AC Electrodeposition of PEDOT Films in Protic Ionic Liquids for Long-Term Stable Organic Electrochemical Transistors
Abstract
:1. Introduction
2. Results and discussion
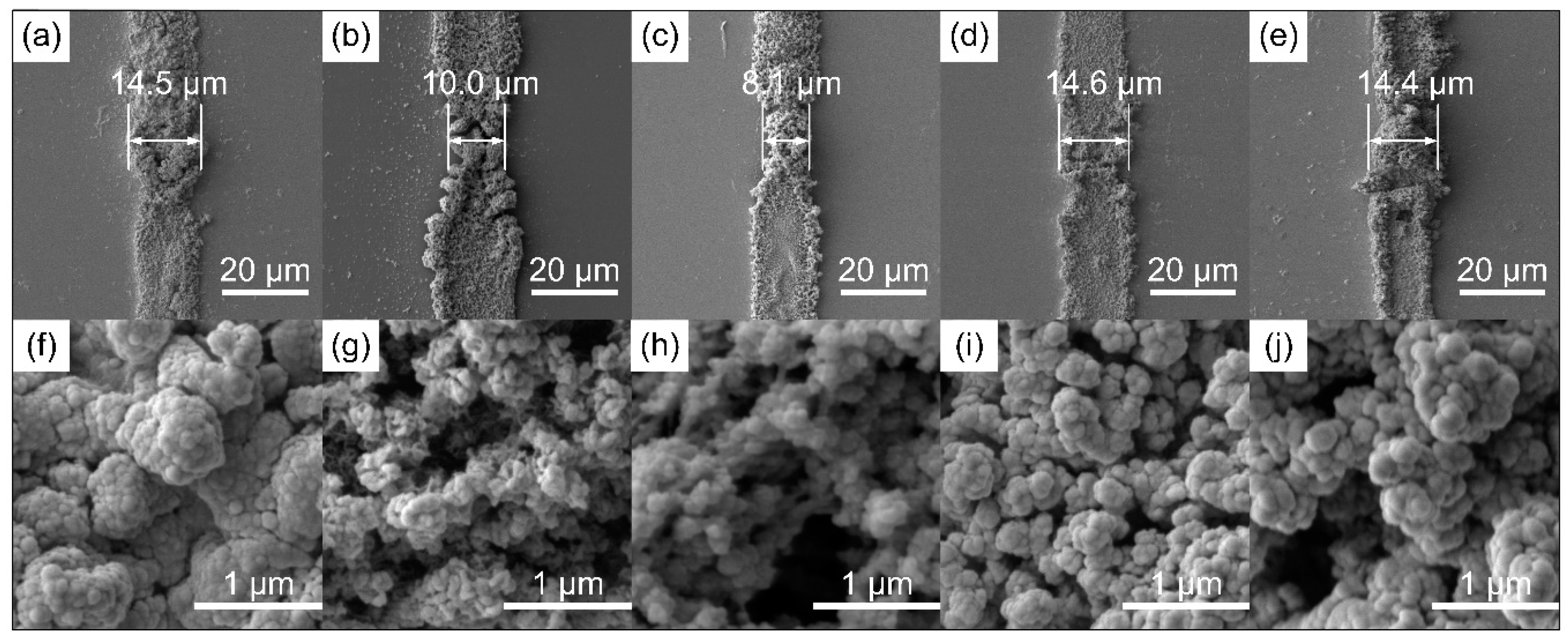
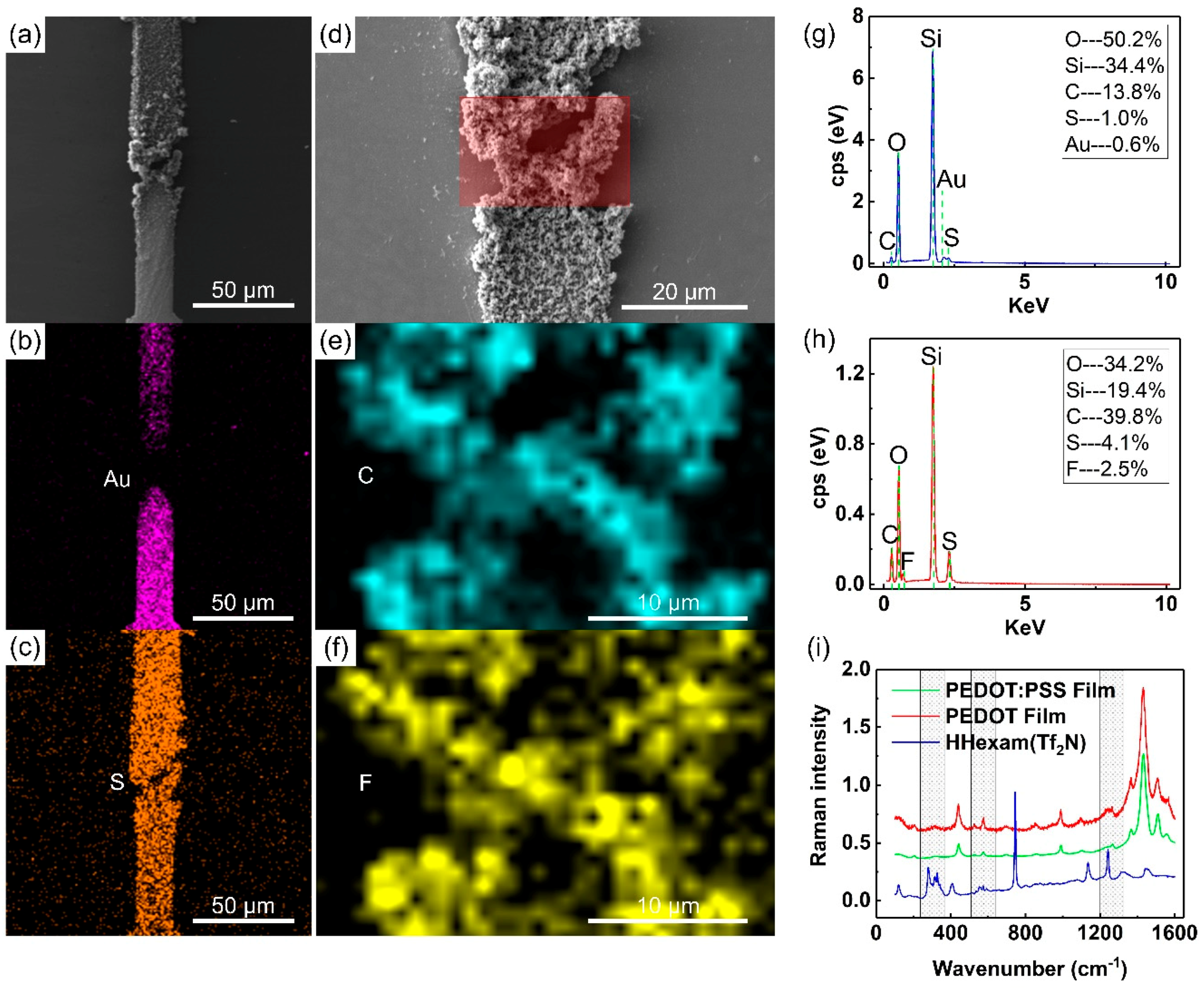
2.1. Chemical and Morphology
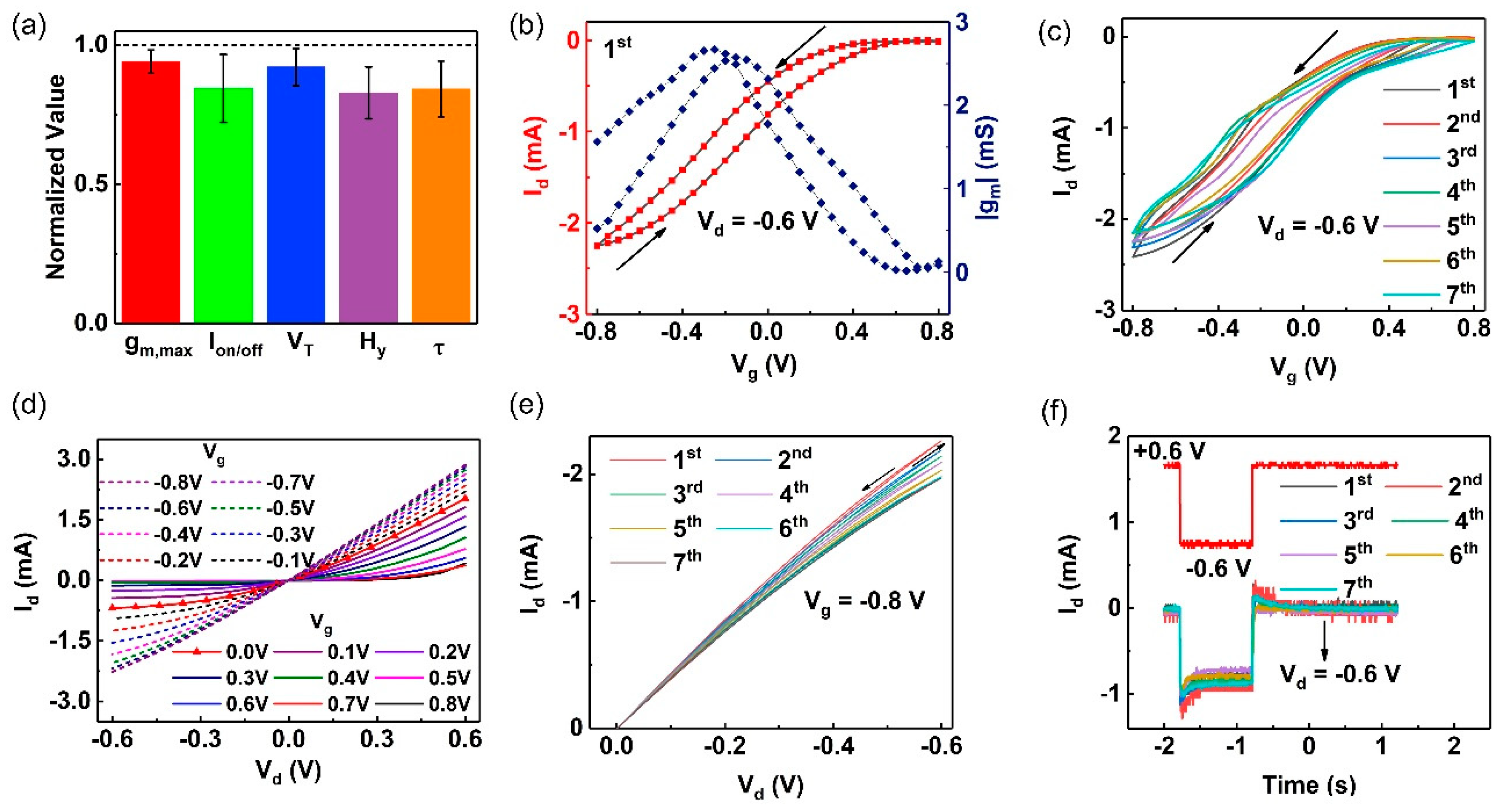
2.2. Long-Term Performance
3. Materials and Methods
3.1. Chemicals
3.2. ACDE on Microelectrodes Chip
3.3. Morphology, Structure, and OECTs Characterization
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sessolo, M.; Rivnay, J.; Bandiello, E.; Malliaras, G.G.; Bolink, H.J. Ion-selective organic electrochemical transistors. Adv. Mater. 2014, 26, 4803–4807. [Google Scholar] [CrossRef] [PubMed]
- Jiajun, S.; Peng, L.; Yi-Fan, R.; Wei-Wei, Z.; Weiwei, W.; Jin, H.; Shanming, K.; Xierong, Z.; Jing-Juan, X.; Hong-Yuan, C.; et al. Organic photo-electrochemical transistor-based biosensor: A proof-of-concept study toward highly sensitive DNA detection. Adv. Healthc. Mater. 2018, 7, 379–384. [Google Scholar]
- Peng, J.; He, T.; Sun, Y.; Liu, Y.; Cao, Q.; Wang, Q.; Tang, H. An organic electrochemical transistor for determination of microRNA21 using gold nanoparticles and a capture DNA probe. Mikrochim. Acta 2018, 185, 408. [Google Scholar] [CrossRef] [PubMed]
- Campana, A.; Cramer, T.; Simon, D.T.; Berggren, M.; Biscarini, F. Electrocardiographic recording with conformable organic electrochemical transistor fabricated on resorbable bioscaffold. Adv. Mater. 2014, 26, 3874–3878. [Google Scholar] [CrossRef]
- Fan, X.; Nie, W.; Tsai, H.; Wang, N.; Huang, H.; Cheng, Y.; Wen, R.; Ma, L.; Yan, F.; Xia, Y. PEDOT:PSS for flexible and stretchable electronics: modifications, strategies, and applications. Adv. Sci. 2019, 6, 1900813. [Google Scholar] [CrossRef]
- Inal, S.; Rivnay, J.; Hofmann, A.I.; Uguz, I.; Mumtaz, M.; Katsigiannopoulos, D.; Brochon, C.; Cloutet, E.; Hadziioannou, G.; Malliaras, G.G. Organic electrochemical transistors based on PEDOT with different anionic polyelectrolyte dopants. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 147–151. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Ouyang, J. Solution-processed metallic conducting polymer films as transparent electrode of optoelectronic devices. Adv. Mater. 2012, 24, 2436–2440. [Google Scholar] [CrossRef]
- Wu, F.; Li, P.; Sun, K.; Zhou, Y.; Chen, W.; Fu, J.; Li, M.; Lu, S.; Wei, D.; Tang, X.; et al. Conductivity enhancement of PEDOT:PSS via addition of chloroplatinic acid and its mechanism. Adv. Electron. Mater. 2017, 3, 1700047. [Google Scholar] [CrossRef]
- DeFranco, J.A.; Schmidt, B.S.; Lipson, M.; Malliaras, G.G. Photolithographic patterning of organic electronic materials. Org. Electron. 2006, 7, 22–28. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, K.; Lee, K.H.; Frisbie, C.D. Performance and stability of aerosol-jet-printed electrolyte-gated transistors based on poly(3-hexylthiophene). ACS Appl. Mater. Interfaces 2013, 5, 6580–6585. [Google Scholar] [CrossRef]
- Ji, J.; Li, M.; Chen, Z.; Wang, H.; Jiang, X.; Zhuo, K.; Liu, Y.; Yang, X.; Gu, Z.; Sang, S.; et al. In situ fabrication of organic electrochemical transistors on a microfluidic chip. Nano Res. 2019, 12, 1943–1951. [Google Scholar] [CrossRef]
- Tamburri, E.; Guglielmotti, V.; Orlanducci, S.; Terranova, M.L. Structure and I2/I− redox catalytic behaviour of PEDOT–PSS films electropolymerized in aqueous medium: Implications for convenient counter electrodes in DSSC. Inorg. Chim. Acta 2011, 377, 170–176. [Google Scholar] [CrossRef]
- Tamburri, E.; Orlanducci, S.; Toschi, F.; Terranova, M.L.; Passeri, D. Growth mechanisms, morphology, and electroactivity of PEDOT layers produced by electrochemical routes in aqueous medium. Synth. Met. 2009, 159, 406–414. [Google Scholar] [CrossRef]
- Reyna-González, J.M.; Reyes-López, J.C.; Aguilar-Martínez, M. Silver and silver–copper electrodeposition from a pyridinium-based ionic liquid. Electrochim. Acta 2013, 94, 344–352. [Google Scholar] [CrossRef]
- Xing, S.; Zanella, C.; Deflorian, F. Effect of pulse current on the electrodeposition of copper from choline chloride-ethylene glycol. J. Solid State Electrochem. 2014, 18, 1657–1663. [Google Scholar] [CrossRef]
- Mallet, J.; Molinari, M.; Martineau, F.; Delavoie, F.; Fricoteaux, P.; Troyon, M. Growth of silicon nanowires of controlled diameters by electrodeposition in ionic liquid at room temperature. Nano Lett. 2008, 8, 3468–3474. [Google Scholar] [CrossRef]
- Al Zoubi, M.; Al-Salman, R.; El Abedin, S.Z.; Li, Y.; Endres, F. Electrochemical synthesis of gallium nanowires and macroporous structures in an ionic liquid. ChemPhysChem. 2011, 12, 2751–2754. [Google Scholar]
- You, Y.H.; Gu, C.D.; Wang, X.L.; Tu, J.P. Electrodeposition of Ni–Co alloys from a deep eutectic solvent. Surf. Coat. Technol. 2012, 206, 3632–3638. [Google Scholar] [CrossRef]
- Fashu, S.; Gu, C.D.; Zhang, J.L.; Zheng, H.; Wang, X.L.; Tu, J.P. Electrodeposition, morphology, composition, and corrosion performance of Zn-Mn coatings from a deep eutectic solvent. J. Mater. Eng. Perform. 2014, 24, 434–444. [Google Scholar] [CrossRef]
- Serafín, V.; Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. A novel hybrid platform for the preparation of disposable enzyme biosensors based on poly(3,4-ethylenedioxythiophene) electrodeposition in an ionic liquid medium onto gold nanoparticles-modified screen-printed electrodes. J. Electroanal. Chem. 2011, 656, 152–158. [Google Scholar]
- Song, Z.; Sheng, G.; Cui, Y.; Li, M.; Song, Z.; Ding, C.; Luo, X. Low fouling electrochemical sensing in complex biological media by using the ionic liquid-doped conducting polymer PEDOT: Application to voltammetric determination of dopamine. Mikrochim. Acta 2019, 186, 220. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, J.; Lu, B.; Zhang, S.; Qin, L.; Mo, D.; Zhen, S. Poly(thieno [3,4-b]-1,4-oxathiane): Medium effect on electropolymerization and electrochromic performance. Langmuir 2014, 30, 15581–15589. [Google Scholar] [CrossRef] [PubMed]
- Er, H.; Xu, Y.; Zhao, H. Properties of mono-protic ionic liquids composed of hexylammonium and hexylethylenediaminium cations with trifluoroacetate and bis (trifluoromethylsulfonyl) imide anions. J. Mol. Liq. 2019, 276, 379–384. [Google Scholar] [CrossRef]
- Takemura, S.; Kawakami, S.; Harada, M.; Iida, M. Solvation structure of a copper(II) ion in protic ionic liquids comprising N-hexylethylenediamine. Inorg. Chem. 2014, 53, 9667–9678. [Google Scholar] [CrossRef] [PubMed]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of conducting polymerss—Persistent models and new concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, V.; Bayon, C.; Descamps, E.; Bergaud, C. Morphology and conductivity of PEDOT layers produced by different electrochemical routes. Synth. Met. 2014, 189, 7–16. [Google Scholar] [CrossRef]
- Heinze, A.R.J.; Pagels, M.; Geschke, B. On the origin of the so-called nucleation loop during electropolymerization of conducting polymers. Phys. Chem. B 2007, 111, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.S.; Ackerson, B.J.; Grischkowsky, D.R.; Flanders, B.N. Directional growth of metallic and polymeric nanowires. Nanotechnology 2009, 20, 235307. [Google Scholar] [CrossRef]
- Patra, S.; Barai, K.; Munichandraiah, N. Scanning electron microscopy studies of PEDOT prepared by various electrochemical routes. Synth. Met. 2008, 158, 430–435. [Google Scholar] [CrossRef]
- Zeglio, E.; Inganas, O. Active materials for organic electrochemical transistors. Adv. Mater. 2018, 30, e1800941. [Google Scholar] [CrossRef]
- Luo, X.; Cui, X.T. Electrochemical deposition of conducting polymer coatings on magnesium surfaces in ionic liquid. Acta Biomater. 2011, 7, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Khodagholy, D.; Gurfinkel, M.; Stavrinidou, E.; Leleux, P.; Herve, T.; Sanaur, S.; Malliaras, G.G. High speed and high density organic electrochemical transistor arrays. Appl. Phys. Lett. 2011, 99, 163304. [Google Scholar] [CrossRef]
- Bidoky, F.Z.; Frisbie, C.D. Parasitic capacitance effect on dynamic performance of aerosol-jet-printed sub 2 V poly(3-hexylthiophene) electrolyte-gated transistors. ACS Appl. Mater. Interfaces 2016, 8, 27012–27017. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.; Zhu, X.; Han, D.; Li, M.; Zhang, Q.; Shu, Y.; Cheng, Z.; Zhang, W.; Hua, E.; Sang, S. AC Electrodeposition of PEDOT Films in Protic Ionic Liquids for Long-Term Stable Organic Electrochemical Transistors. Molecules 2019, 24, 4105. https://doi.org/10.3390/molecules24224105
Ji J, Zhu X, Han D, Li M, Zhang Q, Shu Y, Cheng Z, Zhang W, Hua E, Sang S. AC Electrodeposition of PEDOT Films in Protic Ionic Liquids for Long-Term Stable Organic Electrochemical Transistors. Molecules. 2019; 24(22):4105. https://doi.org/10.3390/molecules24224105
Chicago/Turabian StyleJi, Jianlong, Xiaoxian Zhu, Dan Han, Mangmang Li, Qiang Zhang, Yang Shu, Zhengdong Cheng, Wendong Zhang, Er Hua, and Shengbo Sang. 2019. "AC Electrodeposition of PEDOT Films in Protic Ionic Liquids for Long-Term Stable Organic Electrochemical Transistors" Molecules 24, no. 22: 4105. https://doi.org/10.3390/molecules24224105
APA StyleJi, J., Zhu, X., Han, D., Li, M., Zhang, Q., Shu, Y., Cheng, Z., Zhang, W., Hua, E., & Sang, S. (2019). AC Electrodeposition of PEDOT Films in Protic Ionic Liquids for Long-Term Stable Organic Electrochemical Transistors. Molecules, 24(22), 4105. https://doi.org/10.3390/molecules24224105






