New Indole Glycosides from Aesculus chinensis var. chekiangensis and Their Neuroprotective Activities
Abstract
:1. Introduction
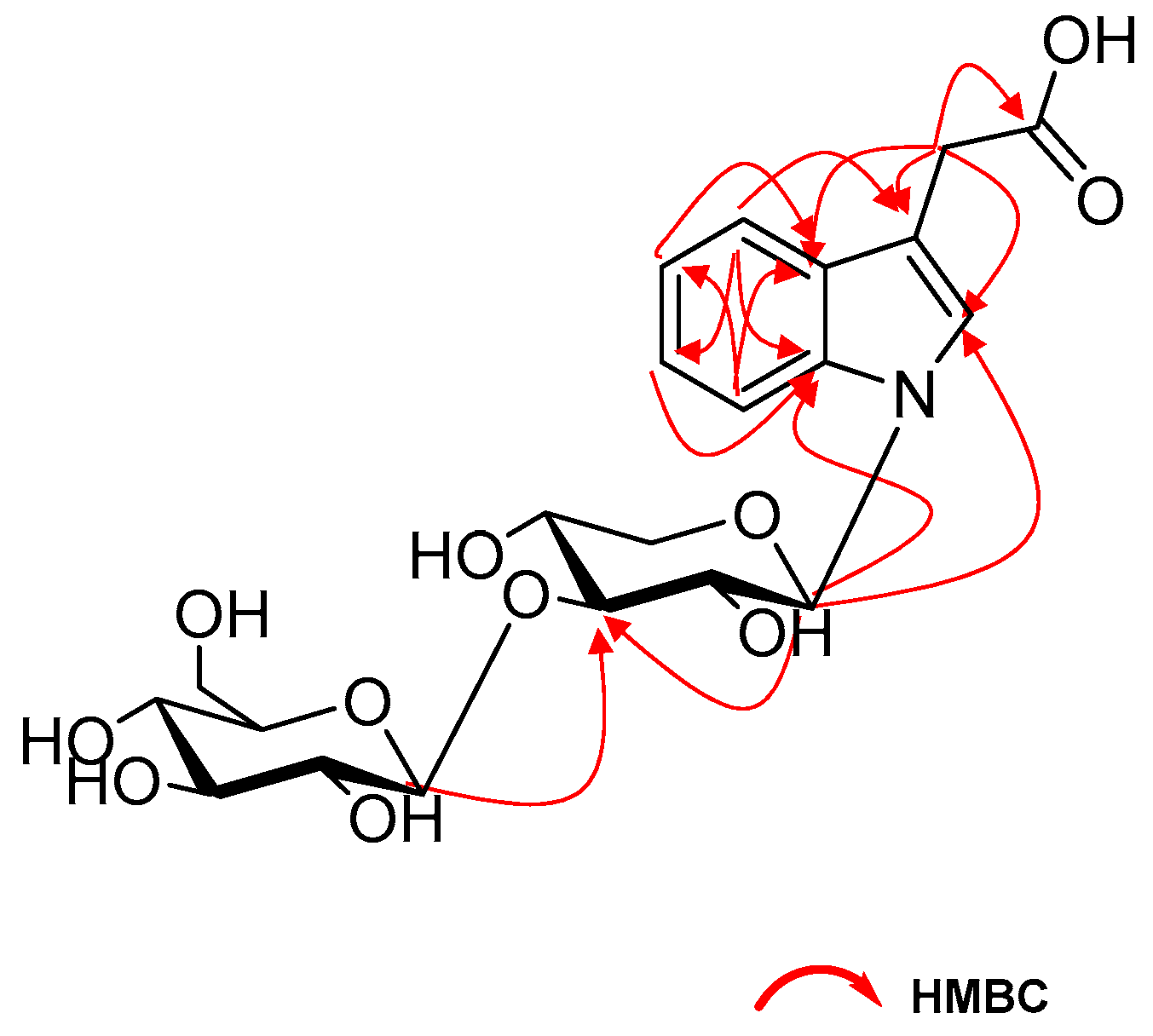
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. N-[β-d-glucopyranosyl(1→3)]-β-d-xylopyranosyl-indole-3-acetic Acid (1)
3.3.2. N-[β-d-glucopyranosyl(1→3)]-β-d-xylopyranosyl-indole-3-methyl Acetate (2)
3.3.3. N-[β-d-glucopyranosyl(1→3)]-β-d-xylopyranosyl-indole-3-carbaldehyde (3)
3.3.4. N-[β-d-glucopyranosyl(1→3)-[β-d-glucopyranosyl(1-4)]-β-d-xylopyranosyl-indole-3-acetic Acid (4)
3.3.5. N-β-d-xylopyranosyl-indole-3-acetic Acid (5)
3.3.6. N-[β-d-glucopyranosyl(1→2)]-β-d-xylopyranosyl-indole-3-acetic Acid (6)
3.3.7. Hydrolysis and Determination of Absolute Configuration of Sugars
3.4. Neuroprotective Effect Assay
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.; Li, S.; Zhang, S.; Gorenstein, D. Triterpenoid saponins from the fruits of Aesculus pavia. Phytochemistry 2006, 67, 784–794. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, J.; Cui, Y.; Liu, X.; Ma, C.; Hattori, M.; Zhang, L. Anti-HIV-1 protease triterpenoid saponins from the seeds of Aesculus chinensis. J. Nat. Prod. 1999, 62, 1510–1513. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, F.; Zhang, X.; Zhu, M.; Wang, T.; Fan, H. Escin attenuates cognitive deficits and hippocampal injury after transient global cerebral ischemia in mice via regulating certain inflammatory genes. Neurochem. Int. 2010, 57, 119–127. [Google Scholar] [CrossRef]
- Wang, Y.W.; Wang, S.J.; Zhou, Y.N.; Pan, S.H.; Sun, B. Escin augments the efficacy of gemcitabine through down-regulation of nuclear factor-κb and nuclear factor-κb-regulated gene products in pancreatic cancer both in vitro and in vivo. J. Cancer Res. Clin. 2012, 138, 785–797. [Google Scholar] [CrossRef]
- Zhao, J.; Yangi, X.W.; Cul, Y.X.; Liu, X.H.; Ouyangz, S.H. A New Triterpenoid Oligoglycoside Escin IVe from the Seeds of Aesculus Chinensis. Chin. Chem. Lett. 1999, 6, 473–476. [Google Scholar]
- Jie, G.; XiuWei, Y. Studies on Triterpenoid Saponins of Seeds of Aesculus chinensis Bunge var. chekiangensis (Hu et Fang) Fang. J. Chin. Pharm. Sci. 2004, 13, 87–91. [Google Scholar]
- Voutquenne, L.; Guinot, P.; Froissard, C.; Thoison, O.; Litaudon, M.; Lavaud, C. Haemolytic acylated triterpenoid saponins from Harpullia austro-caledonica. Phytochemistry 2005, 59, 825–832. [Google Scholar] [CrossRef]
- Ireneusz, K.; Bogdan, J.; Barbara, S.; Anna, S.; Sonia, P.; Cosimo, P.; Federico, F.; Chlodwig, F.; Wieslaw, O. Flavonoids in horse chestnut (Aesculus hippocastanum) seeds and powdered waste water byproducts. J. Agric. Food Chem. 2007, 55, 8485–8490. [Google Scholar]
- Wei, F.; Ma, S.; Ly, M.; But, P.P.; Lin, R.C.; Khan, I.A. Antiviral flavonoids from the seeds of Aesculus chinensis. J. Nat. Prod. 2004, 67, 650–653. [Google Scholar] [CrossRef]
- Niu, X.; Wang, Y.; Li, W.; Zhang, H.; Wang, X.; Mu, Q.; He, Z.; Yao, H. Esculin exhibited anti-inflammatory activities in vivo and regulated TNF-α and IL-6 production in LPS-stimulated mouse peritoneal macrophages in vitro through MAPK pathway. Int. Immunopharmacol. 2015, 29, 779–786. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef]
- Kai, K.; Kyo, W.; Hisashi, M. Metabolism of indole-3-acetic acid in rice: Identification and characterization of N-beta-D-glucopyranosyl indole-3-acetic acid and its conjugates. Phytochemistry 2007, 68, 2512–2522. [Google Scholar] [CrossRef]
- Bernd, S.; Thomas, H. Isolation, structure determination, and sensory activity of mouth-drying and astringent nitrogen-containing phytochemicals isolated from red currants (Ribes rubrum). J. Agric. Food Chem. 2007, 55, 1405–1410. [Google Scholar]
- Lee, M.Y.; Lin, H.Y.; Cheng, F.; Chiang, W.; Kuo, Y.H. Isolation and characterization of new lactam compounds that inhibit lung and colon cancer cells from adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) bran. Food Chem. Toxicol. 2008, 46, 1933–1939. [Google Scholar] [CrossRef]
- Elsayed, Y.; Refaat, J.; Abdelmohsen, U.R.; Ahmed, S.; Fouad, M.A. Retraction Note to: Rhodozepinone, a new antitrypanosomal azepino-diindole alkaloid from the marine sponge-derived bacterium Rhodococcus sp. UA13. Med. Chem. Res. 2019, 28, 105. [Google Scholar] [CrossRef]
- Bano, S.; Ali, M.S.; Ahmad, V.U. Marine Natural Products; VI. A Halogenated Chamigrene Epoxide from the Red Alga Laurencia pinnatifida. Planta Med. 1987, 53, 508. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, W.; Xia, G.; Oppong, M.B.; Ding, L.; Li, P.; Qiu, F. Methods for determination of absolute configuration of monosaccharides. Chin. Herb. Med. 2018, 10, 14–22. [Google Scholar] [CrossRef]
- Tan, Y.Z.; Yong, Y.; Dong, Y.H.; Wang, R.J.; Li, H.X.; Zhang, H.; Guo, D.L.; Zhang, S.J.; Dong, X.P.; Xie, X.F. A new secoiridoid glycoside and a new sesquiterpenoid glycoside from Valeriana jatamansi with neuroprotective activity. Phytochem Lett. 2016, 17, 177–180. [Google Scholar] [CrossRef] [Green Version]
- Zou, W.; Yan, M.; Xu, W.; Huo, H.; Sun, L.; Zheng, Z.; Liu, X. Cobalt chloride induces PC12 cells apoptosis through reactive oxygen species and accompanied by AP-1 activation. J. Neurosci. Res. 2001, 64, 646–653. [Google Scholar] [CrossRef]
- Elreadi, M.Z.; Eid, S.; Ashour, M.L.; Tahrani, A.; Wink, M. Modulation of multidrug resistance in cancer cells by chelidonine and Chelidonium majus alkaloids. Phytomedicine 2013, 20, 282–294. [Google Scholar] [CrossRef]
- Xia, Y.Z.; Yang, L.; Wang, Z.D.; Guo, C.; Zhang, C.; Geng, Y.D.; Kong, L.Y. Schisandrin A enhances the cytotoxicity of doxorubicin by the inhibition of nuclear factor-kappa B signaling in a doxorubicin-resistant human osteosarcoma cell line. RSC Adv. 2015, 5, 13972–13984. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| NO. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 2 | 7.41 (1H, s) | 7.44 (1H, s) | 8.37 (1H, s) | 7.41 (1H, s) | 7.25 (1H, s) | 7.47 (1H, s) |
| 4 | 7.53 (1H, d, J = 7.8 Hz) | 7.54 (1H, d, J = 7.8 Hz) | 8.18 (1H, d, J = 7.7 Hz) | 7.53 (1H, d, J = 7.8 Hz) | 7.54 (1H, d, J = 7.9 Hz) | 7.54 (1H, d, J = 7.9 Hz) |
| 5 | 7.08 (1H, ddd, J = 7.5, 7.0, 0.9 Hz) | 7.11 (1H, ddd, J = 7.5, 7.0, 0.9 Hz) | 7.26 (1H, ddd, J = 7.7, 7.3, 0.9 Hz) | 7.08 (1H, ddd, J = 7.5, 7.0, 1.2 Hz) | 7.06 (1H, ddd, J = 7.9, 7.0, 0.9 Hz) | 7.08 (1H, ddd, J = 7.5, 7.0, 1.2 Hz) |
| 6 | 7.16 (1H, ddd, J = 8.2, 7.0, 1.2 Hz) | 7.19 (1H, ddd, J = 8.2, 7.0, 1.1 Hz) | 7.31 (1H, ddd, J = 8.1, 7.3, 1.1 Hz) | 7.16 (1H, ddd, J = 8.2, 7.0, 1.2 Hz) | 7.15 (1H, ddd, J = 8.2, 7.0, 1.1 Hz) | 7.17 (1H, ddd, J = 8.2, 7.0, 1.2 Hz) |
| 7 | 7.48 (1H, d, J = 8.3 Hz) | 7.51 (1H, d, J = 8.3 Hz) | 7.62 (1H, d, J = 8.1 Hz) | 7.48 (1H, d, J = 8.2 Hz) | 7.48 (1H, d, J = 8.3 Hz) | 7.51 (1H, d, J = 8.3 Hz) |
| 8 | 3.72 (2H, s) | 3.79 (2H, s) | 9.91 (1H, s) | 3.71 (2H, s) | 3.66 (2H, s) | 3.72 (2H, s) |
| 10 | 3.71 (3H, s) | |||||
| Xyl-p | Xyl-p | Xyl-p | Xyl-p | Xyl-p | Glc-p | |
| 1′ | 5.45 (1H, d, J = 9.0 Hz) | 5.48 (1H, d, J = 9.0 Hz) | 5.58 (1H, d, J = 9.0 Hz) | 5.46 (1H, d, J = 9.0 Hz) | 5.31 (1H, d, J = 9.0 Hz) | 5.56 (1H, d, J = 9.0 Hz) |
| 2′ | 3.75 (1H, m) | 3.78 (1H, m) | 3.80 (1H, m) | 3.93 (1H, d, J = 8.8 Hz) | 3.89 (1H, t, J = 9.0 Hz) | 4.21 (1H, t, J = 9.0 Hz) |
| 3′ | 4.19 (1H, t, J = 8.8 Hz) | 4.21 (1H, t, J = 8.7 Hz) | 4.26 (1H, t, J = 8.6 Hz) | 4.26 (1H, t, J = 8.7 Hz) | 3.54 (1H, t, J = 9.0 Hz) | 3.82 (1H, t, J = 8.8 Hz) |
| 4′ | 3.74 (1H, m) | 3.76 (1H, m) | 3.78 (1H, m) | 3.96 (1H, m) | 3.69 (1H, ddd, J = 10.6, 9.0, 5.5 Hz) | 3.54 (1H, m) |
| 5′ | 3.48 (1H, m) 3.97 (1H, m) | 3.50 (1H, m) 3.99 (1H, dd, J = 11.4, 4.7 Hz) | 3.56 (1H, t, J = 10.5 Hz) 4.06 (1H, dd, J = 11.3, 4.7 Hz) | 3.56 (1H, d, J = 10.8 Hz) 4.15 (1H, dd, J = 11.6, 5.1 Hz) | 3.48 (1H, t, J = 11.0 Hz) 3.97 (1H, dd, J = 11.3, 5.5 Hz) | 3.56 (1H, m) |
| 6′ | 3.70 (1H, m) 3.86 (1H, dd, J = 12.2, 2.0 Hz) | |||||
| Glc-p | Glc-p | Glc-p | Glc-p | Glc-p | ||
| 1″ | 4.35 (1H, d, J = 7.8 Hz) | 4.38 (1H, d, J = 7.8 Hz) | 4.48 (1H, d, J = 7.8 Hz) | 4.37 (1H, d, J = 7.8 Hz) | 4.38 (1H, d, J = 7.8 Hz) | |
| 2″ | 2.95 (1H, dd, J = 9.3, 7.8 Hz) | 2.98 (1H, dd, J = 9.3, 7.8 Hz) | 2.89 (1H, m) | 2.95 (1H, dd, J = 9.3, 7.8 Hz) | 2.96 (1H, dd, J = 9.3, 7.8 Hz) | |
| 3″ | 3.17 (1H, t, J = 9.1 Hz) | 3.20 (1H, t, J = 8.5 Hz) | 3.16 (1H, d, J = 8.9 Hz) | 3.17 (1H, d, J = 9.1 Hz) | 3.20 (1H, t, J = 9.1 Hz) | |
| 4″ | 3.05 (1H, t, J = 9.4 Hz) | 3.06 (1H, t, J = 9.4 Hz) | 2.92 (1H, m) | 3.04 (1H, t, J = 9.4 Hz) | 3.05 (1H, t, J = 9.4 Hz) | |
| 5″ | 2.74 (1H, ddd, J = 9.8, 4.6, 2.5 Hz) | 2.77 (1H, ddd, J = 9.7, 4.7, 2.6 Hz) | 2.87 (1H, m) | 2.73 (1H, ddd, J = 9.9, 4.6, 2.5 Hz) | 2.77 (1H, ddd, J = 9.8, 4.5, 2.5 Hz) | |
| 6″ | 3.10 (1H, dd, J = 11.8, 2.5 Hz) 3.21 (1H, dd, J = 11.7, 4.6 Hz) | 3.13 (1H, dd, J = 11.7, 2.6 Hz) 3.23 (1H, dd, J = 11.1, 4.1 Hz) | 3.16 (1H, d, J = 8.9 Hz) 3.35 (1H, dd, J = 11.6, 2.5 Hz) | 3.10 (1H, dd, J = 11.8, 2.5 Hz) 3.21 (1H, dd, J = 12.2, 4.8 Hz) | 3.14 (1H, dd, J = 11.8, 2.5 Hz) 3.23 (1H, dd, J = 11.8, 4.6 Hz) | |
| Glc-p | ||||||
| 1‴ | 4.43 (1H, d, J = 7.8 Hz) | |||||
| 2‴ | 3.26 (1H, dd, J = 9.2, 7.8 Hz) | |||||
| 3‴ | 3.35 (1H, m) | |||||
| 4‴ | 3.29 (1H, m) | |||||
| 5‴ | 3.33 (1H, m) | |||||
| 6‴ | 3.67 (1H, dd, J = 12.0, 5.9 Hz) 3.89 (1H, dd, J = 12.4, 2.5 Hz) |
| NO. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 2 | 126.4 | 126.5 | 141.4 | 126.4 | 125.0 | 126.4 |
| 3 | 109.8 | 109.4 | 119.7 | 109.9 | 112.2 | 109.7 |
| 3a | 129.8 | 129.6 | 126.4 | 129.8 | 130.2 | 129.7 |
| 4 | 119.8 | 119.8 | 122.5 | 119.9 | 120.0 | 119.8 |
| 5 | 120.9 | 120.9 | 124.1 | 120.9 | 120.7 | 120.9 |
| 6 | 122.9 | 122.9 | 125.0 | 122.9 | 122.9 | 122.9 |
| 7 | 111.7 | 111.7 | 112.8 | 111.7 | 111.3 | 111.7 |
| 7a | 138.2 | 138.2 | 139.0 | 138.2 | 138.3 | 138.4 |
| 8 | 31.9 | 31.7 | 187.7 | 31.9 | 33.8 | 31.9 |
| 9 | 176.0 | 174.4 | 176.0 | 180.1 | 175.9 | |
| 10 | 52.5 | |||||
| Xyl-p | Xyl-p | Xyl-p | Xyl-p | Xyl-p | Glc-p | |
| 1′ | 86.1 | 86.1 | 86.5 | 86.0 | 87.5 | 85.2 |
| 2′ | 79.0 | 79.0 | 78.9 | 77.3 | 73.7 | 81.2 |
| 3′ | 81.1 | 81.0 | 79.8 | 80.5 | 78.8 | 78.9 |
| 4′ | 70.9 | 70.9 | 70.7 | 77.6 | 71.1 | 71.2 |
| 5′ | 69.4 | 69.4 | 69.7 | 67.1 | 69.5 | 80.5 |
| 6′ | 62.6 | |||||
| Glc-p | Glc-p | Glc-p | Glc-p | Glc-p | ||
| 1″ | 105.0 | 105.0 | 104.5 | 104.9 | 104.9 | |
| 2″ | 75.8 | 75.7 | 75.5 | 75.7 | 75.7 | |
| 3″ | 77.7 | 77.7 | 77.7 | 77.8 | 77.7 | |
| 4″ | 70.8 | 70.9 | 71.1 | 70.8 | 70.8 | |
| 5″ | 77.4 | 77.4 | 77.6 | 77.2 | 77.4 | |
| 6″ | 62.0 | 62.1 | 62.3 | 62.0 | 62.1 | |
| Glc-p | ||||||
| 1‴ | 103.4 | |||||
| 2‴ | 74.6 | |||||
| 3‴ | 77.9 | |||||
| 4‴ | 71.5 | |||||
| 5‴ | 78.1 | |||||
| 6‴ | 62.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Cao, S.; Huang, W.; Li, P.; Kang, N.; Ding, L.; Qiu, F. New Indole Glycosides from Aesculus chinensis var. chekiangensis and Their Neuroprotective Activities. Molecules 2019, 24, 4063. https://doi.org/10.3390/molecules24224063
Zhang N, Cao S, Huang W, Li P, Kang N, Ding L, Qiu F. New Indole Glycosides from Aesculus chinensis var. chekiangensis and Their Neuroprotective Activities. Molecules. 2019; 24(22):4063. https://doi.org/10.3390/molecules24224063
Chicago/Turabian StyleZhang, Nan, Shijie Cao, Weixing Huang, Pan Li, Ning Kang, Liqin Ding, and Feng Qiu. 2019. "New Indole Glycosides from Aesculus chinensis var. chekiangensis and Their Neuroprotective Activities" Molecules 24, no. 22: 4063. https://doi.org/10.3390/molecules24224063
APA StyleZhang, N., Cao, S., Huang, W., Li, P., Kang, N., Ding, L., & Qiu, F. (2019). New Indole Glycosides from Aesculus chinensis var. chekiangensis and Their Neuroprotective Activities. Molecules, 24(22), 4063. https://doi.org/10.3390/molecules24224063





