Curcumin Derivatives Verify the Essentiality of ROS Upregulation in Tumor Suppression
Abstract
1. Introduction
2. Results
2.1. Design and Synthesis of Novel Curcumin Derivatives
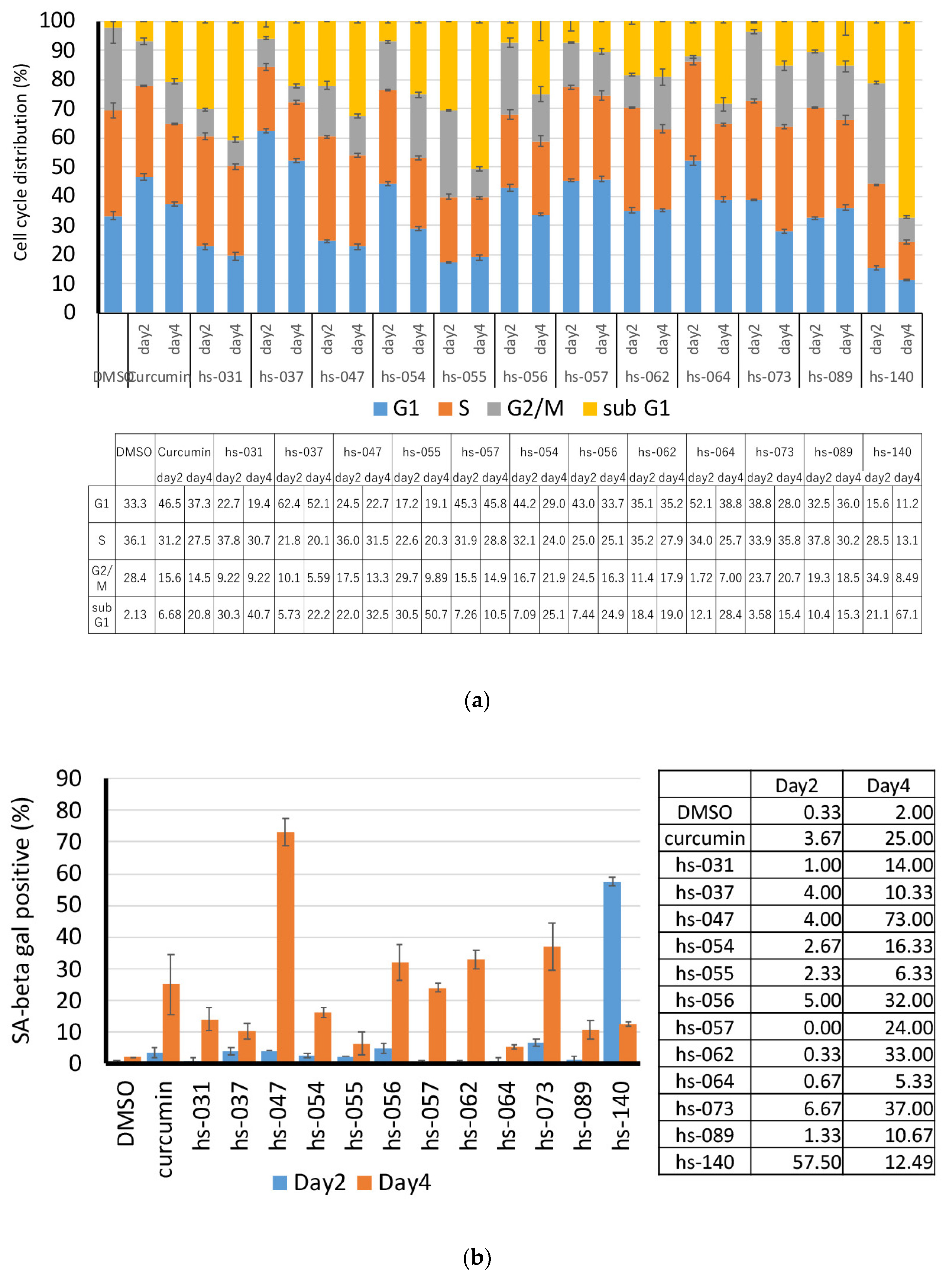
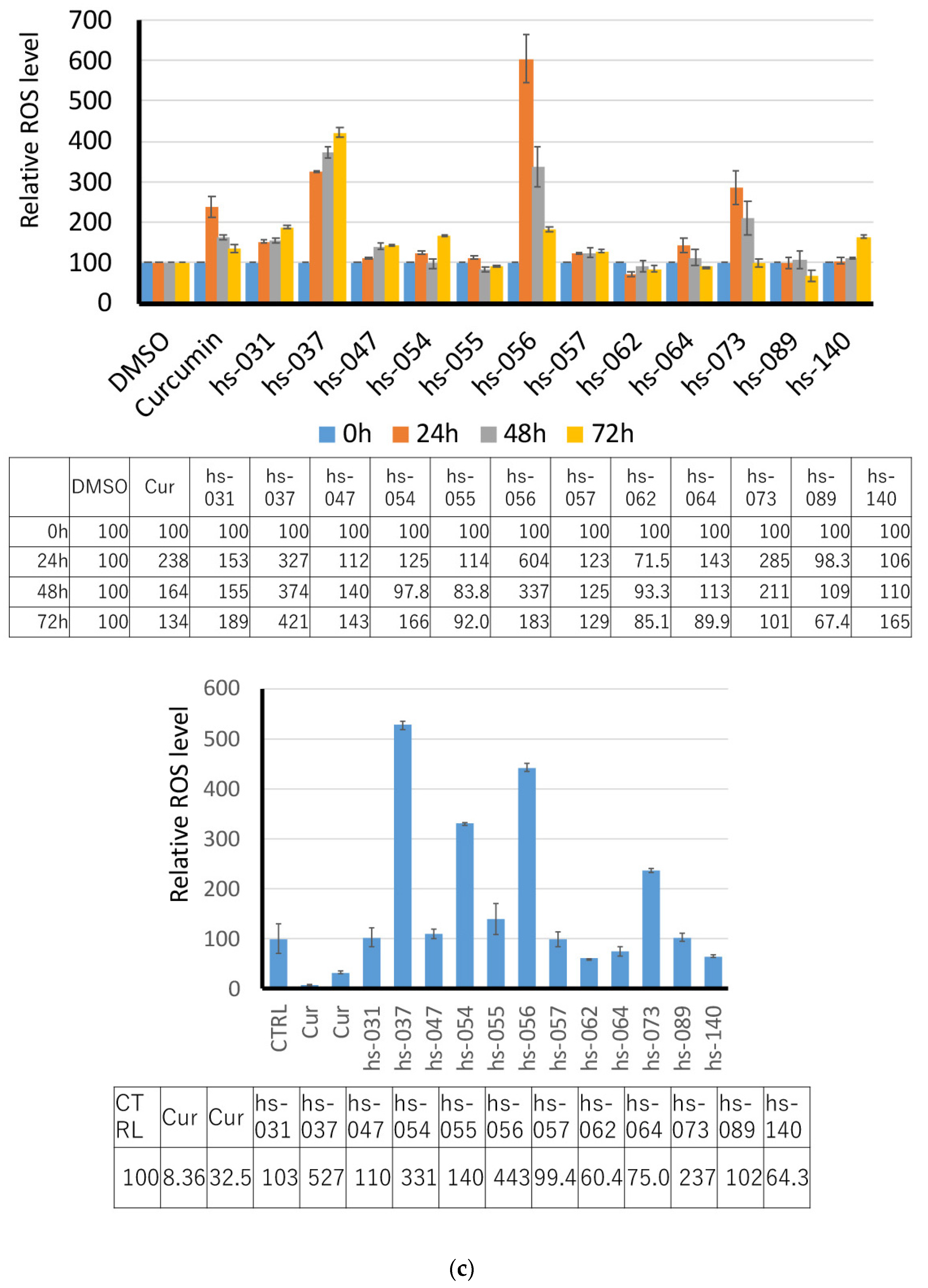
2.2. Growth Inhibition of Human Tumor Cells and ROS Induction by Novel Curcumin Derivatives
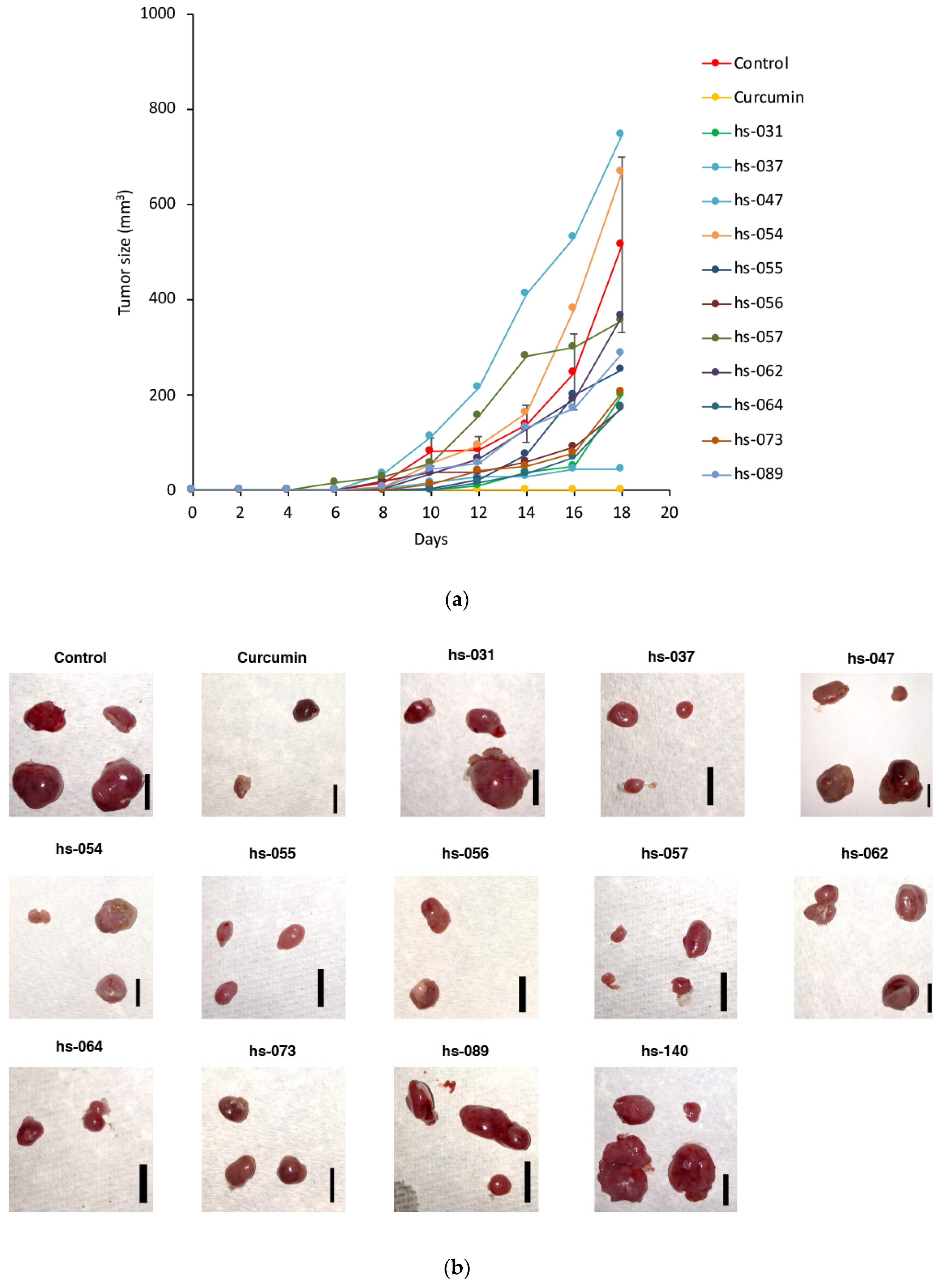
2.3. Anti-Tumorigenic Activity of Novel Curcumin Derivatives
2.4. Clustering Analysis of Properties of Novel Curcumin Derivatives
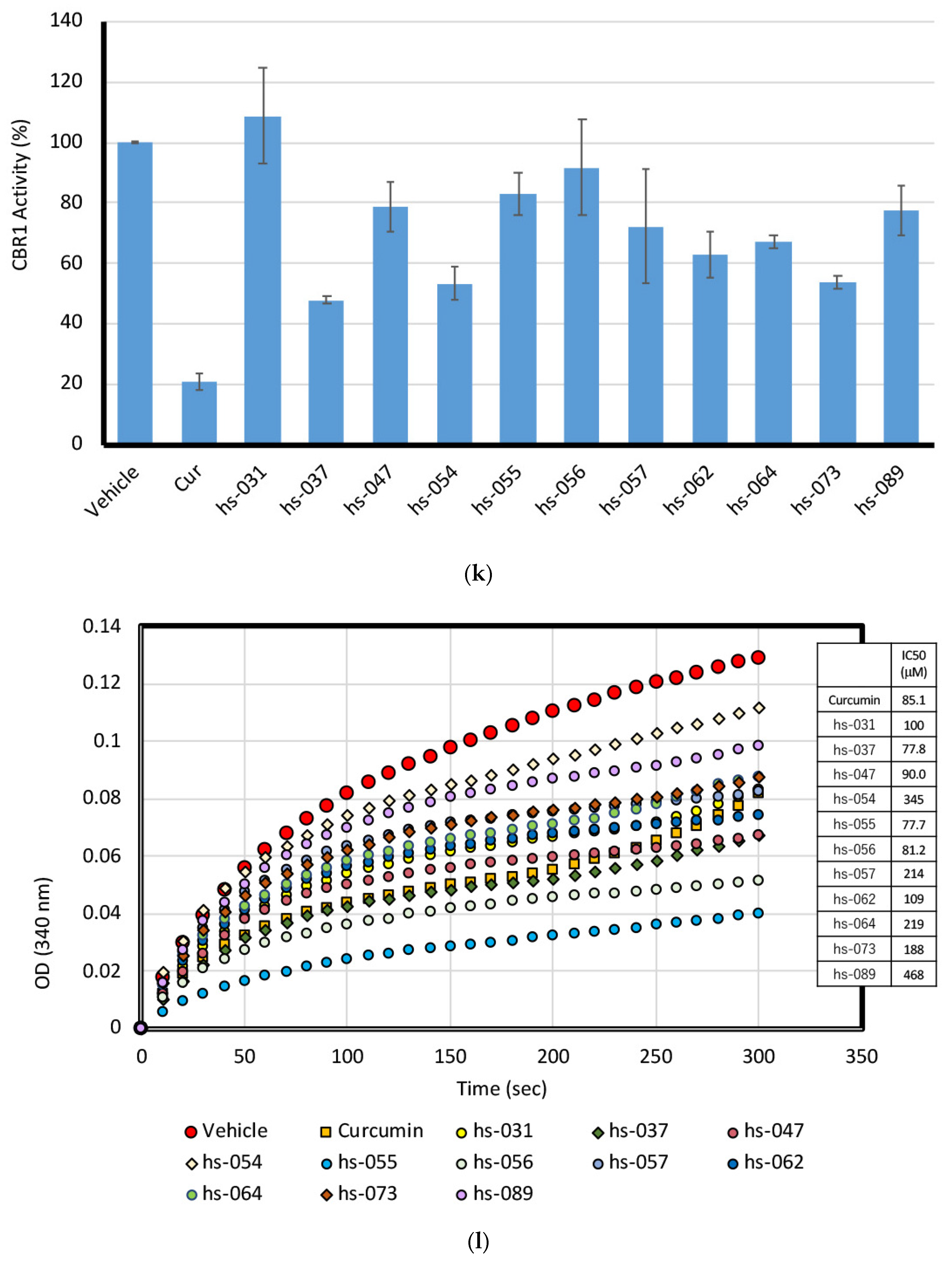
2.5. Binding Properties of Novel Curcumin Derivatives to ROS Metabolic Enzymes and Clustering Analysis
2.6. Mode of Inhibitory Action of Novel Curcumin Derivatives on ROS Metabolic Enzymes
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.1.1. C7-Curcuminoids, hs-031 and hs-037
4.1.2. 1-Aryl-1,3-diketones
4.1.3. C5-Curcuminoids
4.2. Proliferation Assays
4.3. Protein Analyses
4.4. CBR and GST Enzyme Assays
4.5. Tumorigenicity Test
4.6. Statistical Analysis
4.7. Prediction of Binding Sites of ROS Metabolic Enzymes for Curcumin and Curcumin Derivatives via a Docking Study
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, M.; Yu, Z.X.; Ferrans, V.J.; Irani, K.; Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 1995, 270, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Karin, M. Is NF-kappaB the sensor of oxidative stress? FASEB J. 1999, 13, 1137–1143. [Google Scholar] [CrossRef]
- Irani, K.; Xia, Y.; Zweier, J.L.; Sollott, S.J.; Der, C.J.; Fearon, E.R.; Sundaresan, M.; Finkel, T.; Goldschmidt-Clermont, P.J. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 1997, 275, 1649–1652. [Google Scholar] [CrossRef]
- Schreck, R.; Albermann, K.; Baeuerle, P.A. Nuclear factor kappa B: An oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic. Res. Commun. 1992, 17, 221–237. [Google Scholar] [CrossRef]
- Felty, Q.; Singh, K.P.; Roy, D. Estrogen-induced G1/S transition of G0-arrested estrogen-dependent breast cancer cells is regulated by mitochondrial oxidant signaling. Oncogene 2005, 24, 4883–4893. [Google Scholar] [CrossRef]
- Storz, P.; Toker, A. 3’-phosphoinositide-dependent kinase-1 (PDK-1) in PI 3-kinase signaling. Front. Biosci. 2002, 7, d886–d902. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 2015, 27, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Larasati, Y.A.; Yoneda-Kato, N.; Nakamae, I.; Yokoyama, T.; Meiyanto, E.; Kato, J.Y. Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Sci. Rep. 2018, 8, 2039. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.M. Reactive carbonyls and oxidative stress: Potential for therapeutic intervention. Pharmacol. Ther. 2007, 115, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Yakabe, K.; Yoshidomi, K.; Sueoka, K.; Nawata, S.; Yokoyama, Y.; Tsuchida, S.; Al-Mulla, F.; Sugino, N. Decreased carbonyl reductase 1 expression promotes malignant behaviours by induction of epithelial mesenchymal transition and its clinical significance. Cancer Lett. 2012, 323, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione transferases: Substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M. The aldo-keto reductases (AKRs): Overview. Chem. Biol. Interact 2015, 234, 236–246. [Google Scholar] [CrossRef]
- Wang, Y.; Kuramitsu, Y.; Ueno, T.; Suzuki, N.; Yoshino, S.; Iizuka, N.; Akada, J.; Kitagawa, T.; Oka, M.; Nakamura, K. Glyoxalase I (GLO1) is up-regulated in pancreatic cancerous tissues compared with related non-cancerous tissues. Anticancer Res. 2012, 32, 3219–3222. [Google Scholar]
- Siegel, D.; Yan, C.; Ross, D. NAD(P)H:quinone oxidoreductase 1 (NQO1) in the sensitivity and resistance to antitumor quinones. Biochem. Pharmacol. 2012, 83, 1033–1040. [Google Scholar] [CrossRef]
- Nicolussi, A.; D’Inzeo, S.; Capalbo, C.; Giannini, G.; Coppa, A. The role of peroxiredoxins in cancer. Mol. Clin. Oncol. 2017, 6, 139–153. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014, 6, 221ra15. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.L.; Saha, A.; Liu, J.; Tadi, S.; Tiziani, S.; Yan, W.; Triplett, K.; Lamb, C.; Alters, S.E.; Rowlinson, S.; et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat. Med. 2017, 23, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Zhou, Y.; Zhang, H.; Demizu, Y.; Chen, Z.; Pelicano, H.; Chiao, P.J.; Achanta, G.; Arlinghaus, R.B.; Liu, J.; et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 2006, 10, 241–252. [Google Scholar] [CrossRef]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Lo Russo, L.; A, D.E.L.; Laino, L.; Lo Muzio, L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp. Ther. Med. 2015, 10, 1615–1623. [Google Scholar] [CrossRef]
- Sa, G.; Das, T. Anti cancer effects of curcumin: Cycle of life and death. Cell. Div. 2008, 3, 14. [Google Scholar] [CrossRef]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta 2008, 1780, 673–679. [Google Scholar] [CrossRef]
- Heger, M. Drug screening: Don’t discount all curcumin trial data. Nature 2017, 543, 40. [Google Scholar] [CrossRef]
- Zaidi, A.; Lai, M.; Cavenagh, J. Long-term stabilisation of myeloma with curcumin. BMJ Case Rep. 2017, 2017. [Google Scholar] [CrossRef]
- Alves, L.V.; do Canto-Cavalheiro, M.M.; Cysne-Finkelstein, L.; Leon, L. In vitro antiproliferative effects of several diaryl derivatives on Leishmania spp. Biol. Pharm. Bull. 2003, 26, 453–456. [Google Scholar] [CrossRef]
- Caldarelli, A.; Penucchini, E.; Caprioglio, D.; Genazzani, A.A.; Minassi, A. Synthesis and tubulin-binding properties of non-symmetrical click C5-curcuminoids. Bioorg. Med. Chem. 2013, 21, 5510–5517. [Google Scholar] [CrossRef] [PubMed]
- Appiah-Opong, R.; de Esch, I.; Commandeur, J.N.; Andarini, M.; Vermeulen, N.P. Structure-activity relationships for the inhibition of recombinant human cytochromes P450 by curcumin analogues. Eur. J. Med. Chem. 2008, 43, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, A.E.; Ikawati, Z.; Sardjiman; Maeyama, K. Effects of benzylidenecyclopentanone analogues of curcumin on histamine release from mast cells. Biol. Pharm. Bull 2009, 32, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Meiyanto, E.; Putri, D.D.; Susidarti, R.A.; Murwanti, R.; Sardjiman; Fitriasari, A.; Husnaa, U.; Purnomo, H.; Kawaichi, M. Curcumin and its analogues (PGV-0 and PGV-1) enhance sensitivity of resistant MCF-7 cells to doxorubicin through inhibition of HER2 and NF-kB activation. Asian Pac. J. Cancer Prev. 2014, 15, 179–184. [Google Scholar] [CrossRef]
- Meiyanto, E.; Septisetyani, E.P.; Larasati, Y.A.; Kawaichi, M. Curcumin Analog Pentagamavunon-1 (PGV-1) Sensitizes Widr Cells to 5-Fluorouracil through Inhibition of NF-kappaB Activation. Asian Pac. J. Cancer Prev. 2018, 19, 49–56. [Google Scholar]
- Lestari, B.; Nakamae, I.; Yoneda-Kato, N.; Morimoto, T.; Kanaya, S.; Yokoyama, T.; Shionyu, M.; Shirai, T.; Meiyanto, E.; Kato, J.Y. Pentagamavunon-1 (PGV-1) inhibits ROS metabolic enzymes and suppresses tumor cell growth by inducing M phase (prometaphase) arrest and cell senescence. Sci. Rep. 2019, 9, 14867. [Google Scholar] [CrossRef]
- Deck, L.M.; Hunsaker, L.A.; Vander Jagt, T.A.; Whalen, L.J.; Royer, R.E.; Vander Jagt, D.L. Activation of anti-oxidant Nrf2 signaling by enone analogues of curcumin. Eur. J. Med. Chem. 2018, 143, 854–865. [Google Scholar] [CrossRef]
- Popic, V.V.; Korneev, S.M.; Nikolaev, V.A.; Korobitsyna, I.K. An Improved Synthesis of 2-Diazo-1,3-Diketones. Synth. Stuttg. 1991, 3, 195–198. [Google Scholar] [CrossRef]
- Saito, M.; Takemura, N.; Shirai, T. Classification of Ligand Molecules in PDB with Fast Heuristic Graph Match Algorithm COMPLIG. J. Mol. Biol. 2012, 424, 379–390. [Google Scholar] [CrossRef]
- Banerjee, S.; Ji, C.; Mayfield, J.E.; Goel, A.; Xiao, J.; Dixon, J.E.; Guo, X. Ancient drug curcumin impedes 26S proteasome activity by direct inhibition of dual-specificity tyrosine-regulated kinase 2. Proc. Natl. Acad. Sci. USA 2018, 115, 8155–8160. [Google Scholar] [CrossRef]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Yoneda-Kato, N.; Tomoda, K.; Umehara, M.; Arata, Y.; Kato, J.Y. Myeloid leukemia factor 1 regulates p53 by suppressing COP1 via COP9 signalosome subunit 3. Embo. J. 2005, 24, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Vainio, M.J.; Johnson, M.S. Generating conformer ensembles using a multiobjective genetic algorithm. J. Chem. Inf. Model. 2007, 47, 2462–2474. [Google Scholar] [CrossRef]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Biol. 2003, 10, 980. [Google Scholar] [CrossRef]
- Tanaka, M.; Bateman, R.; Rauh, D.; Vaisberg, E.; Ramachandani, S.; Zhang, C.; Hansen, K.C.; Burlingame, A.L.; Trautman, J.K.; Shokat, K.M.; et al. An unbiased cell morphology-based screen for new, biologically active small molecules. PLoS Biol. 2005, 3, e128. [Google Scholar] [CrossRef]
- Harshbarger, W.; Gondi, S.; Ficarro, S.B.; Hunter, J.; Udayakumar, D.; Gurbani, D.; Singer, W.D.; Liu, Y.; Li, L.; Marto, J.A.; et al. Structural and Biochemical Analyses Reveal the Mechanism of Glutathione S-Transferase Pi 1 Inhibition by the Anti-cancer Compound Piperlongumine. J. Biol. Chem. 2017, 292, 112–120. [Google Scholar] [CrossRef]
- Couture, J.F.; Legrand, P.; Cantin, L.; Luu-The, V.; Labrie, F.; Breton, R. Human 20alpha-hydroxysteroid dehydrogenase: Crystallographic and site-directed mutagenesis studies lead to the identification of an alternative binding site for C21-steroids. J. Mol. Biol. 2003, 331, 593–604. [Google Scholar] [CrossRef]
- Leung, K.K.; Shilton, B.H. Chloroquine binding reveals flavin redox switch function of quinone reductase 2. J. Biol. Chem. 2013, 288, 11242–11251. [Google Scholar] [CrossRef]
- Cameron, A.D.; Ridderstrom, M.; Olin, B.; Kavarana, M.J.; Creighton, D.J.; Mannervik, B. Reaction mechanism of glyoxalase I explored by an X-ray crystallographic analysis of the human enzyme in complex with a transition state analogue. Biochemistry 1999, 38, 13480–13490. [Google Scholar] [CrossRef]
- Cho, K.J.; Park, Y.; Khan, T.; Lee, J.H.; Kim, S.; Seok, J.H.; Chung, Y.B.; Cho, A.E.; Choi, Y.; Chang, T.-S.; et al. Crystal Structure of Dimeric Human Peroxiredoxin-1 C83S Mutant. Bull. Korean Chem. Soc. 2015, 36, 1543–1545. [Google Scholar] [CrossRef]
- Le Guilloux, V.; Schmidtke, P.; Tuffery, P. Fpocket: An open source platform for ligand pocket detection. BMC Bioinform. 2009, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |










| Compound | CLogP (1) | Growth Suppression (2) | Cell Death (3) | 1/GI50 (4) | Washout Growth Suppression (5) | Tumor Suppression (6) |
|---|---|---|---|---|---|---|
| Curcumin | 2.94 | 4.83 | 10.80 | 0.08 | 20.00 | 100.00 |
| hs-031 | 4.59 | 7.83 | 11.80 | 0.10 | 20.33 | 40.32 |
| hs-037 | 1.91 | 8.33 | 15.00 | 0.31 | 28.00 | 95.16 |
| hs-047 | 4.03 | −2.20 | 2.17 | 0.07 | −99.00 | −179.29 |
| hs-054 | 1.81 | 3.17 | 8.00 | 0.08 | 15.20 | 10.16 |
| hs-055 | 3.20 | 7.00 | 8.33 | 0.05 | 29.00 | 69.35 |
| hs-056 | 3.35 | −23.80 | 1.33 | 0.03 | −98.00 | 85.48 |
| hs-057 | 3.32 | 2.00 | 4.33 | 0.11 | −21.20 | 96.77 |
| hs-062 | 1.88 | 5.33 | 1.33 | 0.06 | −24.50 | −101.61 |
| hs-064 | 2.98 | 4.50 | 2.33 | 0.08 | 23.83 | 82.26 |
| hs-073 | 2.47 | −20.70 | 0.50 | 0.03 | −101.00 | 17.74 |
| hs-089 | 3.68 | 2.50 | 2.83 | 0.10 | 2.20 | 38.71 |
| hs-140 | 2.65 | 8.50 | 4.83 | 0.06 | 12.00 | −206.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamae, I.; Morimoto, T.; Shima, H.; Shionyu, M.; Fujiki, H.; Yoneda-Kato, N.; Yokoyama, T.; Kanaya, S.; Kakiuchi, K.; Shirai, T.; et al. Curcumin Derivatives Verify the Essentiality of ROS Upregulation in Tumor Suppression. Molecules 2019, 24, 4067. https://doi.org/10.3390/molecules24224067
Nakamae I, Morimoto T, Shima H, Shionyu M, Fujiki H, Yoneda-Kato N, Yokoyama T, Kanaya S, Kakiuchi K, Shirai T, et al. Curcumin Derivatives Verify the Essentiality of ROS Upregulation in Tumor Suppression. Molecules. 2019; 24(22):4067. https://doi.org/10.3390/molecules24224067
Chicago/Turabian StyleNakamae, Ikuko, Tsumoru Morimoto, Hiroki Shima, Masafumi Shionyu, Hisayo Fujiki, Noriko Yoneda-Kato, Takashi Yokoyama, Shigehiko Kanaya, Kiyomi Kakiuchi, Tsuyoshi Shirai, and et al. 2019. "Curcumin Derivatives Verify the Essentiality of ROS Upregulation in Tumor Suppression" Molecules 24, no. 22: 4067. https://doi.org/10.3390/molecules24224067
APA StyleNakamae, I., Morimoto, T., Shima, H., Shionyu, M., Fujiki, H., Yoneda-Kato, N., Yokoyama, T., Kanaya, S., Kakiuchi, K., Shirai, T., Meiyanto, E., & Kato, J.-y. (2019). Curcumin Derivatives Verify the Essentiality of ROS Upregulation in Tumor Suppression. Molecules, 24(22), 4067. https://doi.org/10.3390/molecules24224067






