Rapid Characterization and Discovery of Chemical Markers for Discrimination of Xanthii Fructus by Gas Chromatography Coupled to Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
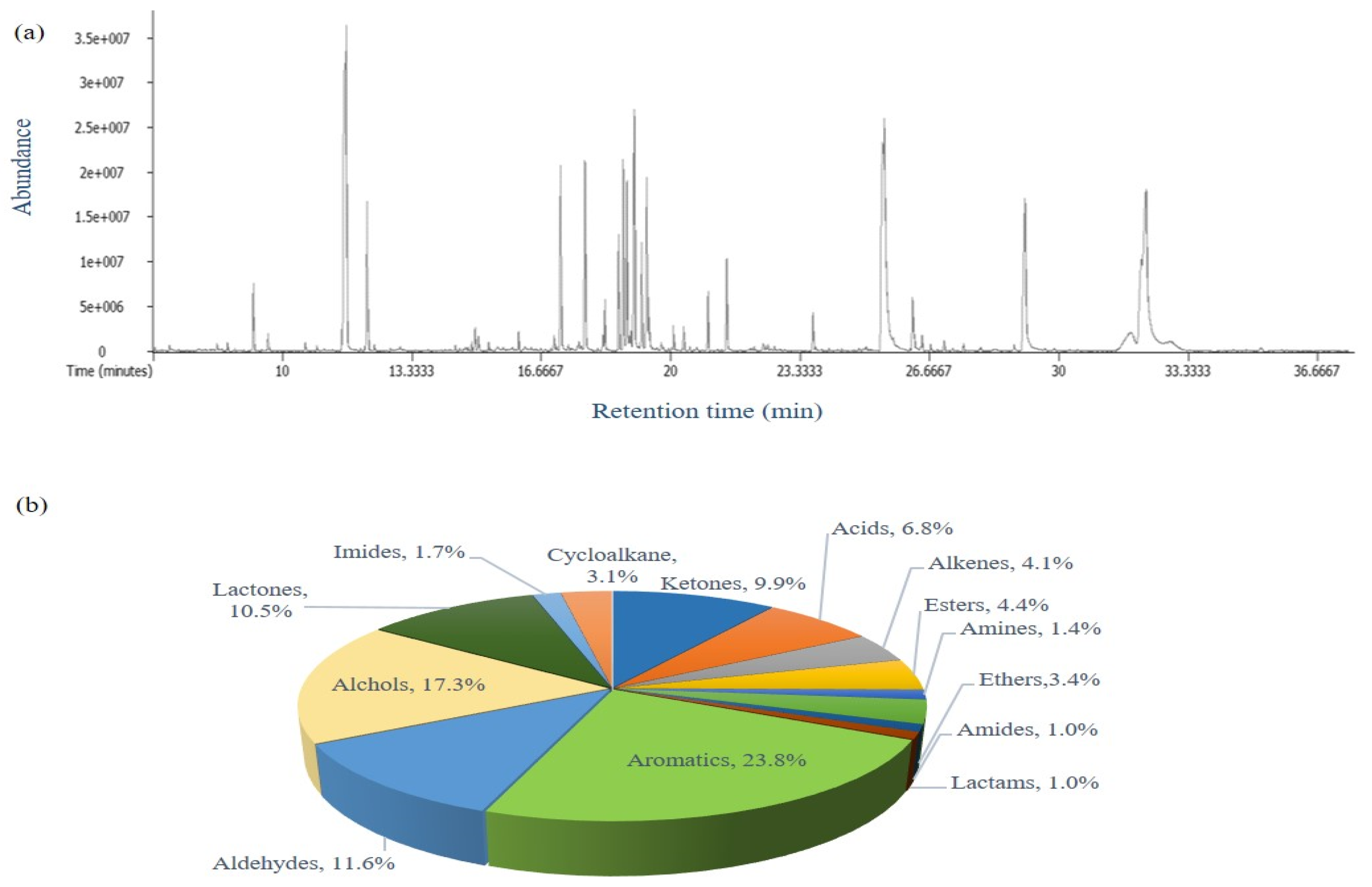
2.1. Analysis of VOCs by HS-SPME/GC-TOF MS
2.1.1. Chemical Profiling of Volatile Organic Compounds (VOCs)
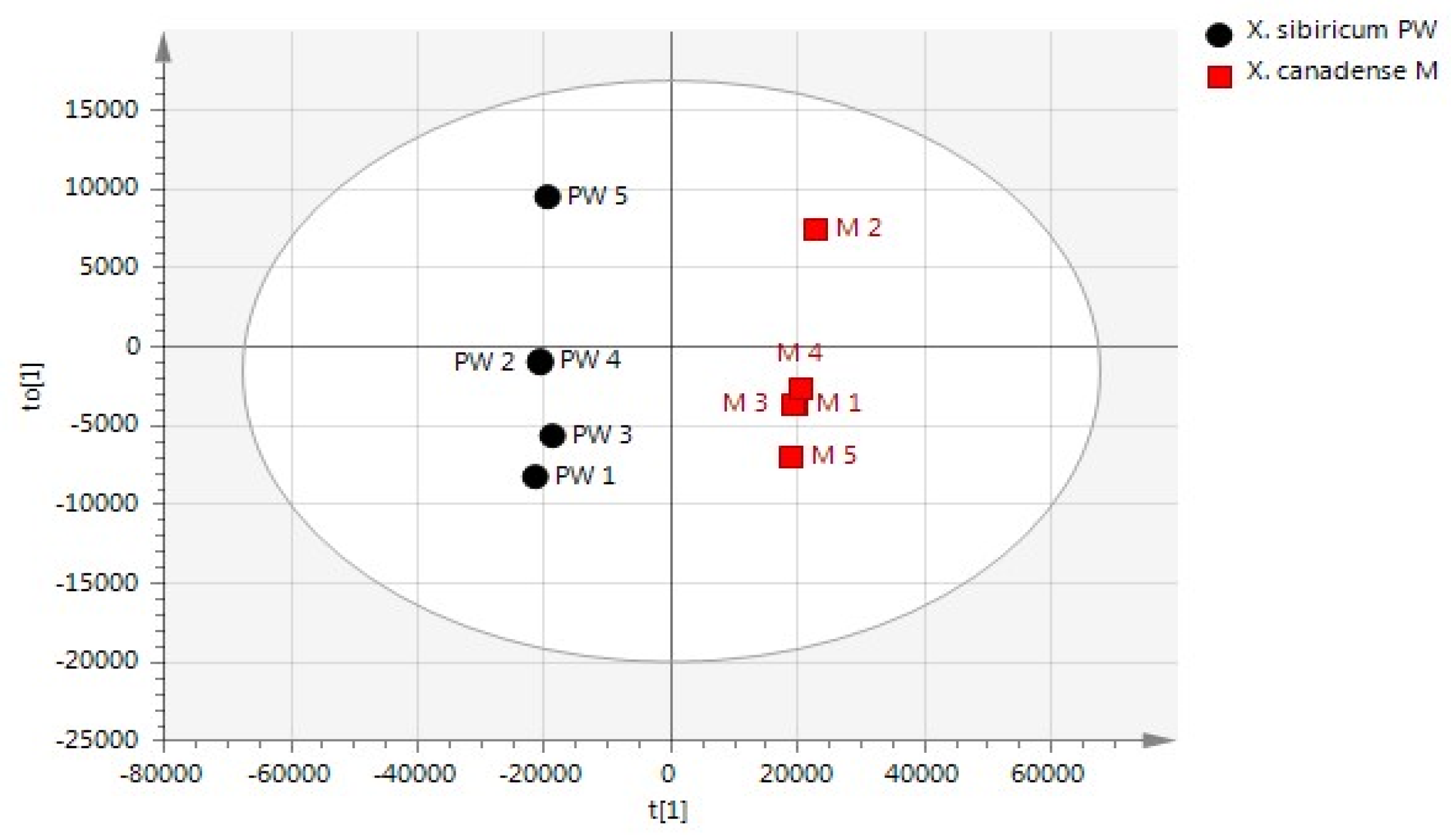
2.1.2. Statistical Analysis of VOC Profiles
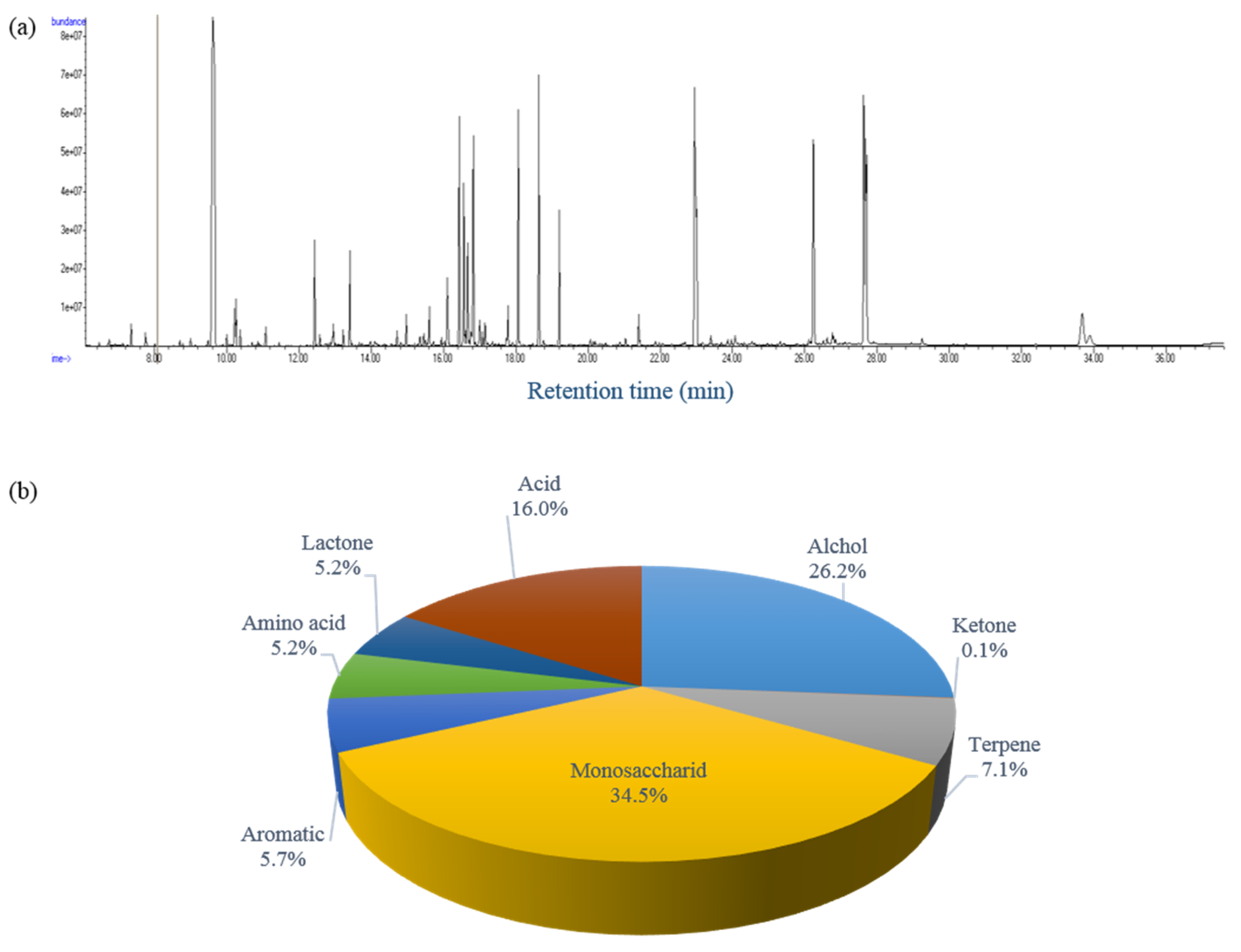
2.2. Analysis of Polar Metabolites by GC-TOF MS
2.2.1. Chemical Profiling of Polar Metabolites
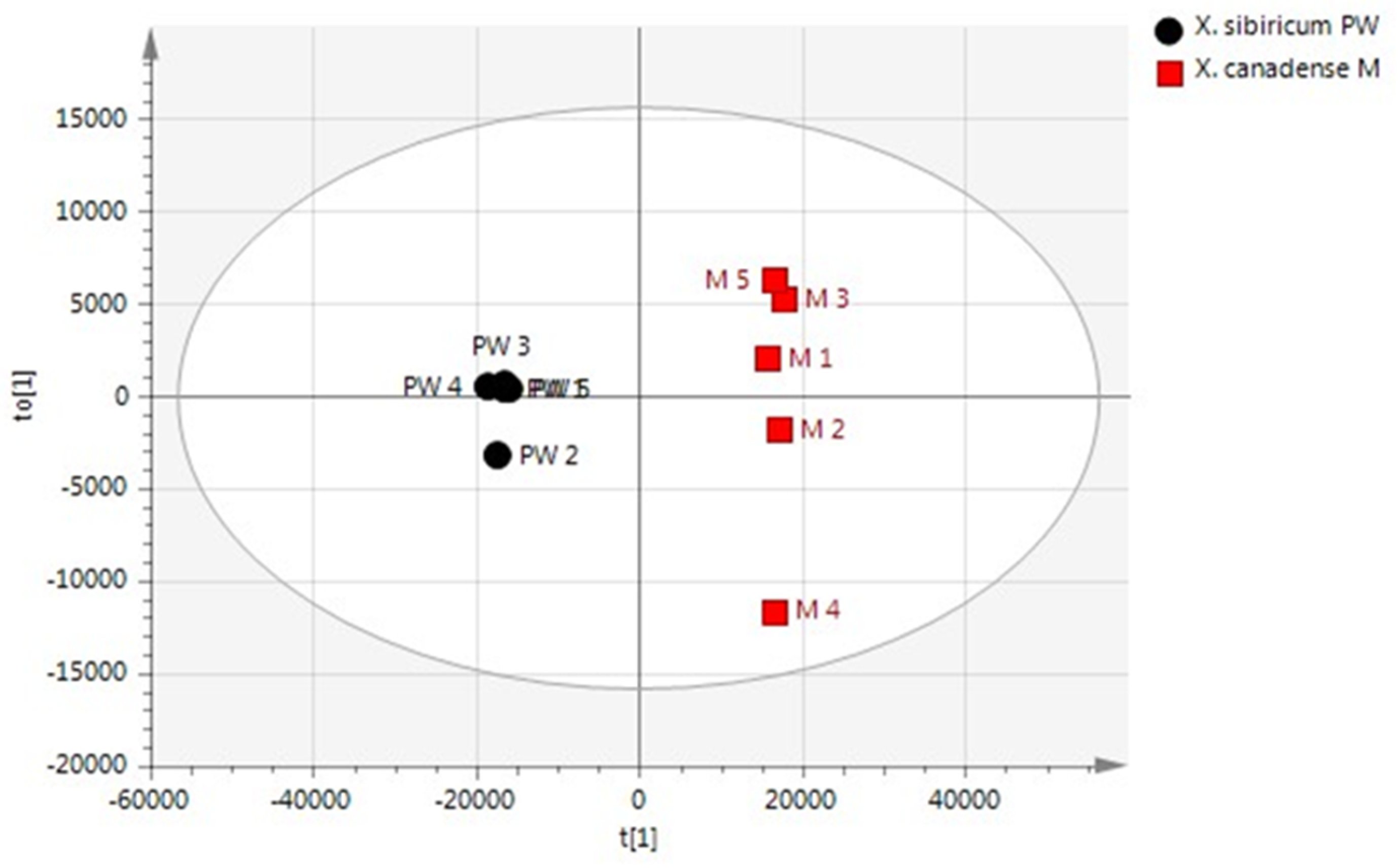
2.2.2. Statistical Analysis of Polar Metabolites
2.3. Quantitative Determination of the Chemical Markers
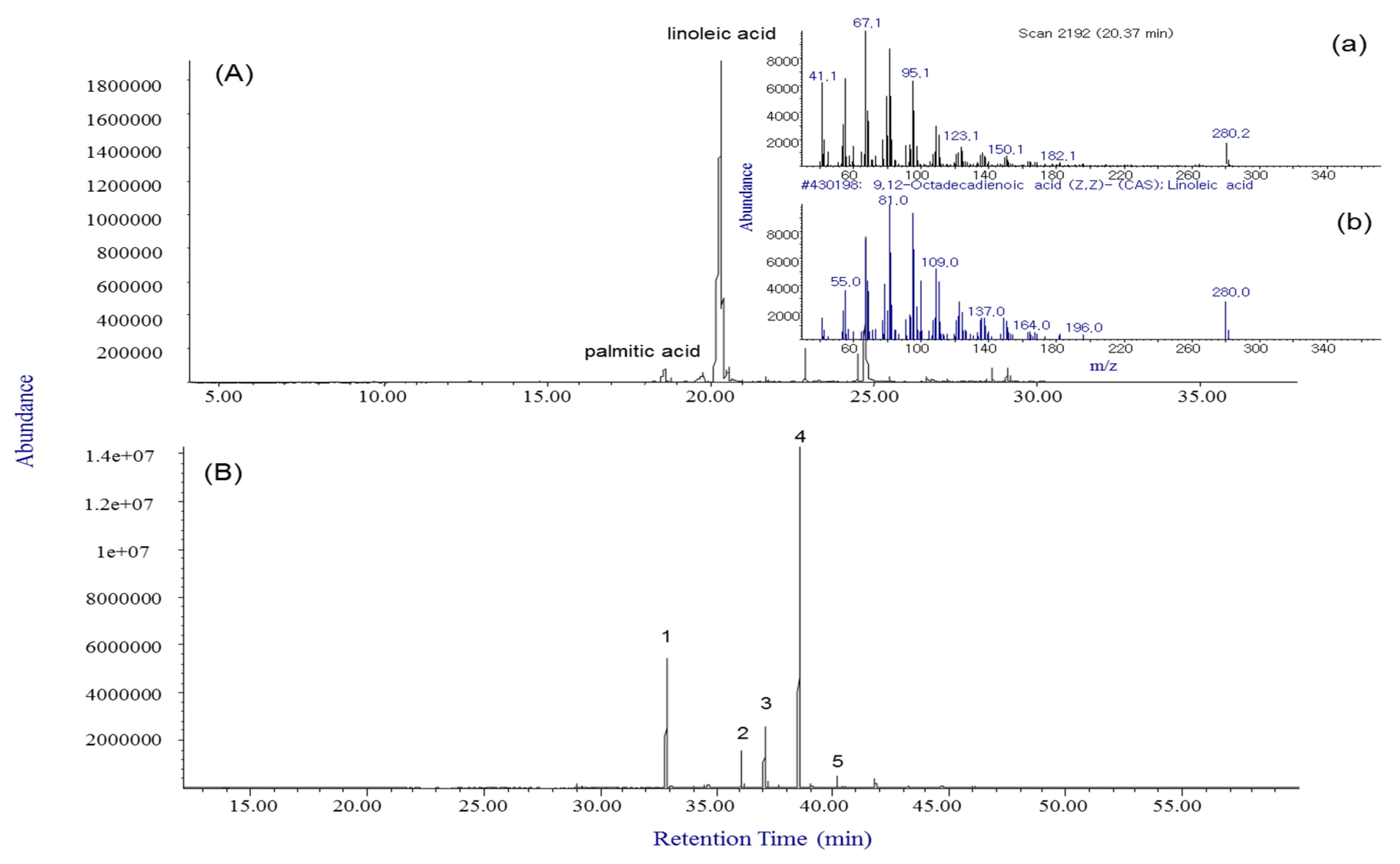
2.4. Fatty Acid Compositions of Xanthii Fructus by GC-MS Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
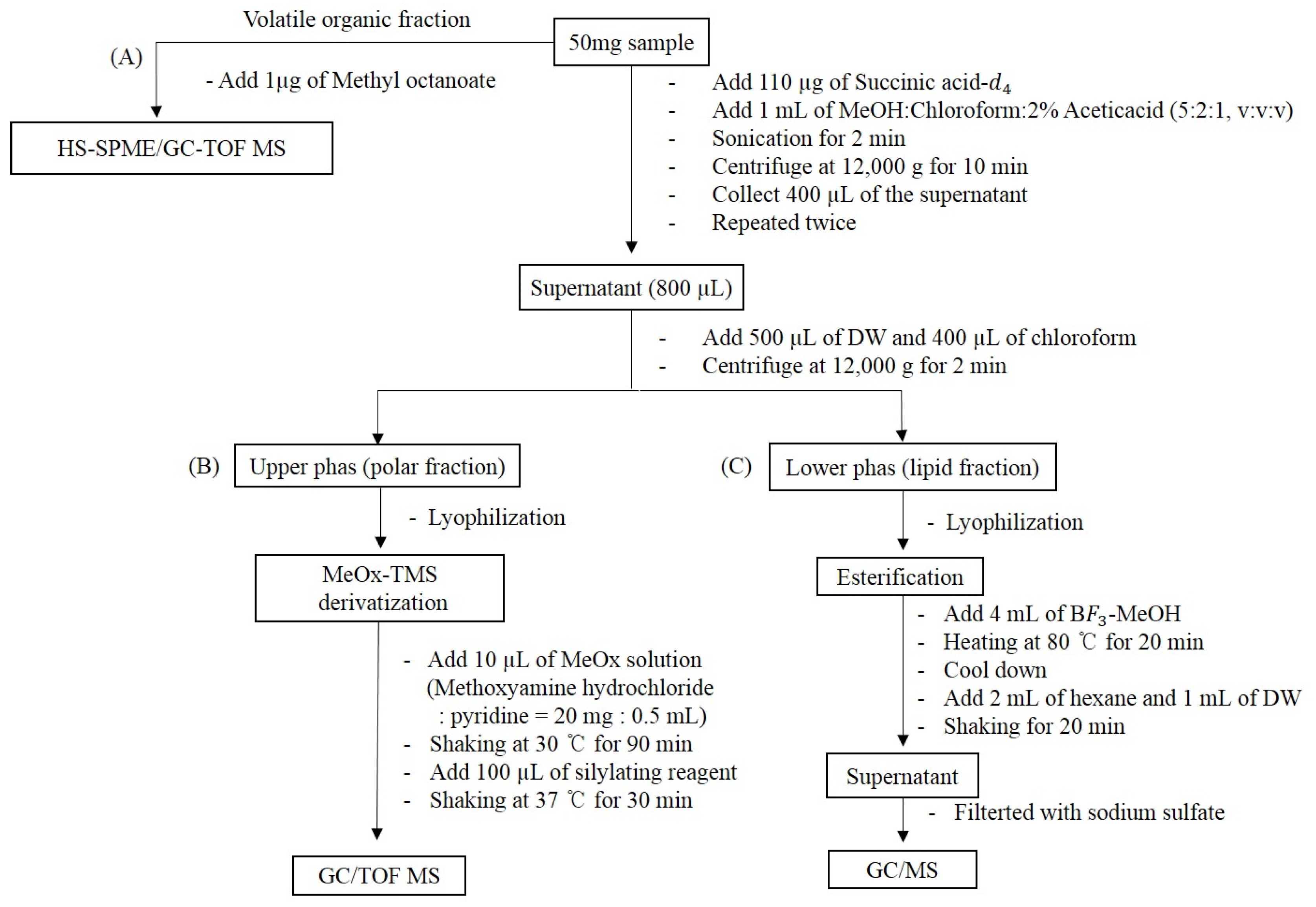
4.2. Preparation of Samples
4.3. Preparation of Standards
4.4. GC-TOF MS Analysis
4.5. Statistical Analysis
4.6. Analysis of Fatty Acid Composition by GC-MS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Amin, S.; Barkatullah, H.K. Pharmacology of Xanthium species. A review. J. Phytopharm. 2016, 5, 126–127. [Google Scholar]
- Goodarzi, M.; Russell, P.J.; Vander Heyden, Y. Similarity analyses of chromatographic herbal fingerprints: A review. Analytica Chimica Acta 2013, 804, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.; Saluja, A.K. Phytopharmacological review of Xanthium strumarium L. (Cocklebur). Int. J. Green Pharm. 2010, 4. [Google Scholar] [CrossRef]
- Kan, S.; Chen, G.; Han, C.; Chen, Z.; Song, X.; Ren, M.; Jiang, H. Chemical constituents from the roots of Xanthium sibiricum. Nat. Prod. Res. 2011, 25, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Z.; LI, N. Chemical constituents and bioactivity of Xanthium sibiricum Patrin. ex Widder. J. Sci. Teachers’College Univer. 2016, 10. [Google Scholar]
- Sakuda, Y.; Tahara, T. The Constituents of Essential Oil from Xanthium canadense Mill. J. Japan Oil Chem. Soc. 1982, 31, 151–153. [Google Scholar] [CrossRef]
- Li, N.; Zhang, W.-Z. Study on chemical constituents of Xanthium sibiricum Patrin ex Widder. J. Qiqihar Univer. 2016, 4, 13. [Google Scholar]
- Tang, J.S.; Jiang, C.Y.; Liu, Y.; Zhang, X.Y.; Shao, H.; Zhang, C. Allelopathic potential of volatile organic compounds released by Xanthium sibiricum Patrin ex Widder. Allelopathy J. 2019, 47, 233–241. [Google Scholar] [CrossRef]
- Henson, I.; Wareing, P. Cytokinins in Xanthium strumarium L.: Distribution in the plant and production in the root system. J. Exp. Botany 1976, 27, 1268–1278. [Google Scholar] [CrossRef]
- Kim, I.T.; Park, Y.M.; Won, J.H.; Jung, H.J.; Park, H.J.; Choi, J.W.; Lee, K.T. Methanol extract of Xanthium strumarium L. possesses anti-inflammatory and anti-nociceptive activities. Bio. Pharm. Bull. 2005, 28, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhao, Y.; Han, P.; Yue, W.; Ma, X.Q.; Rahman, K.; Han, T. Anti-arthritic activity of Xanthium strumarium L. extract on complete Freund’s adjuvant induced arthritis in rats. J. Ethnopharmacol. 2014, 155, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Hoseini-Alfatemi, S.M.; Sharifi-Rad, M.; Sharifi-Rad, M.; Iriti, M.; Sharifi-Rad, M.; Sharifi-Rad, R.; Raeisi, S. Phytochemical compositions and biological activities of essential oil from Xanthium strumarium L. Molecules 2015, 20, 7034–7047. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Agarwala, M. Phytochemical analysis of some medicinal plants. J. Phytol. 2011, 3. [Google Scholar]
- Yoon, J.H.; Lim, H.J.; Lee, H.J.; Kim, H.D.; Jeon, R.; Ryu, J.H. Inhibition of lipopolysaccharide-induced inducible nitric oxide synthase and cyclooxygenase-2 expression by xanthanolides isolated from Xanthium strumarium. Bioorg. Med. Chem. Lett. 2008, 18, 2179–2182. [Google Scholar] [CrossRef] [PubMed]
- Iordache, A.; Culea, M.; Gherman, C.; Cozar, O. Characterization of some plant extracts by GC–MS. Nucl. Ins. Methods Phys. Res. 2009, 267, 338–342. [Google Scholar] [CrossRef]
- Taherpour, A.A.; Khaef, S.; Yari, A.; Nikeafshar, S.; Fathi, M.; Ghambari, S. Chemical composition analysis of the essential oil of Mentha piperita L. from Kermanshah, Iran by hydrodistillation and HS/SPME methods. J. Analy. Sci. Technol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Boyacı, E.; Rodriguez-Lafuente, A.; Gorynski, K.; Mirnaghi, F.; Souza-Silva, E.A.; Hein, D.; Pawliszyn, J. Sample preparation with solid phase microextraction and exhaustive extraction approaches: Comparison for challenging cases. Analytica Chimica Acta 2015, 873, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Manzo, A.; Panseri, S.; Vagge, I.; Giorgi, A. Volatile Fingerprint of Italian Populations of Orchids Using Solid Phase Microextraction and Gas Chromatography Coupled with Mass Spectrometry. Molecules 2014, 19, 7913–7936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Zhou, L.D.; Chen, H.; Wang, C.Z.; Xia, Z.N.; Yuan, C.S. Solid-phase microextraction technology for in vitro and in vivo metabolite analysis. TrAC Trends Analy. Chem. 2016, 80, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Zhang, Q.Y.; Zhang, H.; Wen, J.; Wang, Y.; Huang, B.K.; Qin, L.P. Authentication and quantitative analysis on the chemical profile of Xanthium fruit (Cang-Er-Zi) by high-performance liquid chromatography-diode-array detection tandem mass spectrometry method. Analytica Chimica Acta 2009, 634, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Burruezo, A.; Kollmannsberger, H.; González-Mas, M.C.; Nitz, S.; Fernando, N. HS-SPME Comparative Analysis of Genotypic Diversity in the Volatile Fraction and Aroma-Contributing Compounds of Capsicum Fruits from the annuum−chinense−frutescens Complex. J. Agric. Food Chem. 2010, 58, 4388–4400. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Nam, P.W.; Lee, S.J.; Lee, K.G. Antioxidant activities of volatile and non-volatile fractions of selected traditionally brewed Korean rice wines. J. Ins. Brewing 2014, 120, 537–542. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. PCA as a practical indicator of OPLS-DA model reliability. Curr. Metab. 2016, 4, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Kim, J.K.; Han, J.G.; Kong, W.S.; Kim, S.Y.; Yang, Y.J.; Kim, S.H. Potential geo-discriminative tools to trace the origins of the dried slices of shiitake (Lentinula edodes) using stable isotope ratios and OPLS-DA. Food Chem. 2019, 295, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Tambellini, N.; Zaremberg, V.; Turner, R.; Weljie, A. Evaluation of Extraction Protocols for Simultaneous Polar and Non-Polar Yeast Metabolite Analysis Using Multivariate Projection Methods. Metabolites 2013, 3, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Fiehn, O. Seven Golden Rules for heuristic filtering of molecular formulas obtained by accurate mass spectrometry. BMC Bioinform. 2007, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.; Samwel, P.M.; Limbu, M.; Chacha, J. Mwita Fatty Acid Composition and Levels of Selected Polyunsaturated Fatty Acids in Four Commercial Important Freshwater Fish Species from Lake Victoria, Tanzania. J. Lipids 2014, 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.L.; Beaudette, L.A.; Hart, M.; Moutoglis, P.; Klironomos, J.N.; Lee, H.; Trevors, J.T. Methods of studying soil microbial diversity. J. Microbiol. Methods 2004, 58, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, P.; Fry, F.S.; Curtis, S.K.; Al-Khaldi, S.F.; Mossoba, M.M.; Yurawecz, M.P.; Dunkel, V.C. Use of Fatty Acid Profiles to Identify Food-Borne Bacterial Pathogens and Aerobic Endospore-Forming Bacilli. J. Agric. Food Chem. 2005, 53, 3735–3742. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, H.H.; Sen, N.; Holman, R.T. Characterization of unsaturated fatty acids by gas-liquid chromatography. J. Amer. Oil Chem. Soc. 1965, 42, 537–540. [Google Scholar] [CrossRef]
- Zhang, X.J.; Huang, L.L.; Cai, X.J.; Li, P.; Wang, Y.T.; Wan, J.B. Fatty acid variability in three medicinal herbs of Panaxspecies. Chem. Central J. 2013, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Vibha, J.B.; Choudhary, K.; Singh, M.; Rathore, M.S.; Shekhawat, N.S. A study on pharmacokinetics and therapeutic efficacy of Glycyrrhiza glabra: A miracle medicinal herb. Botany Res. Int. 2009, 2, 157–163. [Google Scholar]
- Tanaka, K.; Taniguchi, S.; Tamaoki, D.; Yoshitomi, K.; Akimitsu, K.; Gomi, K. Multiple roles of plant volatiles in jasmonate-induced defense response in rice. Plant Signaling Behavior 2014, 9, e29247. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Khan, A.L.; Ali, L.; Khan, A.R.; Waqas, M.; Hussain, J.; Shin, J.H. Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M1021. J. Microbiol. 2015, 53, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kahriman, N.; Tosun, G.; Terzioglu, S.; Karaoglu, S.A.; Yayli, N. Chemical composition and antimicrobial activity of the essential oils from the flower, leaf, and stem of Senecio pandurifolius. Records Nat. Prod. 2011, 5, 82. [Google Scholar]
- Shebl, M. Coordination behavior of new bis (tridentate ONO, ONS and ONN) donor hydrazones towards some transition metal ions: Synthesis, spectral, thermal, antimicrobial and antitumor studies. J. Mol. Struc. 2017, 1128, 79–93. [Google Scholar] [CrossRef]
- Sharma, K.; Pasricha, V.; Satpathy, G.; Gupta, R.K. Evaluation of phytochemical and antioxidant activity of raw Pyrus communis (l), an underexploited fruit. J. Pharm. Phytochem. 2015, 3. [Google Scholar]
- Goicoechea, E.; Van Twillert, K.; Duits, M.; Brandon, E.D.; Kootstra, P.R.; Blokland, M.H.; Guillén, M.D. Use of an in vitro digestion model to study the bioaccessibility of 4-hydroxy-2-nonenal and related aldehydes present in oxidized oils rich in omega-6 acyl groups. J. Agric. Food Chem. 2008, 56, 8475–8483. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.S.; Du, S.S.; Liu, Q.Z.; Liu, Q.R.; Liu, Z.L. Composition and insecticidal activity of the essential oil of Artemisia igniaria Maxim. Flowering aerial parts against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Med. Plants Res. 2012, 6, 3188–3192. [Google Scholar]
- Holt, S.; Miks, M.H.; de Carvalho, B.T.; Foulquié-Moreno, M.R.; Thevelein, J.M. The molecular biology of fruity and floral aromas in beer and other alcoholic beverages. FEMS Microbiol. Rev. 2018, 43, 193–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, K.H.; Nam, S. Antioxidant activities of volatile aroma components from Cudrania tricuspidata (carr.) bureau extracts. J. Korean Soc. Food Sci. Nutr. 2012, 41, 1493–1501. [Google Scholar] [CrossRef] [Green Version]
- Rohloff, J. Analysis of phenolic and cyclic compounds in plants using derivatization techniques in combination with GC-MS-based metabolite profiling. Molecules 2015, 20, 3431–3462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Mataix, J. Fatty acid composition of nuts–implications for cardiovascular health. British J. Nutr. 2006, 96, S29–S35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abate, S.; Ahn, Y.G.; Kind, T.; Cataldi, T.R.; Fiehn, O. Determination of elemental compositions by gas chromatography/time-of-flight mass spectrometry using chemical and electron ionization. Rapid Commun. Mass Spectr. 2010, 24, 1172–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Not available. |
| Class | Compound | X. canadense M | X. sibiricum PW | Linear Range (ng/mg) | Calibration Curve | |||
|---|---|---|---|---|---|---|---|---|
| Concentration (ng/mg) | RSD (%) | Concentration (ng/mg) | RSD (%) | Equation | γ2 | |||
| VOCs (ng/mg) | Benzeneethanol | 3.82 | 20.11 | 0.65 | 10.15 | 0.005~25 | y = 0.0003x − 0.004 | 0.9974 |
| Benzaldehyde | 0.10 | 20.17 | n.d. | y = 0.0013x + 0.0137 | 0.9994 | |||
| 1H-Pyrrole-2-carboxaldehyde | 2.38 | 8.38 | 0.69 | 8.47 | y = 0.0003x − 0.0158 | 0.9984 | ||
| 3-Octen-2-one | 0.57 | 13.37 | n.d. | y = 0.0002x − 0.004 | 0.9978 | |||
| Butyrolactone | 57.12 | 12.76 | 31.54 | 1.20 | y = 0.000002x − 0.0002 | 0.9951 | ||
| γ-Caprolactone | 11.05 | 9.19 | 0.79 | 4.76 | y = 0.00007x − 0.0049 | 0.9974 | ||
| δ-Hexalactone | 0.37 | 1.83 | 0.32 | 0.21 | y = 0.0073x − 0.4665 | 0.9981 | ||
| Pantolactone | 19.10 | 22.31 | 2.03 | 5.02 | y = 0.00001x + 0.0009 | 0.9969 | ||
| γ-Octalactone | 2.15 | 7.79 | 0.31 | 4.93 | y = 0.0001x − 0.0049 | 0.9983 | ||
| Polar Metabolites (ng/mg) | Ethylene glycol | 102.70 | 0.17 | n.d. | 20~2000 | y = 0.0009x − 4.5373 | 0.9953 | |
| l-(−)-Arabitol | 511.18 | 2.15 | 3684.11 | 2.36 | 20~10000 | y = 0.0001x − 2.014 | 0.9955 | |
| d-Mannitol | 424.32 | 5.31 | 4404.07 | 13.64 | 20~2000 | y = 0.00006x − 0.2687 | 0.9921 | |
| Scyllo-inositol | 1080.15 | 1.41 | 650.46 | 5.46 | 20~4000 | y = 0.0001x − 1.4494 | 0.9947 | |
| Succinic acid | 750.50 | 3.09 | 259.24 | 2.73 | 20~4000 | y = 0.00005x − 0.5167 | 0.995 | |
| d-Glyceric acid | 267.90 | 6.19 | 205.64 | 8.27 | 20~2000 | y = 0.0001x − 0.6062 | 0.9939 | |
| Fumaric acid | 185.21 | 10.72 | n.d. | 20~2000 | y = 0.00003x − 0.1361 | 0.995 | ||
| Malic acid | 422.72 | 9.63 | n.d. | 20~4000 | y = 0.00008x − 0.832 | 0.994 | ||
| Azelaic acid | 353.06 | 13.43 | n.d. | 20~2000 | y = 0.000008x − 0.0378 | 0.9931 | ||
| Gluconic acid | 141.72 | 10.74 | n.d. | 20~2000 | y = 0.00003x + 0.1344 | 0.9915 | ||
| d-Psicofuranose | 866.10 | 5.26 | 3748.90 | 1.57 | 20~10000 | y = 0.0001x −2.1083 | 0.9948 | |
| Fatty Acids | X. canadense M | X. sibiricum PW | ||||
|---|---|---|---|---|---|---|
| Common Name | Symbol | GC RT | % | %RSD | % | %RSD |
| Lauric | C12:0 | 27.35 | 0.1 | 0.7 | 0.1 | 2.2 |
| Tridecanoic | C13:0 | 29.27 | 0.3 | 6.9 | 0.3 | 3.9 |
| Palmitic | C16:0 | 34.06 | 0.3 | 3.6 | 0.2 | 5.6 |
| Saturated fatty acids (SFA) | 0.7 | 0.6 | ||||
| Myristoleic | C14:1 | 31.11 | 0.2 | 6.1 | 0.2 | 1.5 |
| Cis-10-pentadecanoic | C15:1 | 32.86 | 19.4 | 0.3 | 20.4 | 1.0 |
| Palmitoleic | C16:1 | 34.50 | 0.3 | 1.1 | 0.3 | 2.5 |
| Cis-10-heptadecenoic | C17:1 | 36.07 | 5.0 | 0.9 | 5.9 | 0.9 |
| Elaidic | C18:1n9t | 37.09 | 8.1 | 0.6 | 20.3 | 0.3 |
| Cis-11-eicosanoic | C20:1n9 | 40.19 | 1.7 | 1.2 | 0.8 | 4.5 |
| Nervonic | C24:1n9 | 45.72 | 0.2 | 12.8 | 1.7 | 2.4 |
| Monounsaturated (MUFA) | 34.9 | 49.6 | ||||
| Linolelaidic | C18:2n6t | 38.58 | 61.5 | 0.2 | 48.6 | 0.3 |
| Linoleic | C18:2n6c | 39.04 | 0.6 | 9.4 | 0.3 | 8.2 |
| Gamma-linolenic | C18:3n3-6 | 39.96 | 0.1 | 15.2 | 0.1 | 2.3 |
| Linolenic | C18:3n3-3 | 40.40 | 0.3 | 28.3 | - | - |
| Cis-11,14-eicosadienoic | C20:2 | 41.81 | 1.2 | 2.1 | 0.6 | 2.9 |
| Cis-11,14,17-eicosatrienoic | C20:3n3 | 43.22 | 0.3 | 7.0 | 0.1 | 20.9 |
| Cis-13,16-docosadienoic | C22:2 | 44.68 | 0.4 | 5.8 | 0.1 | 9.6 |
| Polyunsaturated (PUFA) | 64.4 | 49.8 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Jung, Y.; Jeon, S.H.; Hwang, G.-S.; Ahn, Y.G. Rapid Characterization and Discovery of Chemical Markers for Discrimination of Xanthii Fructus by Gas Chromatography Coupled to Mass Spectrometry. Molecules 2019, 24, 4079. https://doi.org/10.3390/molecules24224079
Kim H, Jung Y, Jeon SH, Hwang G-S, Ahn YG. Rapid Characterization and Discovery of Chemical Markers for Discrimination of Xanthii Fructus by Gas Chromatography Coupled to Mass Spectrometry. Molecules. 2019; 24(22):4079. https://doi.org/10.3390/molecules24224079
Chicago/Turabian StyleKim, Hayoung, Youngae Jung, So Hyeon Jeon, Geum-Sook Hwang, and Yun Gyong Ahn. 2019. "Rapid Characterization and Discovery of Chemical Markers for Discrimination of Xanthii Fructus by Gas Chromatography Coupled to Mass Spectrometry" Molecules 24, no. 22: 4079. https://doi.org/10.3390/molecules24224079
APA StyleKim, H., Jung, Y., Jeon, S. H., Hwang, G.-S., & Ahn, Y. G. (2019). Rapid Characterization and Discovery of Chemical Markers for Discrimination of Xanthii Fructus by Gas Chromatography Coupled to Mass Spectrometry. Molecules, 24(22), 4079. https://doi.org/10.3390/molecules24224079






