clin. Med. 1973, 82, 704–712. [Google Scholar]
- DeGowin, R.L.; Gibson, D.P. Prostaglandin-mediated enhancement of erythroid colonies by marrow stromal cells (MSC). Exp. Hematol. 1981, 9, 274–280. [Google Scholar]
- Lu, L.; Pelus, L.M.; Broxmeyer, H.E. Modulation of the expression of HLA-DR (Ia) antigens and the proliferation of human erythroid (BFU-E) and multipotential (CFU-GEMM) progenitor cells by prostaglandin E. Exp. Hematol. 1984, 12, 741–748. [Google Scholar]
- Lu, L.; Pelus, L.M.; Broxmeyer, H.E.; Moore, M.A.; Wachter, M.; Walker, D.; Platzer, E. Enhancement of the proliferation of human marrow eyrthroid (BFU-E) progenitor cells by prostaglandin E requires the participation of OKT8-positive T lymphocytes and is associated with the density expression of major histocompatibility complex class II antigens on BFU-E. Blood 1986, 68, 126–133. [Google Scholar] [PubMed]
- Lu, L.; Pelus, L.M.; Piacibello, W.; Moore, M.A.; Hu, W.; Broxmeyer, H.E. Prostaglandin E acts at two levels to enhance colony formation in vitro by erythroid (BFU-E) progenitor cells. Exp. Hematol. 1987, 15, 765–771. [Google Scholar] [PubMed]
- Nocka, K.H.; Ottman, O.G.; Pelus, L.M. The role of marrow accessory cell populations in the augmentation of human erythroid progenitor cell (BFU-E) proliferation by prostaglandin E. Leuk. Res. 1989, 13, 527–534. [Google Scholar] [CrossRef]
- Pelus, L.M. Association between colony-forming units-granulocyte macrophage expression of Ia-like (HLA-DR) antigen and control of granulocyte and macrophage production. A new role for prostaglandin E. J. Clin. Investig. 1982, 70, 568–578. [Google Scholar] [CrossRef]
- Dupuis, F.; Gachard, N.; Allegraud, A.; Praloran, V.; Denizot, Y. Prostaglandin E2 stimulates the growth of human blood CD34+ progenitors. Prostaglandins Other Lipid Mediat. 1998, 55, 179–186. [Google Scholar] [CrossRef]
- Hoggatt, J.; Singh, P.; Sampath, J.; Pelus, L.M. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood 2009, 113, 5444–5455. [Google Scholar] [CrossRef]
- Pelus, L.M.; Hoggatt, J.; Singh, P. Pulse exposure of haematopoietic grafts to prostaglandin E2 in vitro facilitates engraftment and recovery. Cell Prolif. 2011, 44 (Suppl. 1), 22–29. [Google Scholar] [CrossRef]
- Donnelly, E.H.; Nemhauser, J.B.; Smith, J.M.; Kazzi, Z.N.; Farfán, E.B.; Chang, A.S. Acute radiation syndrome: Assessment and management. South. Med. J. 2010, 103, 541–544. [Google Scholar] [CrossRef]
- Coleman, C.N.; Blakely, W.F.; Fike, J.R.; Mac Vittie, T.J.; Metting, N.F.; Mitchell, J.B.; Moulder, J.E.; Preston, R.J.; Seed, T.M.; Stone, H.B.; et al. Molecular and cellular biology of moderate-dose radiation and potential mechanisms of radiation protection: Report of a workshop at Bethesda, Maryland, 17–18 December 2001. Radiat. Res. 2003, 159, 812–834. [Google Scholar] [CrossRef]
- Waselenko, J.K.; MacVittie, T.J.; Blakely, W.F.; Pesik, N.; Wiley, A.L.; Dickerson, W.E.; Tsu, H.; Confer, D.L.; Coleman, C.N.; Seed, T.; et al. Strategic National Stockpile Working Group. Medical management of the acute radiation syndrome. Recommendations of the Strategic National Stockpile Radiation Working Group. Ann. Intern. Med. 2004, 40, 1037–1051. [Google Scholar] [CrossRef]
- Hall, E.J. Radiobiology for Radiologists, 5th ed.; Lippincott Williams & Wilkins: New York, NY, USA, 2000. [Google Scholar]
- Prasad, K.N. Handbook of Radiobiology, 5th ed.; CRC Press, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Bond, V.P.; Fliedner, T.M.; Archambeau, J.O. Mammalian Radiation Lethality; Academic Press: New York, NY, USA; London, UK, 1965; p. 107. [Google Scholar]
- Bond, V.P.; Robertson, J.S. Vertebrate radiobiology (lethal actions and associated effects). Ann. Rev. Nucl. Sci. 1957, 7, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Hanson, W.R. Radiation protection of the murine intestine by misoprotol, a prostaglandin-E1 analog, given alone or with WR-2721, is stereospecific. Prostaglandins Leukot. Essent. Fatty Acids 1988, 32, 101–105. [Google Scholar] [PubMed]
- Hanson, W.R.; Ainsworth, E.J. 16,16-dimethyl prostaglandin E2 induces radioprotection in murine intestinal and hematopoietic stem-cells. Radiat. Res. 1985, 103, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Walden, T.L.; Patchen, M.; Snyder, J.L. 16,16-dimethyl prostaglandin E2 increases survival in mice following irradiation. Radiat. Res. 1987, 109, 440–444. [Google Scholar] [CrossRef]
- Hanson, W.R.; Zhen, W.N.; Geng, L.; Hunter, N.; Milas, L. The prostaglandin E1 analog, misoprostol, a normal tissue protector, does not protect 4 murine tumors in-vivo from radiation injury. Radiat. Res. 1995, 142, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shao, L.; Hendrickson, H.P.; Liu, L.; Chang, J.; Luo, Y.; SEng, J.; Pouliot, M.; Authier, S.; Zhou, D.; et al. Total body irradiation in the “hematopoietic” dose range induces substantial injury intestinal in jury in non-human primates. Radiat. Res. 2015, 184, 545–553. [Google Scholar] [CrossRef]
- Smith, W.L. The eicosanoids and their biochemical mechanisms of action. Biochem. J. 1989, 259, 315–324. [Google Scholar] [CrossRef]
- Frölich, J.C. A classification of NSAIDs according to the relative inhibition of cyclooxygenase isoenzymes. Trends Pharmacol. Sci. 1997, 18, 30–34. [Google Scholar] [CrossRef]
- Marnett, L.J.; Rowlinson, S.W.; Goodwin, D.C.; Kalgutkar, A.S.; Lanzo, C.A. Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J. Biol. Chem. 1999, 274, 22903–22906. [Google Scholar] [CrossRef]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isoenzymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef]
- Smith, W.L.; Urade, Y.; Jakobsson, P.-J. Enzymes of the cyclooxygenase pathways of prostanoid synthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar] [CrossRef] [PubMed]
- Blain, H.; Jouzeau, J.Y.; Netter, P.; Jeandel, C. Non-steroidal anti-inflammatory drugs (NSAIDs) drugs with selective inhibitory activity on cyclooxygenase 2. Interest and future prospects. La Revue de Medecine Interne 2000, 21, 978–988. [Google Scholar] [CrossRef]
- Patrignani, P.; Patrono, C. Cyclooxygenase inhibitors: From pharmacology to clinical read-outs. Biochim. Biophys. Acta 2015, 1851, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Hoggatt, J.; Pelus, L.M. How beneficial is the use of NSAIDs in stem cell transplantation? Expert Opin. Pramacother. 2013, 14, 2453–2456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lanas, A.; Panés, J.; Piqué, J.M. Clinical implications of COX-1 and/or COX-2 inhibition for the distal gastrointestinal tract. Curr. Phar. Des. 2003, 9, 2253–2266. [Google Scholar] [CrossRef]
- Cohn, S.M.; Schloeman, S.; Tessner, T.; Seibert, K.; Stenson, W.F. Crypt stem cell survival in the mouse intestinal epithelium is regulated by prostaglandin synthesis through cyclooxygenase-1. J. Clin. Invetsig. 1997, 99, 1367–1379. [Google Scholar] [CrossRef]
- Maruyama, T.; Nakai, H. Investigation of prostanoid synthesis. J. Synth. Org. Chem. Jpn. 2007, 65, 481–491. [Google Scholar] [CrossRef]
- Boorman, G.A.; Luster, M.I.; Dean, J.H.; Luebke, R.W. Effect of indomethacin on the bone marrow and immune system of the mouse. J. Clin. Lab. Immunol. 1982, 7, 119–126. [Google Scholar]
- Takahashi, H.K.; Iwagako, H.; Tamura, R.; Xue, D.; Sano, M.; Mori, S.; Yoshino, T.; Tanaka, N.; Nishibori, M. Unique regulation profile of prostaglandin E1 on mononuclear cells. J. Pharmacol. Exp. Therap. 2003, 307, 1188–1195. [Google Scholar] [CrossRef]
- Chang, D.M.; Baptiste, P.; Chur, P.H. The effect of antirheumatic drugs on interleukin-1 (IL-1) activity and IL-1 inhibitor production by human monocytes. J. Rheumatol. 1990, 17, 1148–1157. [Google Scholar]
- Lozanski, G.; Ballou, S.P.; Kushner, I. Effect of flurbiprofen on cytokine production by human monocytes and U-937 and THP-1 cell lines. J. Rheumatol. 1992, 19, 921–926. [Google Scholar] [PubMed]
- Hofer, N.; Pospíšil, M. Stimulated recovery of perturbed hemartopoiesis by inhibition of prostaglandin production—Promising therapeutic strategy. Cent. Eur. J. Biol. 2006, 1, 584–593. [Google Scholar]
- Nishiguchi, I.; Furuta, Y.; Hunter, N.; Murray, D.; Milas, L. Radioprotection of haematopoietic tissue by indomethacin. Radiat. Res. 1990, 122, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, M.; Netíková, J.; Kozubík, A.; Pipalová, I. Effect of indomethacin, diclofenac sodium and sodium salicylate on peripheral blood cell counts in sublethally gamma-irradiated mice. Strahlenther. Onkol. 1989, 165, 627–631. [Google Scholar]
- Hofer, M.; Pospíšil, M.; Pipalová, I.; Holá, J. Modulation of haemopoietic radiation response of mice by diclofenac in fractionated treatment. Physiol. Res. 1996, 45, 213–220. [Google Scholar]
- Hofer, M.; Pospíšil, M.; Viklická, Š.; Vacek, A.; Pipalová, I.; Bartoníčková, A. Hematopoietic recovery in repeatedly irradiated mice can be enhanced by a repeatedly administered combination of diclofenac and glucan. J. Leukoc. Biol. 1993, 53, 185–189. [Google Scholar] [CrossRef]
- Fedoročko, P.; Macková, N.O. Combined modality radioprotection: Enhancement of survival and hematopoietic recovery by the joint use of liposomal muramyl tripeptide phosphatidylethanolamine (MTP-PE) and indomethacin. Int. J. Immunopharmacol. 1996, 18, 329–337. [Google Scholar] [CrossRef]
- Fedoročko, P.; Macková, N.O. Radioprotective effects of combination of bronchovaxom, a macrophage activator, and indomethacin, an inhibitor of prostaglandin production: Relationships to myelopoiesis. Eur. J. Haematol. 1996, 56, 54–61. [Google Scholar] [CrossRef]
- Kozubík, A.; Pospíšil, M.; Netíková, J. Possibilities of the combined use of non-steroidal anti-inflammatory drugs and sulfhydryl compounds in radioprotection. Strahlenther. Onkol. 1991, 167, 186–190. [Google Scholar]
- Floersheim, G.L. Allopurinol, indomethacin and riboflavin enhance radiation lethality in mice. Radiat. Res. 1994, 139, 240–247. [Google Scholar] [CrossRef]
- Hofer, M.; Popsíšil, M.; Tkadleček, L.; Viklická, Š.; Pipalová, I. Low survival of mice following lethal gamma-irradiation after administration of inihibitors of prostaglandin synthesis. Physiol. Res. 1992, 41, 157–161. [Google Scholar] [PubMed]
- Wang, J.Y.; Yamasaki, S.; Takeuchi, K.; Okabe, S. Delayed healing of acetic acid-induced gastric ulcers in rats by indomethacin. Gastroenterology 1989, 96, 393–402. [Google Scholar] [CrossRef]
- Akarca, U.S. Gastrointestinal effects of selective and non-selective non-steroidal anti-inflammatory drugs. Curr. Pharm. Des. 2005, 11, 1779–1793. [Google Scholar] [CrossRef]
- Shoup, M.; He, L.K.; Liu, H.; Shankar, R.; Gamelli, R. Cyclooxygenase-2 inhibitor NS-398 improved survival and restores leukocyte counts in burn infection. J. Trauma Inj. Infect. Crit. Care 1998, 45, 215–220. [Google Scholar] [CrossRef]
- Ogino, K.; Hatanaka, K.; Kawamura, M.; Ohno, T.; Harada, Y. Meloxicam inhibits prostaglandin E2 generation via cyclooxygenase 2 in the inflammatory site but not that via cyclooxygenase 1 in the stomach. Pharmacology 2000, 61, 244–250. [Google Scholar] [CrossRef]
- Hofer, M.; Pospíšil, M.; Znojil, V.; Holá, J.; Vacek, A.; Weiterová, L.; Štreitová, D.; Kozubík, A. Meloxicam, an inhibitor of cyclooxygenase-2, supports hematopoietic recovery in gamma-irradiated mice. Radiat. Res. 2006, 166, 556–560. [Google Scholar] [CrossRef]
- Hofer, M.; Pospíšil, M.; Holá, J.; Vack, A.; Štreitová, D.; Znojil, V. Inhibition of cyclooxygenase 2 in mice increases production of G-CSF and induces radioprotection. Radiat. Res. 2008, 170, 566–571. [Google Scholar] [CrossRef]
- Hofer, M.; Pospíšil, M.; Znojil, V.; Holá, J.; Vacek, A.; Štreitová, D. Meloxicam, an inhibitor of cyclooxygenase-2, increases the level of serum G-CSF and might be usable as an auxiliary means in G-CSF therapy. Physiol. Res. 2008, 57, 307–310. [Google Scholar]
- Hofer, M.; Pospíšil, M.; Dušek, L.; Hoferová, Z.; Weiterová, L. A single dose of an inhibitor of cyclooxygenase 2, meloxicam, administered shortly after irradiation increases survival of lethally irradiated mice. Radiat. Res. 2011, 176, 269–272. [Google Scholar] [CrossRef]
- Jiao, W.; Kiang, J.G.; Cary, L.; Elliot, T.B.; Pellmar, T.C.; Lednay, G.D. COX-2 inhibitors are contraindicated for treatment of combined injury. Radiat. Res. 2009, 172, 686–697. [Google Scholar] [CrossRef]
- Del Tacca, M.; Colucci, R.; Fornal, M.; Blandizzi, C. Efficacy and tolerability of meloxicam, a COX-2 preferential nonsteroidal anti-inflammatory drug—A review. Clin. Drug Investig. 2002, 22, 799–818. [Google Scholar] [CrossRef]
- Hofer, M.; Pospíšil, M.; Dušek, L.; Hoferová, Z.; Komůrková, D. Agonist of the adenosine A3 receptor, IB-MECA, and inhibitor of cyclooxygenase-2, meloxicam, given alone or in a combination early after total body irradiation enhance survival of γ-irradiated mice. Radiat. Environ. Biophys. 2014, 53, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Hérodin, F.; Bourin, P.; Mayol, J.F.; Lataillade, J.J.; Drouet, M. Short-term injection of antiapoptotic cytokine combination soon after lethal γ-irradiation promotes survival. Blood 2003, 101, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- Hérodin, F.; Drouet, M. Myeloprotection following cytotoxic dmage: The sooner, the better. Exp. Hematol. 2008, 36, 769–770. [Google Scholar] [CrossRef]
- Anning, P.B.; Coles, B.; Morton, J.; Wang, H.; Uddin, J.; Morrox, J.D.; Dey, S.K.; Marnett, L.J.; O’Donnell, V.B. Nitric oxide deficiency promotes vascular side effects of cyclooxygenase inhibitors. Blood 2006, 108, 4059–4062. [Google Scholar] [CrossRef]
- Staerkel, P.; Horsmans, Y. Meloxicam-induced liver toxicity. Acta Gastro-Enterol. Belg. 1999, 62, 255–256. [Google Scholar]
- Hoggatt, J.; Singh, P.; Stilger, K.N.; Plett, P.A.; Sampson, C.H.; Chua, H.L.; Orschell, C.M.; Pelus, L.M. Recovery from hematopoietic injury by modulating prostaglandin E2 signaling post-irradiation. Blood Cells Mol. Dis. 2013, 50, 147–153. [Google Scholar] [CrossRef]
- Weiss, J.F.; Kumar, K.S.; Walden, T.L.; Neta, R.; Landauer, M.R.; Clark, E.P. Advances in radioprotection through the use of combined agent regimens. Int. J. Radiat. Biol. 1990, 57, 709–722. [Google Scholar] [CrossRef]
- Hoseinimehr, S.A. Trends in development of radioprotective agents. Drug Discov. Today 2007, 12, 794–805. [Google Scholar] [CrossRef]
- Hofer, M.; Hoferová, Z.; Depeš, D.; Falk, M. Combining pharmacological countermeasures to attenuate the acute radiation syndrome—A concise review. Molecules 2017, 22, 834. [Google Scholar] [CrossRef]
- Lorenz, M.; Slaughter, H.S.; Wescott, D.M.; Carter, S.I.; Schnyder, B.; Dinchuk, J.E.; Car, B.D. Cyclooxygenase-2 is essential for normal recovery from 5-fluorouracil-induced myelotoxicity in mice. Exp. Hematol. 1989, 10, 1494–1502. [Google Scholar] [CrossRef]
- Hofer, M.; Hoferová, Z.; Dušek, L.; Souček, K.; Gruzdev, A. Hematological profile of untreated or ionizing radiation exposed cyclooxygenase-2-deficient mice. Physiol. Res. 2017, 66, 673–676. [Google Scholar] [PubMed]
- Hofer, M.; Hoferová, Z.; Gruzdev, A.; Dušek, L.; Falk, M. Impaired post-irradiation survival of cyclooxygenase-2-deficient mice. Physiol. Res. 2018, 67, 809–812. [Google Scholar] [CrossRef] [PubMed]

| Species | Mouse | Rat | Dog | Monkey (Macaca) | Rabbit | Guinea Pig | Hamster | Pig | Goat | Man |
|---|---|---|---|---|---|---|---|---|---|---|
| LD50/30 (Gy) | 6.4 | 7.1 | 2.5 | 6.0 | 7.5 | 4.5 | 6.1 | 2.5 | 2.4 | 3.0 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofer, M.; Hoferová, Z.; Falk, M. Brief Story on Prostaglandins, Inhibitors of their Synthesis, Hematopoiesis, and Acute Radiation Syndrome. Molecules 2019, 24, 4019. https://doi.org/10.3390/molecules24224019
Hofer M, Hoferová Z, Falk M. Brief Story on Prostaglandins, Inhibitors of their Synthesis, Hematopoiesis, and Acute Radiation Syndrome. Molecules. 2019; 24(22):4019. https://doi.org/10.3390/molecules24224019
Chicago/Turabian StyleHofer, Michal, Zuzana Hoferová, and Martin Falk. 2019. "Brief Story on Prostaglandins, Inhibitors of their Synthesis, Hematopoiesis, and Acute Radiation Syndrome" Molecules 24, no. 22: 4019. https://doi.org/10.3390/molecules24224019
APA StyleHofer, M., Hoferová, Z., & Falk, M. (2019). Brief Story on Prostaglandins, Inhibitors of their Synthesis, Hematopoiesis, and Acute Radiation Syndrome. Molecules, 24(22), 4019. https://doi.org/10.3390/molecules24224019





