Spruce Bark—A Source of Polyphenolic Compounds: Optimizing the Operating Conditions of Supercritical Carbon Dioxide Extraction
Abstract
1. Introduction
2. Results and Discussion
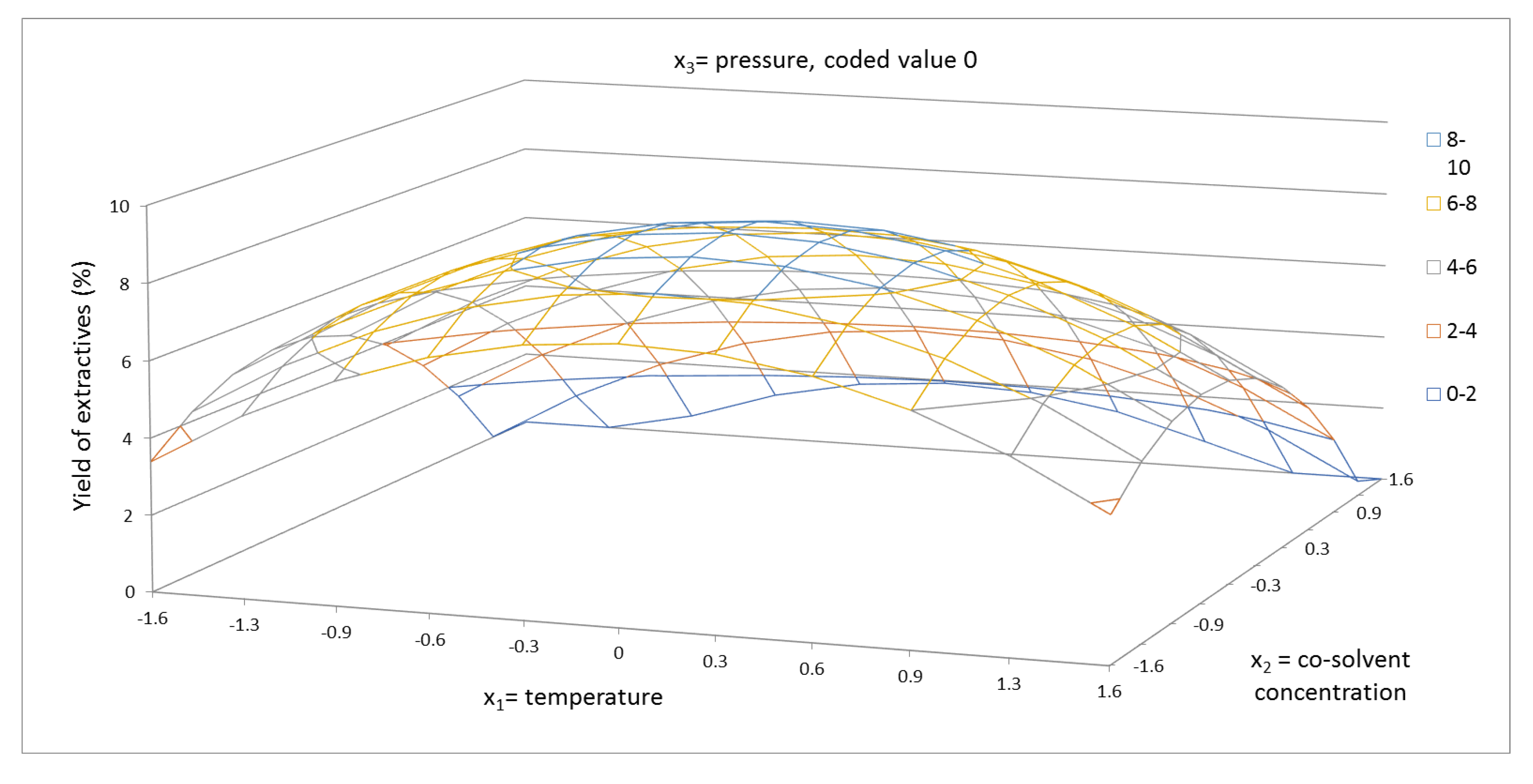
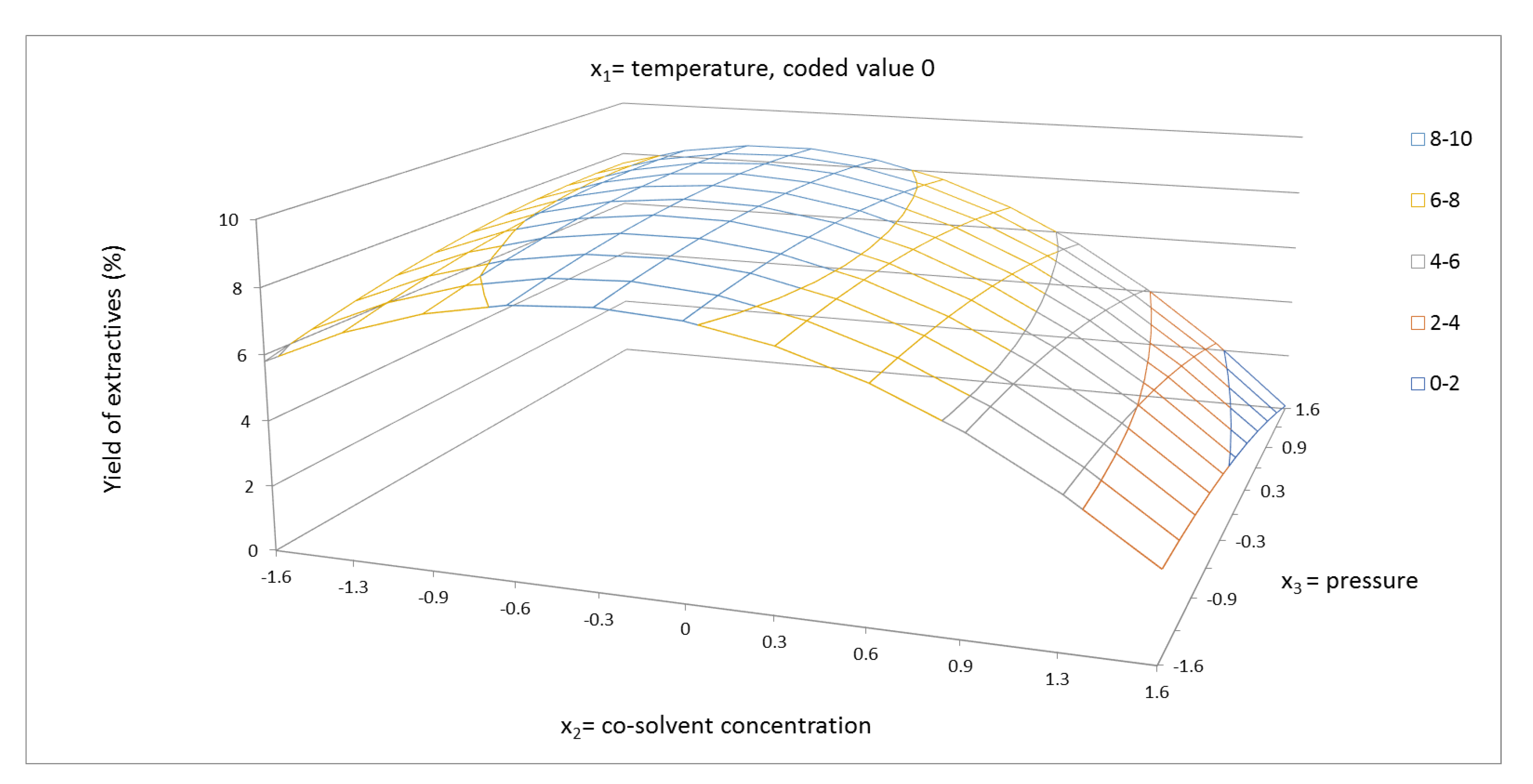
2.1. Yield of Extractives
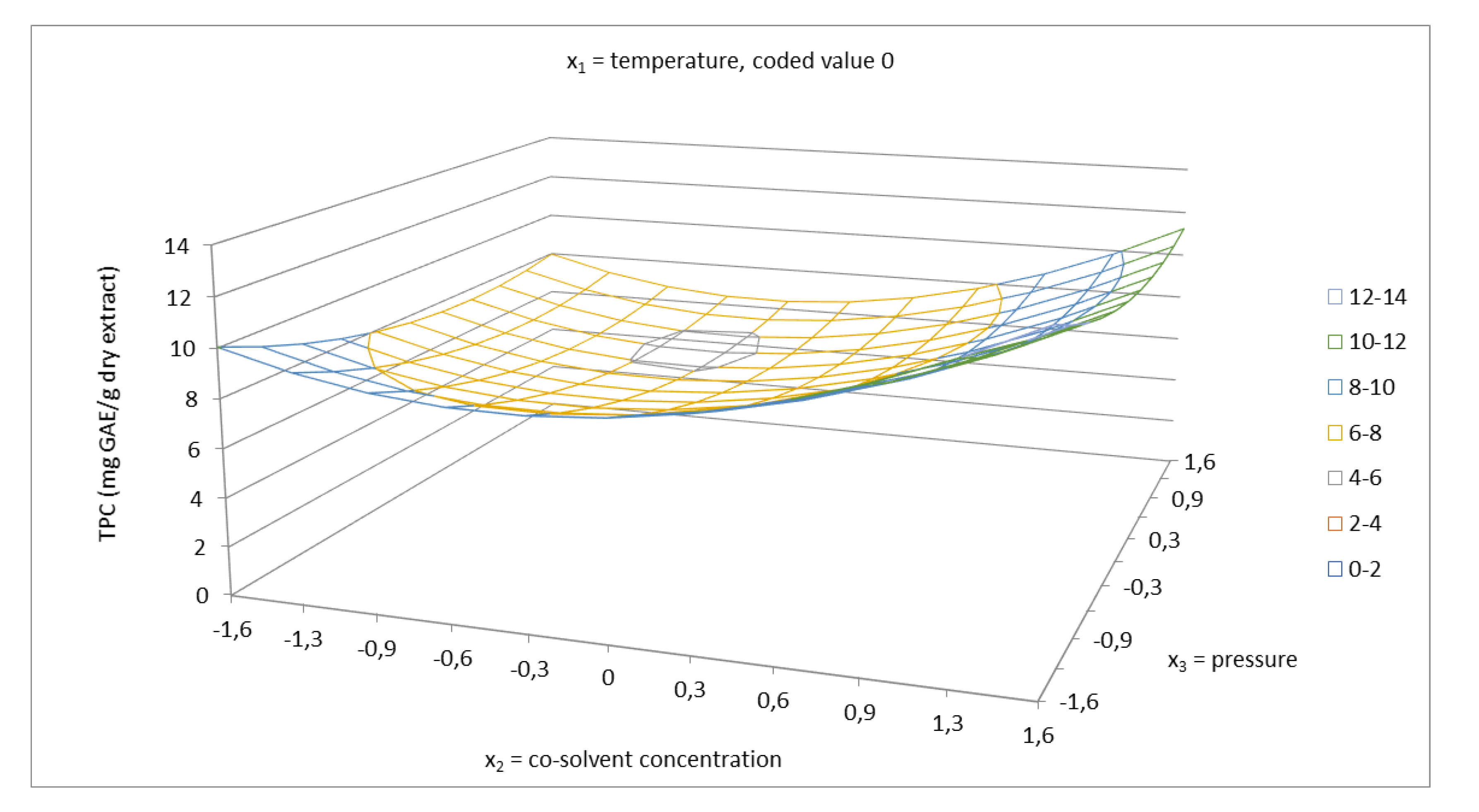
2.2. Total Phenolics Content and Antioxidant Capacity of Extracts
2.3. Extraction Optimization
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials
3.3. Supercritical Extraction with CO2
3.4. Determination of Extractives Yield
3.5. Determination of Total Phenolics Content
3.6. Determination of Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kemppainen, K.; Siika-aho, M.; Pattathil, S.; Giovando, S. Spruce bark as an industrial source of condensed tannins and non-cellulosic sugars. Ind. Crops Prod. 2014, 52, 158–168. [Google Scholar] [CrossRef]
- Harkin, J.M.; Rowe, J.W. United States Forest Service research note Forest Products Laboratory. Available online: https://www.fpl.fs.fed.us/documnts/fplrn/fplrn091.pdf (accessed on 8 August 2019).
- Miranda, I.; Gominho, J.; Mirra, I.; Pereira, H. Chemical characterization of barks from Picea abies and Pinus sylvestris after fractioning into different particle sizes. Ind. Crops Prod. 2012, 36, 395–400. [Google Scholar] [CrossRef]
- Räisänen, T.; Athanassiadis, D. Semanticscholar. Available online: https://www.semanticscholar.org/paper/Basic-chemical-composition-of-the-biomass-of-pine%2C-R%C3%A4is%C3%A4nen-Athanassiadis/f17423a8129685dd0a874ad47d16431862505d7b (accessed on 7 August 2019).
- Pietarinen, S.P.; Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Holmbom, B.R. Knotwood and bark extracts: Strong antioxidants from waste materials. J. Wood Sci. 2006, 52, 436–444. [Google Scholar] [CrossRef]
- Conde, E.; Cadahia, E.; Diez-Barra, R.; García-Vallejo, M. Polyphenolic composition of bark extracts fromEucalyptus camaldulensis, E. globulus and E. rudis. Holz als Roh-und Werkstoff 1996, 54, 175–181. [Google Scholar] [CrossRef]
- Şen, A.; Miranda, I.; Santos, S.; Graça, J.; Pereira, H. The chemical composition of cork and phloem in the rhytidome of Quercus cerris bark. Ind. Crops Prod. 2010, 31, 417–422. [Google Scholar] [CrossRef]
- Valentín, L.; Kluczek-Turpeinen, B.; Willför, S.; Hemming, J.; Hatakka, A.; Steffen, K.; Tuomela, M. Scots pine (Pinus sylvestris) bark composition and degradation by fungi: Potential substrate for bioremediation. Bioresour. Technol. 2010, 101, 2203–2209. [Google Scholar] [CrossRef]
- Tsao, R.J.N. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Delikanli. Phenolics in human health. Int. J. Chem. Eng. Appl. 2014, 5, 393. [Google Scholar]
- Nascimento, M.; Santana, A.; Maranhão, C.; Oliveira, L.; Bieber, L. Phenolic extractives and natural resistance of wood. In Biodegradation-Life of Science; Chamy, R., Rosenkranz, F., Eds.; InTech: Rijeka, Croatia, 2013; pp. 349–370. [Google Scholar]
- Laurova, M.; Vybohova, E.; Mamonova, M. Heartwood and Sapwood Liphophilic Extractives of Oak (Quercus petraea (Mattusch.) Liebl.). Acta Facultatis-Xylologiae 2007, 2, 17. [Google Scholar]
- Jablonsky, M.; Nosalova, J.; Sladkova, A.; Haz, A.; Kreps, F.; Valka, J.; Miertus, S.; Frecer, V.; Ondrejovic, M.; Sima, J.J.B.a. Valorisation of softwood bark through extraction of utilizable chemicals. A review. Biotechnol. Adv. 2017, 35, 726–750. [Google Scholar] [CrossRef]
- Caron, A.; Altaner, C.M.; Gardiner, B.; Jarvis, M.C. Distribution of extractives in Sitka spruce (Picea sitchensis) grown in the northern UK. Eur. J. Wood Wood Prod. 2013, 71, 697–704. [Google Scholar] [CrossRef]
- Hon, D.N.-S.; Shiraishi, N. Wood and cellulosic chemistry, revised, and expanded; Marcel Dekker: New York, NY, USA, 2000; Chapter 7. [Google Scholar]
- Kanadaswami, C.; Lee, L.; Lee, P.; Hwang, J.; Ke, F.; Huang, Y.; Lee, M. The antitumor activities of flavonoids. In Vivo 2005, 19, 895–909. [Google Scholar]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Ghitescu, R.-E.; Volf, I.; Carausu, C.; Bühlmann, A.-M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef]
- Lazar, L.; Talmaciu, A.I.; Volf, I.; Popa, V.I. Kinetic modeling of the ultrasound-assisted extraction of polyphenols from Picea abies bark. Ultrason. Sonochem 2016, 32, 191–197. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. Supercritical Fluids and Ultrasound Assisted Extractions Applied to Spruce Bark Conversion. Environ. Eng. Manage. J. 2015, 14, 615–623. [Google Scholar]
- Co, M.; Fagerlund, A.; Engman, L.; Sunnerheim, K.; Sjöberg, P.J.; Turner, C. Extraction of antioxidants from spruce (Picea abies) bark using eco-friendly solvents. Phytochem. Anal. 2012, 23, 1–11. [Google Scholar] [CrossRef]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Products. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crops Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Sládková, A.; Benedeková, M.; Stopka, J.; Šurina, I.; Ház, A.; Strižincová, P.; Čižová, K.; Škulcová, A.; Burčová, Z.; Kreps, F. Yield of polyphenolic substances extracted from spruce (Picea abies) bark by microwave-assisted extraction. BioResources 2016, 11, 9912–9921. [Google Scholar] [CrossRef]
- Jablonsky, M.; Vernarecová, M.; Ház, A.; Dubinyová, L.; Skulcova, A.; Sladková, A.; Surina, I.J. Extraction of phenolic and lipophilic compounds from spruce (Picea abies) bark using accelerated solvent extraction by ethanol. Wood Res. 2015, 60, 583–590. [Google Scholar]
- Talmaciu, A.I.; Ravber, M.; Volf, I.; Knez, Ž.; Popa, V.I. Isolation of bioactive compounds from spruce bark waste using sub-and supercritical fluids. J. Supercrit. Fluids 2016, 117, 243–251. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Alexy, P.; Viselka, M. STATIS Program for Planning and Evaluation of Experiments Food Technology; Department of Plastics and Rubber, Faculty of Chemical and Food Technology, SUT: Bratislava, Slovakia, 1998. [Google Scholar]
- Destandau, E.; Michel, T.; Elfakir, C. Microwave-assisted extraction. In Natural Product Extraction: Principles and applications; RSC Publishing: Cambridge, UK, 2013; pp. 113–156. [Google Scholar]
- Xiao, W.; Han, L.; Shi, B. Microwave-assisted extraction of flavonoids from Radix Astragali. Sep. Purif. Technol. 2008, 62, 614–618. [Google Scholar] [CrossRef]
- Markom, M.; Hasan, M.; Daud, W.R.W.; Singh, H.; Jahim, J.; Technology, P. Extraction of hydrolysable tannins from Phyllanthus niruri Linn.: Effects of solvents and extraction methods. Sep. Purif. Technol. 2007, 52, 487–496. [Google Scholar] [CrossRef]
- Katayama, S.; Zhao, L.; Yonezawa, S.; Iwai, Y. Modification of the surface of cotton with supercritical carbon dioxide and water to support nanoparticles. J. Supercrit. Fluids 2012, 61, 199–205. [Google Scholar] [CrossRef]
- Leitão, N.; Prado, G.; Veggi, P.; Meireles, M.; Pereira, C. Anacardium occidentale L. leaves extraction via SFE: Global yields, extraction kinetics, mathematical modeling and economic evaluation. J. Supercrit. Fluids 2013, 78, 114–123. [Google Scholar] [CrossRef]
- Monrad, J.K.; Howard, L.R.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical solvent extraction of anthocyanins from dried red grape pomace. J. Agric. food chem. 2010, 58, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.; Villaverde, J.J.; Silva, C.M.; Neto, C.P.; Silvestre, A.J. Supercritical fluid extraction of phenolic compounds from Eucalyptus globulus Labill bark. J. Supercrit. Fluids 2012, 71, 71–79. [Google Scholar] [CrossRef]
- Strizincova, P.; Ház, A.; Sládková, A.; Jablonský, M.; Surina, I. Optimization conditions of extraction. In Proceedings of the FP1306 COST Action Third Workshop and Fourth MC Meeting, Torremolinos, Spain, 27–28 March 2017; p. 89. [Google Scholar]
- Mandal, S.C.; Mandal, V.; Das, A.K. Essentials of botanical extraction: Principles and applications, 1st ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Baldosano, H.Y.; Castillo, M.B.M.G.; Elloran, C.D.H.; Bacani, F.T. Effect of particle size, solvent and extraction time on tannin extract from Spondias purpurea bark through soxhlet extraction. In Proceedings of the DLSU Research Congress, De La Salle University, Manila, Philippines, 2–4 March 2015; pp. 1–6. [Google Scholar]
- Sapkale, G.; Patil, S.; Surwase, U.; Bhatbhage, P. Supercritical fluid extraction. Int. J. Chem. Sci. 2010, 8, 729–743. [Google Scholar]
- Garcia-Gonzalez, L.; Geeraerd, A.H.; Spilimbergo, S.; Elst, K.; Van Ginneken, L.; Debevere, J.; Van Impe, J.; Devlieghere, F. High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future. Int. J. Food Microbiol. 2007, 117, 1–28. [Google Scholar] [CrossRef]
- Wimmer, Z.; Zarevúcka, M. A review on the effects of supercritical carbon dioxide on enzyme activity. Int. J. Mol. Sci. 2010, 11, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Čolnik, M.; Primožič, M.; Knez, Ž.; Leitgeb, M. Use of non-conventional cell Disruption Method for extraction of Proteins from Black Yeasts. Front. Bioeng. Biotechnol. 2016, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jia, L.; Kan, J.; Jin, C.-H. In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food chem. Toxicol. 2013, 51, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Kraujalis, P.; Venskutonis, P.R.; Ibáñez, E.; Herrero, M. Optimization of rutin isolation from Amaranthus paniculatus leaves by high pressure extraction and fractionation techniques. J. Supercrit. Fluids 2015, 104, 234–242. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- International Standard ISO. Available online: https://www.iso.org/standard/8288.html (accessed on 15 September 2019).
- Zougagh, M.; Valcárcel, M.; Rıos, A. Supercritical fluid extraction: A critical review of its analytical usefulness. TrAC Trends Anal. Chem. 2004, 23, 399–405. [Google Scholar] [CrossRef]
- Veggi, P.C.; Prado, J.M.; Bataglion, G.A.; Eberlin, M.N.; Meireles, M.A.A. Obtaining phenolic compounds from jatoba (Hymenaea courbaril L.) bark by supercritical fluid extraction. J. Supercrit. Fluids 2014, 89, 68–77. [Google Scholar] [CrossRef]
- Martinez-Correa, H.A.; Magalhães, P.M.; Queiroga, C.L.; Peixoto, C.A.; Oliveira, A.L.; Cabral, F.A. Extracts from pitanga (Eugenia uniflora L.) leaves: Influence of extraction process on antioxidant properties and yield of phenolic compounds. J. Supercrit. Fluids 2011, 55, 998–1006. [Google Scholar] [CrossRef]
- Supercritical Fluids. Available online: http://www.supercriticalfluids.com/wp-content/uploads/Spec-Sheet-SFT-150.pdf (accessed on 10 September 2019).
- Yu, L.; Haley, S.; Perret, J.; Harris, M. Antioxidant properties of hard winter wheat extracts. Food chem. 2002, 78, 457–461. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Gressler, V.; Moura, S.; Flores, A.F.; Flores, D.C.; Colepicolo, P.; Pinto, E. Antioxidant and antimicrobial properties of 2-(4, 5-dihydro-1H-pyrazol-1-yl)-pyrimidine and 1-carboxamidino-1H-pyrazole derivatives. J. Braz. Chem. Soc. 2010, 21, 1477–1483. [Google Scholar] [CrossRef]
- Mareček, V.; Mikyška, A.; Hampel, D.; Čejka, P.; Neuwirthová, J.; Malachová, A.; Cerkal, R. ABTS and DPPH methods as a tool for studying antioxidant capacity of spring barley and malt. J. Cereal Sci. 2017, 73, 40–45. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |







| Name of Factors | Factors | Coded Variable Level | ||||
|---|---|---|---|---|---|---|
| −1.682 | −1 | 0 | 1 | 1.682 | ||
| Temperature (°C) | x1 | 40 | 52 | 70 | 88 | 100 |
| Cosolvent concentration (%) | x2 | 40.0 | 51.5 | 68.3 | 85.1 | 96.6 |
| Pressure (psi) | x3 | 1050 | 2660 | 5025 | 7390 | 9000 |
| Run | Coded Levels of Factors | Real Levels of Factors | |||||||
|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | t (°C) | c (%) | p (psi) | YE (%) | TPC a | TEAC b | |
| 1 | −1 | −1 | −1 | 52.16 | 51.47 | 2661.74 | 7.78 | 6.61 | 0.75 |
| 2 | 1 | −1 | −1 | 87.84 | 51.47 | 2661.74 | 6.68 | 4.41 | 0.75 |
| 3 | −1 | 1 | −1 | 52.16 | 85.13 | 2661.74 | 4.14 | 9.22 | 0.71 |
| 4 | 1 | 1 | −1 | 87.84 | 85.13 | 2661.74 | 4.94 | 8.46 | 0.74 |
| 5 | −1 | −1 | 1 | 52.16 | 51.47 | 7388.26 | 7.80 | 5.82 | 0.74 |
| 6 | 1 | −1 | 1 | 87.84 | 51.47 | 7388.26 | 9.20 | 5.24 | 0.79 |
| 7 | −1 | 1 | 1 | 52.16 | 85.13 | 7388.26 | 3.73 | 9.23 | 0.75 |
| 8 | 1 | 1 | 1 | 87.84 | 85.13 | 7388.26 | 3.99 | 8.46 | 0.69 |
| 9 | −α | 0 | 0 | 40.00 | 68.30 | 5025.00 | 3.78 | 7.39 | 0.76 |
| 10 | α | 0 | 0 | 100.00 | 68.30 | 5025.00 | 4.54 | 4.47 | 0.75 |
| 11 | 0 | −α | 0 | 70.00 | 40.00 | 5025.00 | 5.70 | 10.32 | 0.76 |
| 12 | 0 | α | 0 | 70.00 | 96.60 | 5025.00 | 2.15 | 9.61 | 0.70 |
| 13 | 0 | 0 | α | 70.00 | 68.30 | 1050.00 | 7.82 | 11.30 | 0.74 |
| 14 | 0 | 0 | α | 70.00 | 68.30 | 9000.00 | 6.99 | 5.46 | 0.75 |
| 15 | 0 | 0 | 0 | 70.00 | 68.30 | 5025.00 | 8.14 | 6.24 | 0.74 |
| 16 | 0 | 0 | 0 | 70.00 | 68.30 | 5025.00 | 8.69 | 6.40 | 0.74 |
| 17 | 0 | 0 | 0 | 70.00 | 68.30 | 5025.00 | 8.71 | 6.50 | 0.75 |
| 18 | 0 | 0 | 0 | 70.00 | 68.30 | 5025.00 | 8.70 | 6.10 | 0.74 |
| 19 | 0 | 0 | 0 | 70.00 | 68.30 | 5025.00 | 8.19 | 6.34 | 0.73 |
| 20 | 0 | 0 | 0 | 70.00 | 68.30 | 5025.00 | 8.67 | 6.83 | 0.74 |
| Source of Variability | s | f | s ^ 2 | F | Fc |
|---|---|---|---|---|---|
| s1 | 29.89 | 3 | 9.96 | 133.01 | 5.41 |
| s2 | 51.84 | 6 | 8.64 | 115.34 | 4.95 |
| SE | 0.37 | 5 | 0.07 | 1.00 | - |
| SLF | 6.76 | 5 | 1.35 | 18.05 | 5.05 |
| Coefficients | Yield of Extractives (%) | TPC (mg GAE/g Dry Extract) | ||
|---|---|---|---|---|
| bi | bc | bi | bc | |
| b0 | 8.49 | 0.29 | 6.30 | 0.32 |
| b1 | 0.25 | 0.19 | −0.67 | 0.21 |
| b2 | −1.46 | 0.19 | 0.89 | 0.21 |
| b3 | −0.07 | 0.19 | −0.68 | 0.21 |
| b11 | −1.33 | 0.19 | −0.33 | 0.20 |
| b12 | 0.00 | 0.25 | 0.16 | 0.27 |
| b13 | 0.33 | 0.25 | 0.20 | 0.27 |
| b22 | −1.42 | 0.19 | 1.10 | 0.20 |
| b23 | −0.4 | 0.25 | −0.004 | 0.27 |
| b33 | −0.19 | 0.19 | 0.49 | 0.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strižincová, P.; Ház, A.; Burčová, Z.; Feranc, J.; Kreps, F.; Šurina, I.; Jablonský, M. Spruce Bark—A Source of Polyphenolic Compounds: Optimizing the Operating Conditions of Supercritical Carbon Dioxide Extraction. Molecules 2019, 24, 4049. https://doi.org/10.3390/molecules24224049
Strižincová P, Ház A, Burčová Z, Feranc J, Kreps F, Šurina I, Jablonský M. Spruce Bark—A Source of Polyphenolic Compounds: Optimizing the Operating Conditions of Supercritical Carbon Dioxide Extraction. Molecules. 2019; 24(22):4049. https://doi.org/10.3390/molecules24224049
Chicago/Turabian StyleStrižincová, Petra, Aleš Ház, Zuzana Burčová, Jozef Feranc, František Kreps, Igor Šurina, and Michal Jablonský. 2019. "Spruce Bark—A Source of Polyphenolic Compounds: Optimizing the Operating Conditions of Supercritical Carbon Dioxide Extraction" Molecules 24, no. 22: 4049. https://doi.org/10.3390/molecules24224049
APA StyleStrižincová, P., Ház, A., Burčová, Z., Feranc, J., Kreps, F., Šurina, I., & Jablonský, M. (2019). Spruce Bark—A Source of Polyphenolic Compounds: Optimizing the Operating Conditions of Supercritical Carbon Dioxide Extraction. Molecules, 24(22), 4049. https://doi.org/10.3390/molecules24224049






