Microbial Conversion of Toxic Resin Acids
Abstract
:1. Introduction
2. Distribution and Toxicity of RAs
3. Biodegradation of RAs
4. Biotransformation of RAs to Bioactive Compounds
5. Conclusions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization of The United Nations, (FAO). Forestry Production and Trade (the Database). Available online: http://www.fao.org/faostat/en/#data/FO/visualize (accessed on 9 July 2019).
- Singh, P.; Srivastava, N.; Jagadish, R.; Upadhyay, A. Effect of toxic pollutants from pulp and paper mill on water and soil quality and its remediation. Int. J. Lakes Rivers 2019, 12, 1–20. [Google Scholar]
- Ottavioli, J.; Paoli, M.; Casanova, J.; Tomi, F.; Bighelli, A. Identification and quantitative determination of resin acids from corsican Pinus pinaster Aiton oleoresin using 13C-NMR spectroscopy. Chem. Biodivers. 2019, 16, e1800482. [Google Scholar] [CrossRef]
- Hernández, V.; Silva, M.; Gavilán, J.; Jiménez, B.; Barra, R.; Becerra, J. Resin acids in bile samples from fish inhabiting marine waters affected by pulp mill effluents. J. Chil. Chem. Soc. 2008, 53, 1718–1721. [Google Scholar] [CrossRef]
- Zheng, J.; Nicholson, R. Action of resin acids in nerve ending fractions isolated from fish central nervous system. Environ. Toxicol. Chem. 1998, 17, 1852–1859. [Google Scholar] [CrossRef]
- Rissanen, E.; Krumschnabel, G.; Nikinmaa, M. Dehydroabietic acid, a major component of wood industry effluents, interferes with cellular energetics in rainbow trout hepatocytes. Aquat. Toxicol. 2003, 62, 45–53. [Google Scholar] [CrossRef]
- Söderberg, T.A.; Johansson, A.; Gref, R. Toxic effects of some conifer resin acids and tea tree oil on human epithelial and fibroblast cells. Toxicology 1996, 107, 99–109. [Google Scholar] [CrossRef]
- Sunzel, B.; Söderberg, T.A.; Johansson, A.; Hallmans, G.; Gref, R. The protective effect of zinc on rosin and resin acid toxicity in human polymorphonuclear leukocytes and human gingival fibroblasts in vitro. J. Biomed. Mater. Res. 1997, 37, 20–28. [Google Scholar] [CrossRef]
- Ohmori, K.; Kawamura, Y. Cell transformation activities of abietic acid and dehydroabietic acid: Safety assessment of possible contaminants in paper and paperboard for food contact use. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2009, 26, 568–573. [Google Scholar] [CrossRef]
- Nestmann, E.R.; Lee, E.G.; Mueller, J.C.; Douglas, G.R. Mutagenicity of resin acids identified in pulp and paper mill effluents using the salmonella/mammalian-microsome assay. Environ. Mutagen. 1979, 1, 361–369. [Google Scholar] [CrossRef]
- Kinae, N.; Hashizume, T.; Makita, T.; Tomita, I.; Kimura, I.; Kanamori, H. Studies on the toxicity of pulp and paper mill effluents—I. Mutagenicity of the sediment samples derived from kraft paper mills. Water Res. 1981, 15, 17–24. [Google Scholar] [CrossRef]
- Burčová, Z.; Kreps, F.; Greifová, M.; Jablonský, M.; Ház, A.; Schmidt, Š.; Šurina, I. Antibacterial and antifungal activity of phytosterols and methyl dehydroabietate of Norway spruce bark extracts. J. Biotechnol. 2018, 282, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, Y.; Saito, Y.; Takeya, M.; Goto, M.; Nakagawa-Goto, K. Synthesis of 4-epi-parviflorons A, C, and E: Structure-activity relationship study of antiproliferative abietane derivatives. J. Org. Chem. 2019, 84, 3239–3248. [Google Scholar] [CrossRef] [PubMed]
- Liss, S.N.; Bicho, P.A.; Saddler, J.N. Microbiology and biodegradation of resin acids in pulp mill effluents: A minireview. Can. J. Microbiol. 1997, 75, 599–611. [Google Scholar] [CrossRef]
- Martin, V.J.J.; Yu, Z.; Mohn, W.W. Recent advances in understanding resin acid biodegradation: Microbial diversity and metabolism. Arch. Microbiol. 1999, 172, 131–138. [Google Scholar] [CrossRef]
- Keeling, C.I.; Bohlmann, J. Diterpene resin acids in conifers. Phytochemistry 2006, 67, 2415–2423. [Google Scholar] [CrossRef]
- Rogachev, A.D.; Salakhutdinov, N.F. Chemical composition of Pinus sibirica (Pinaceae). Chem. Biodivers. 2015, 12, 1–53. [Google Scholar] [CrossRef]
- Gonçalves, M.D.; Bortoleti, B.T.S.; Tomiotto-Pellissier, F.; Miranda-Sapla, M.M.; Assolini, J.P.; Carloto, A.C.M.; Carvalho, P.G.C.; Tudisco, E.T.; Urbano, A.; Ambrósio, S.R.; et al. Dehydroabietic acid isolated from Pinus elliottii exerts in vitro antileishmanial action by pro-oxidant effect, inducing ROS production in promastigote and downregulating Nrf2/ferritin expression in amastigote forms of Leishmania amazonensis. Fitoterapia 2018, 128, 224–232. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Cox, R.E.; Oros, D.R.; Otto, A. Terpenoid compositions of resins from Callitris species (Cupressaceae). Molecules 2018, 23, 3384. [Google Scholar] [CrossRef]
- Schicchi, R.; Geraci, A.; Rosselli, S.; Spinella, A.; Maggio, A.; Bruno, M. Phytochemical investigation of the needles of Abies nebrodensis (Lojac.) Mattei. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Smeds, A.; Willför, S. Chemical composition and content of lipophilic seed extractives of some Abies and Picea species. Chem. Biodivers. 2016, 13, 1194–1201. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Atif, M.; Shan, S.A.A.; Sultan, S.; Erum, S.; Khan, S.N. Atta-Ur-Rahman Biotransformation of dehydroabietic acid with microbial cell cultures and α-glucosidase inhibitory activity of resulting metabolites. Int. J. Pharm. Pharm. Sci. 2014, 6, 375–378. [Google Scholar]
- González, M.A.; Perez-Guaita, D.; Correa-Royero, J.; Zapata, B.; Agudelo, L.; Mesa-Arango, A.; Betancur-Galvis, L. Synthesis and biological evaluation of dehydroabietic acid derivatives. Eur. J. Med. Chem. 2010, 45, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Hirai, S.; Goto, T.; Kuroyanagi, K.; Lee, J.Y.; Uemura, T.; Ezaki, Y.; Takahashi, N.; Kawada, T. Dehydroabietic acid, a phytochemical, acts as ligand for PPARs in macrophages and adipocytes to regulate inflammation. Biochem. Biophys. Res. Commun. 2008, 369, 333–338. [Google Scholar] [CrossRef]
- Janocha, S. Umsatz von Harzsäuren Durch die Bakteriellen Cytochrome CYP105A1 und CYP106A2—Strukturelle Grundlagen und Potentielle Anwendungen. Ph.D. Thesis, Univarsität des Saarlandes, Saarbrücken, Germany, 2013. [Google Scholar]
- Plemenkov, V.V.; Appolonova, S.A.; Kirlitsa, D.A. To the question of the native content of resin acids in the galipot of conifers. Chem. Comput. Simulation. Butl. Commun. 2004, 5, 30–32. [Google Scholar]
- Peng, G.; Roberts, J.C. Solubility and toxicity of resin acids. Wat. Res. 2000, 34, 2779–2785. [Google Scholar] [CrossRef]
- Zanella, E. Effect of pH on acute toxicity of dehydroabietic acid and chlorinated dehydroabietic acid to fish and Daphnia. Bull. Environ. Contam. Toxicol. 1983, 30, 133–140. [Google Scholar] [CrossRef]
- Volkman, J.K.; Holdsworth, D.G.; Richardson, D.E. Determination of resin acids by gas chromatography and high-perfomance liquid chromatography in paper mill effluent, river waters and sediments from the upper Derwent Estuary, Tasmania. J. Chromatogr. 1993, 643, 209–219. [Google Scholar] [CrossRef]
- Leppanen, H.; Marttinen, S.; Oikari, A. The use of fish bile metabolite analyses as exposure biomarkers to pulp and paper mill effluents. Chemosphere 1998, 36, 2621–2634. [Google Scholar] [CrossRef]
- Oikari, A.; Holmbom, B.; Bister, H. Uptake of resin acids into tissues of trout (Salmo gairdneri Richardson). Ann. Zool. Fenn. 1982, 19, 61–64. [Google Scholar]
- Lindesjöö, E.; Adolfsson-Erici, M.; Ericson, G.; Förlin, L. Biomarker responses and resin acids in fish chronically exposed to effluents from a total chlorine-free pulp mill during regular production. Ecotoxicol. Environ. Saf. 2002, 53, 238–247. [Google Scholar] [CrossRef]
- Teschke, K.; Demers, P.A.; Davies, H.W.; Kennedy, S.M.; Marion, S.A.; Leung, V. Determinants of exposure to inhalable particulate, wood dust, resin acids, and monoterpenes in a lumber mill environment. Ann. Occup. Hyg. 1999, 43, 247–255. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Rogge, W.F.; Lang, Q.; Ja, R. Molecular characterization of smoke from campfire burning of pine wood (Pinus elliottii). Chemosphere 2000, 2, 107–122. [Google Scholar] [CrossRef]
- Ozaki, A.; Ooshima, T.; Mori, Y. Migration of dehydroabietic and abietic acids from paper and paperboard food packaging into food-simulating solvents and Tenax TA. Food Addit. Contam. 2006, 23, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, S.; Geng, Z.; Wang, D.; Liu, F.; Zhang, M. Analysis of abietic acid and dehydroabietic acid residues in raw ducks and cooked ducks. Poult. Sci. 2014, 93, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, S.; Geng, Z.; Wang, D.; Liu, F.; Zhang, M.; Bian, H.; Xu, W. Simultaneous determination of abietic acid and dehydroabietic acid residues in duck meat by HPLC-PAD-FLD. Food Anal. Methods 2014, 7, 1627–1633. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Ulyanova, O.A.; Kalacheva, G.S. Investigation of dynamics of the content of terpene compounds in pine bark-based composts and their growth-promoting activity. Russ. J. Bioorganic Chem. 2010, 1, 121–126. [Google Scholar]
- Ayars, G.H.; Altman, L.C.; Frazier, E.; Chi, E.Y. The toxicity of constituents of cedar and pine woods pulmonary epitheii. J. Allergy Clin. Immunol. 1989, 83, 610–618. [Google Scholar] [CrossRef]
- Suh, S.J.; Kwak, C.H.; Chung, T.W.; Park, S.J.; Cheeeei, M.; Park, S.S.; Seo, C.S.; Son, J.K.; Chang, Y.C.; Park, Y.G.; et al. Pimaric acid from Aralia cordata has an inhibitory effect on TNF-α-induced MMP-9 production and HASMC migration via down-regulated NF-κB and AP-1. Chem. Biol. Interact. 2012, 199, 112–119. [Google Scholar] [CrossRef]
- Kang, M.-S.; Hirai, S.; Goto, T.; Kuroyanagi, K.; Kim, Y.-I.; Ohyama, K.; Uemura, T.; Lee, J.-Y.; Sakamoto, T.; Ezaki, Y.; et al. Dehydroabietic acid, a diterpene, improves diabetes and hyperlipidemia in obese diabetic KK-Ay mice. BioFactors 2009, 35, 442–448. [Google Scholar] [CrossRef]
- Hjortness, M.K.; Riccardi, L.; Hongdusit, A.; Ruppe, A.; Zhao, M.; Kim, E.Y.; Zwart, P.H.; Sankaran, B.; Arthanari, H.; Sousa, M.C.; et al. Abietane-type diterpenoids inhibit protein tyrosine phosphatases by stabilizing an inactive enzyme conformation. Biochemistry 2018, 57, 5886–5896. [Google Scholar] [CrossRef]
- Huang, X.C.; Wang, M.; Pan, Y.M.; Yao, G.Y.; Wang, H.S.; Tian, X.Y.; Qin, J.K.; Zhang, Y. Synthesis and antitumor activities of novel thiourea α-aminophosphonates from dehydroabietic acid. Eur. J. Med. Chem. 2013, 69, 508–520. [Google Scholar] [CrossRef]
- Thummuri, D.; Guntuku, L.; Challa, V.S.; Ramavat, R.N.; Naidu, V.G.M. Abietic acid attenuates RANKL induced osteoclastogenesis and inflammation associated osteolysis by inhibiting the NF-KB and MAPK signaling. J. Cell. Physiol. 2018, 234, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Wang, S.X.; Cheng, Y.X. Abietane diterpenoids with potent cytotoxic activities from the resins of Populus euphratica. Nat. Prod. Commun. 2019, 14, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Fallarero, A.; Skogman, M.; Kujala, J.; Rajaratnam, M.; Moreira, V.M.; Yli-Kauhaluoma, J.; Vuorela, P. (+)-Dehydroabietic acid, an abietane-type diterpene, inhibits Staphylococcus aureus biofilms in vitro. Int. J. Mol. Sci. 2013, 14, 12054–12072. [Google Scholar] [CrossRef] [Green Version]
- Kostamo, A.; Kukkonen, J.V.K. Removal of resin acids and sterols from pulp mill effluents by activated sludge treatment. Water Res. 2003, 37, 2813–2820. [Google Scholar] [CrossRef]
- Stuthridge, T.R.; Campin, D.N.; Langdon, A.G.; Mackie, K.L.; McFarlane, P.N.; Wilkins, A.L. Treatability of bleached kraft pulp and paper mill wastewaters in a New Zealand aerated lagoon treatment system. Water Sci. Technol. 1991, 24, 309–317. [Google Scholar] [CrossRef]
- Belmonte, M.; Xavier, C.; Decap, J.; Martinez, M.; Sierra-Alvarez, R.; Vidal, G. Improved aerobic biodegradation of abietic acid in ECF bleached kraft mill effluent due to biomass adaptation. J. Hazard. Mater. 2006, 135, 256–263. [Google Scholar] [CrossRef]
- Werker, A.G.; Hall, E.R. Limitations for biological removal of resin acids from pulp mill effluent. Water Sci. Technol. 1999, 40, 281–288. [Google Scholar] [CrossRef]
- Cheremnykh, K.M.; Luchnikova, N.A.; Grishko, V.V.; Ivshina, I.B. Bioconversion of ecotoxic dehydroabietic acid using Rhodococcus actinobacteria. J. Hazard. Mater. 2018, 346, 103–112. [Google Scholar] [CrossRef]
- Bicho, P.A.; Martin, V.; Saddler, J.N. Growth, induction, and substrate-specificity of dehydroabietic acid-degrading bacteria isolated from a kraft mill effluent enrichment. Appl. Environ. Microbiol. 1995, 61, 3245–3250. [Google Scholar]
- Morgan, C.A.; Wyndham, R.C. Isolation and characterization of resin acid degrading bacteria found in effluent from a bleached kraft pulp mill. Can. J. Microbiol. 1996, 42, 423–430. [Google Scholar] [CrossRef]
- Mohn, W.W.; Wilson, A.E.; Bicho, P.; Moore, E.R. Physiological and phylogenetic diversity of bacteria growing on resin acids. Syst. Appl. Microbiol. 1999, 22, 68–78. [Google Scholar] [CrossRef]
- Yu, Z.; Stewart, G.R.; Mohn, W.W. Apparent contradiction: Psychrotolerant bacteria from hydrocarbon-contaminated Arctic tundra soils that degrade diterpenoids synthesized by trees. Appl. Environ. Microbiol. 2000, 66, 5148–5154. [Google Scholar] [CrossRef] [Green Version]
- Cheremnykh, K.M.; Grishko, V.V.; Ivshina, I.B. Bacterial degradation of ecotoxic dehydroabietic acid. Catal. Ind. 2017, 9, 331–338. [Google Scholar] [CrossRef]
- Yu, Z.; Mohn, W.W. Isolation and characterization of thermophilic bacteria capable of degrading dehydroabietic acid. Can. J. Microbiol. 1999, 45, 513–519. [Google Scholar] [CrossRef]
- Lindberg, L.E.; Holmbom, B.R.; Väisänen, O.M.; Weber, A.M.-L.; Salkinoja-Salonen, M.S. Degradation of paper mill water components in laboratory tests with pure cultures of bacteria. Biodegradation 2001, 12, 141–148. [Google Scholar] [CrossRef]
- Mohn, W.W.; Yu, Z.; Moore, E.R.B.; Muttray, A.F. Lessons learned from Sphingomonas species that degrade abietane triterpenoids. J. Ind. Microbiol. Biotechnol. 1999, 23, 374–379. [Google Scholar] [CrossRef]
- Smith, D.J.; Martin, V.J.J.; Mohn, W.W. A cytochrome P450 involved in the metabolism of abietane diterpenoids by Pseudomonas abietaniphila BKME-9. J. Bacteriol. 2004, 186, 3631–3639. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.J.; Park, J.; Tiedje, J.M.; Mohn, W.W. A large gene cluster in Burkholderia xenovorans encoding abietane diterpenoid catabolism. J. Bacteriol. 2007, 189, 6195–6204. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.J.; Patrauchan, M.A.; Florizone, C.; Eltis, L.D.; Mohn, W.W. Distinct roles for two CYP226 family cytochromes P450 in abietane diterpenoid catabolism by Burkholderia xenovorans LB400. J. Bacteriol. 2008, 190, 1575–1583. [Google Scholar] [CrossRef] [Green Version]
- Muttray, A.F.; Yu, Z.; Mohn, W.W. Population dynamics and metabolic activity of Pseudomonas abietaniphila BKME-9 within pulp mill wastewater microbial communities assayed by competitive PCR and RT-PCR. FEMS Microbiol. Ecol. 2001, 38, 21–31. [Google Scholar] [CrossRef]
- Burnes, T.A.; Blanchette, R.A.; Farrell, R.L. Bacterial biodegradation of extractives and patterns of bordered pit membrane attack in pine wood. Appl. Environ. Microbiol. 2000, 66, 5201–5205. [Google Scholar] [CrossRef] [Green Version]
- Kallioinen, A.; Vaari, A.; Rättö, M.; Konn, J.; Siika-Aho, M.; Viikari, L. Effects of bacterial treatments on wood extractives. J. Biotechnol. 2003, 103, 67–76. [Google Scholar] [CrossRef]
- Cámara, B.; Strömpl, C.; Verbarg, S.; Spröer, C.; Pieper, D.H.; Tindall, B.J. Pseudomonas reinekei sp. nov., Pseudomonas moorei sp. nov. and Pseudomonas mohnii sp. nov., novel species capable of degrading chlorosalicylates or isopimaric acid. Int. J. Syst. Evol. Microbiol. 2007, 57, 923–931. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.E.; Moore, E.R.; Mohn, W.W. Isolation and characterization of isopimaric acid-degrading bacteria from a sequencing batch reactor. Appl. Environ. Microbiol. 1996, 62, 3146–3151. [Google Scholar]
- Mohn, W.W. Bacteria obtained from a sequencing batch reactor that are capable of growth on dehydroabietic acid. Appl. Environ. Microbiol. 1995, 61, 2145–2150. [Google Scholar]
- Côté, R.; Otis, C. Étude de la biodégradation de l’acide dehydroabietique par Bacillus psychrophilus. Rev. Des Sci. L’eau 1989, 2, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Martin, V.J.J.; Mohn, W.W. A novel aromatic-ring-hydroxylating dioxygenase from the diterpenoid-degrading bacterium Pseudomonas abietaniphila BKME-9. J. Bacteriol. 1999, 181, 2675–2682. [Google Scholar]
- Martin, V.J.J.; Mohn, W.W. Genetic investigation of the catabolic pathway for degradation of abietane diterpenoids by Pseudomonas abietaniphila BKME-9. J. Bacteriol. 2000, 182, 3784–3793. [Google Scholar] [CrossRef] [Green Version]
- Morgan, C.A.; Wyndham, R.C. Characterization of tdt genes for the degradation of tricyclic diterpenes by Pseudomonas diterpeniphila A19-6a. Can. J. Microbiol. 2002, 48, 49–59. [Google Scholar] [CrossRef]
- Eaton, R.W.; Chapman, P.J. Bacterial metabolism of naphthalene: Construction and use of recombinant bacteria to stody ring cleavage os 1,2-dihydroxynaphthalene and subsequent reactions. J. Bacteriol. 1992, 174, 7542–7554. [Google Scholar] [CrossRef] [Green Version]
- Tavendale, M.H.; McFarlane, P.N.; Mackie, K.L.; Wilkins, A.L.; Langdon, A.G. The fate of resin acids-1. The biotransformation and degradation of deuterium labelled dehydroabietic acid in anaerobic sediments. Chemosphere 1997, 35, 2137–2151. [Google Scholar] [CrossRef]
- Tavendale, M.H.; McFarlane, P.N.; Mackie, K.L.; Wilkins, A.L.; Langdon, A.G. The fate of resin acids-2. The fate of resin acids and resin acid derived neutral compounds in anaerobic sediments. Chemosphere 1997, 35, 2153–2166. [Google Scholar] [CrossRef]
- Meyer, T.; Yang, M.I.; Tran, H.N.; Allen, D.G.; Edwards, E.A. Impact of resin and fatty acids on full-scale anaerobic treatment of pulp and paper mill effluents. Environ. Eng. Sci. 2016, 33, 394–403. [Google Scholar] [CrossRef]
- Aguirre, M.; Vuorenmaa, J.; Valkonen, E.; Kettunen, H.; Callens, C.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F.; Goossens, E. In-feed resin acids reduce matrix metalloproteinase activity in the ileal mucosa of healthy broilers without inducing major effects on the gut microbiota. Vet. Res. 2019, 50, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Savluchinske-Feio, S.; Curto, M.J.M.; Gigante, B.; Roseiro, J.C. Antimicrobial activity of resin acid derivatives. Appl. Microbiol. Biotechnol. 2006, 72, 430–436. [Google Scholar] [CrossRef]
- Savluchinske-Feio, S.; Nunes, L.; Tavares, P.; Silva, A.M.; Roseiro, J.C.; Gigante, B.; João, M.; Curto, M. Activity of dehydroabietic acid derivatives against wood contaminant fungi. J. Microbiol. Methods 2007, 70, 465–470. [Google Scholar] [CrossRef]
- Tolmacheva, I.A.; Tarantin, A.V.; Boteva, A.A.; Anikina, L.V.; Vikharev, Y.; Grishko, V.V.; Tolstikov, A.G. Synthesis and biological activity of nitrogen-containing derivatives of methyl dehydroabietate. Pharm. Chem. J. 2006, 40, 489–493. [Google Scholar] [CrossRef]
- Xu, D.D.; Yan, Y.; Jiang, C.X.; Liang, J.J.; Li, H.F.; Wu, Q.X.; Zhu, Y. Sesquiterpenes and diterpenes with cytotoxic activities from the aerial parts of Carpesium humile. Fitoterapia 2018, 128, 50–56. [Google Scholar] [CrossRef]
- Lee, T.K.; Park, J.Y.; Yu, J.S.; Jang, T.S.; Oh, S.T.; Pang, C.; Ko, Y.J.; Kang, K.S.; Kim, K.H. 7α,15-Dihydroxydehydroabietic acid from Pinus koraiensis inhibits the promotion of angiogenesis through downregulation of VEGF, p-Akt and p-ERK in HUVECs. Bioorganic Med. Chem. Lett. 2018, 28, 1084–1089. [Google Scholar] [CrossRef]
- González, M. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 684–704. [Google Scholar] [CrossRef]
- Parimal, K.; Khale, A.; Pramod, K. Resins from herbal origin and a focus on their applications. Int. J. Pharm. Sci. Res. 2011, 2, 1077–1085. [Google Scholar]
- Yadav, B.K.; Gidwani, B.; Vyas, A. Rosin: Recent advances and potential applications in novel drug delivery system. J. Bioact. Compat. Polym. 2015, 1–16. [Google Scholar] [CrossRef]
- Gonzalez, M.A. Aromatic abietane diterpenoids: Total syntheses and synthetic studies. Tetrahedron 2015, 71, 1883–1908. [Google Scholar] [CrossRef]
- Kugler, S.; Ossowicz, P.; Malarczyk-Matusiak, K.; Wierzbicka, E. Advances in rosin-based chemicals: The latest recipes, applications and future trends. Molecules 2019, 24, 1651. [Google Scholar] [CrossRef] [Green Version]
- Loughlin, W.A. Biotransformations in organic synthesis. Bioresour. Technol. 2000, 74, 49–62. [Google Scholar] [CrossRef]
- Huang, F.X.; Yang, W.Z.; Ye, F.; Tian, J.Y.; Hu, H.B.; Feng, L.M.; Guo, D.A.; Ye, M. Microbial transformation of ursolic acid by Syncephalastrum racemosum (Cohn) Schroter AS 3.264. Phytochemistry 2012, 82, 56–60. [Google Scholar] [CrossRef]
- Gouiric, S.C.; Feresin, G.E.; Tapia, A.A.; Rossomando, P.C.; Schmeda-Hirschmann, G.; Bustos, D.A. 1b,7b-Dihydroxydehydroabietic acid, a new biotransformation product of dehydroabietic acid by Aspergillus niger. World J. Microbiol. Biotechol. 2004, 20, 281–284. [Google Scholar] [CrossRef]
- Özşen, Ö.; Kıran, İ.; Dağ, İ.; Atlı, Ö.; Çiftçi, G.A.; Demirci, F. Biotransformation of abietic acid by fungi and biological evaluation of its metabolites. Process Biochem. 2017, 52, 130–140. [Google Scholar] [CrossRef]
- Tapia, A.A.; Vallejo, M.D.; Gouiric, S.C.; Feresin, G.E.; Rossomando, P.C.; Bustos, D.A. Hydroxylation of dehydroabietic acid by Fusarium species. Phytochemistry 1997, 46, 131–133. [Google Scholar] [CrossRef]
- Van Beek, T.A.; Claassen, F.W.; Dorado, J.; Godejohann, M.; Sierra-Alvarez, R.; Wijnberg, J.B.P.A. Fungal biotransformation products of dehydroabietic acid. J. Nat. Prod. 2007, 70, 154–159. [Google Scholar] [CrossRef]
- Yang, N.-Y.; Liu, L.; Tao, W.-W.; Duan, J.-A.; Tian, L.-J. Phytochemistry diterpenoids from Pinus massoniana resin and their cytotoxicity against A431 and A549 cells. Phytochemistry 2010, 71, 1528–1533. [Google Scholar] [CrossRef]
- Witzig, R.; Aly, H.A.H.; Strömpl, C.; Wray, V.; Junca, H.; Pieper, D.H. Molecular detection and diversity of novel diterpenoid dioxygenase DitA1 genes from proteobacterial strains and soil samples. Environ. Microbiol. 2007, 9, 1202–1218. [Google Scholar] [CrossRef]
- Bleif, S.; Hannemann, F.; Lisurek, M.; von Kries, J.P.; Zapp, J.; Dietzen, M.; Antes, I.; Bernhardt, R. Identification of CYP106A2 as a regioselective allylic bacterial diterpene hydroxylase. ChemBioChem 2011, 12, 576–582. [Google Scholar] [CrossRef]
- Janocha, S.; Zapp, J.; Hutter, M.; Kleser, M.; Bohlmann, J.; Bernhardt, R. Resin acid conversion with CYP105A1: An enzyme with potential for the production of pharmaceutically relevant diterpenoids. ChemBioChem 2013, 14, 467–473. [Google Scholar] [CrossRef]
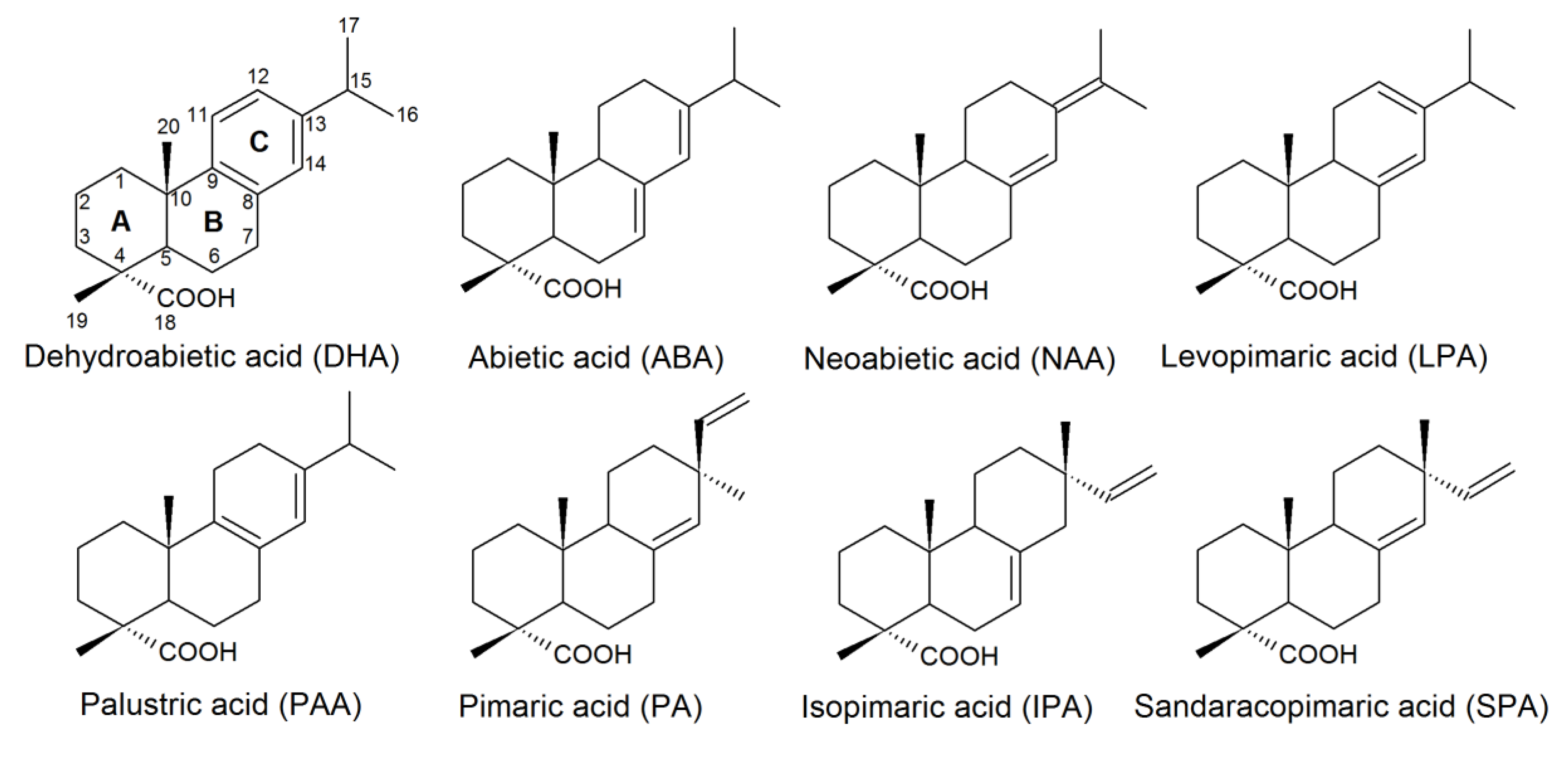
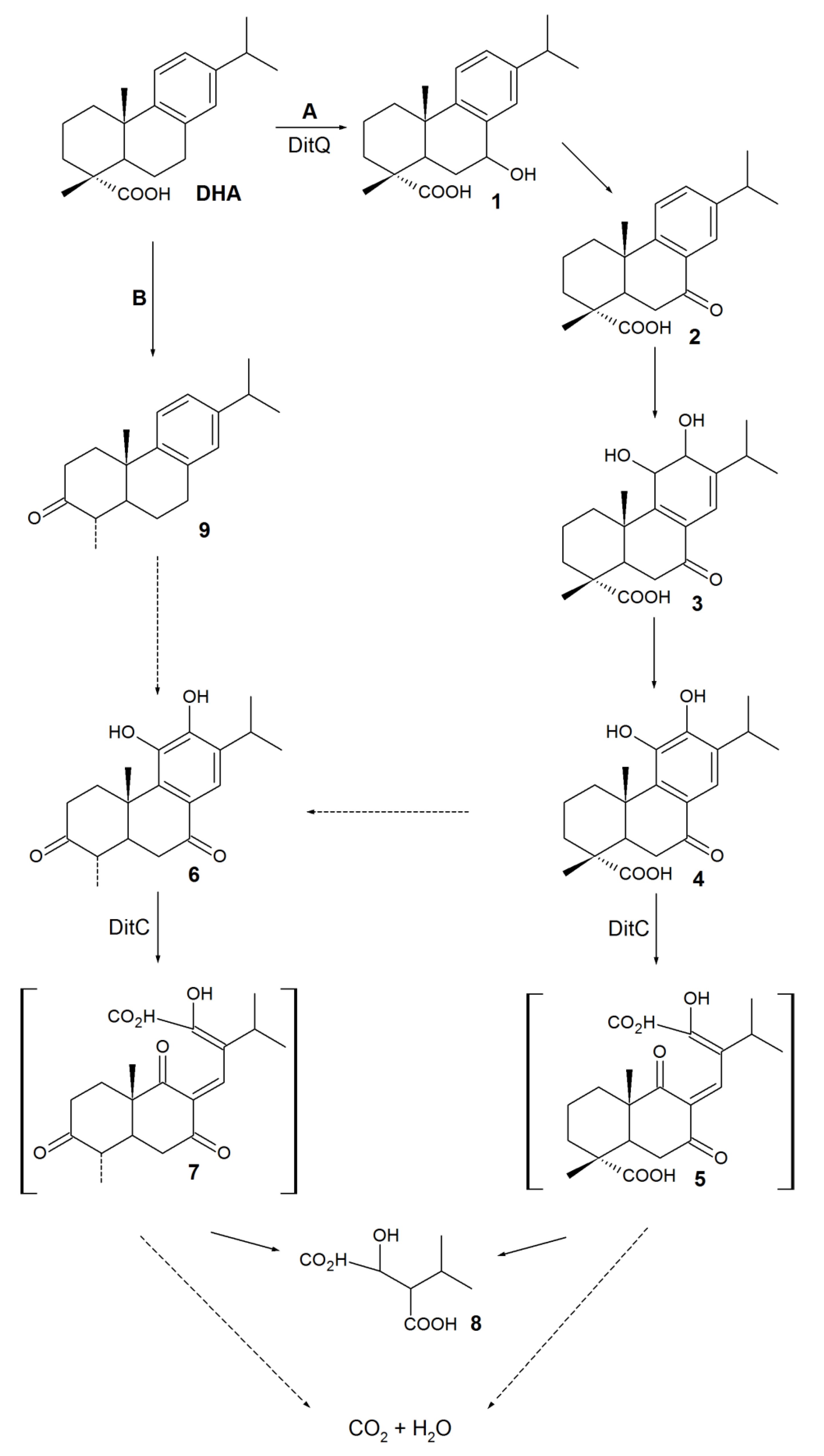

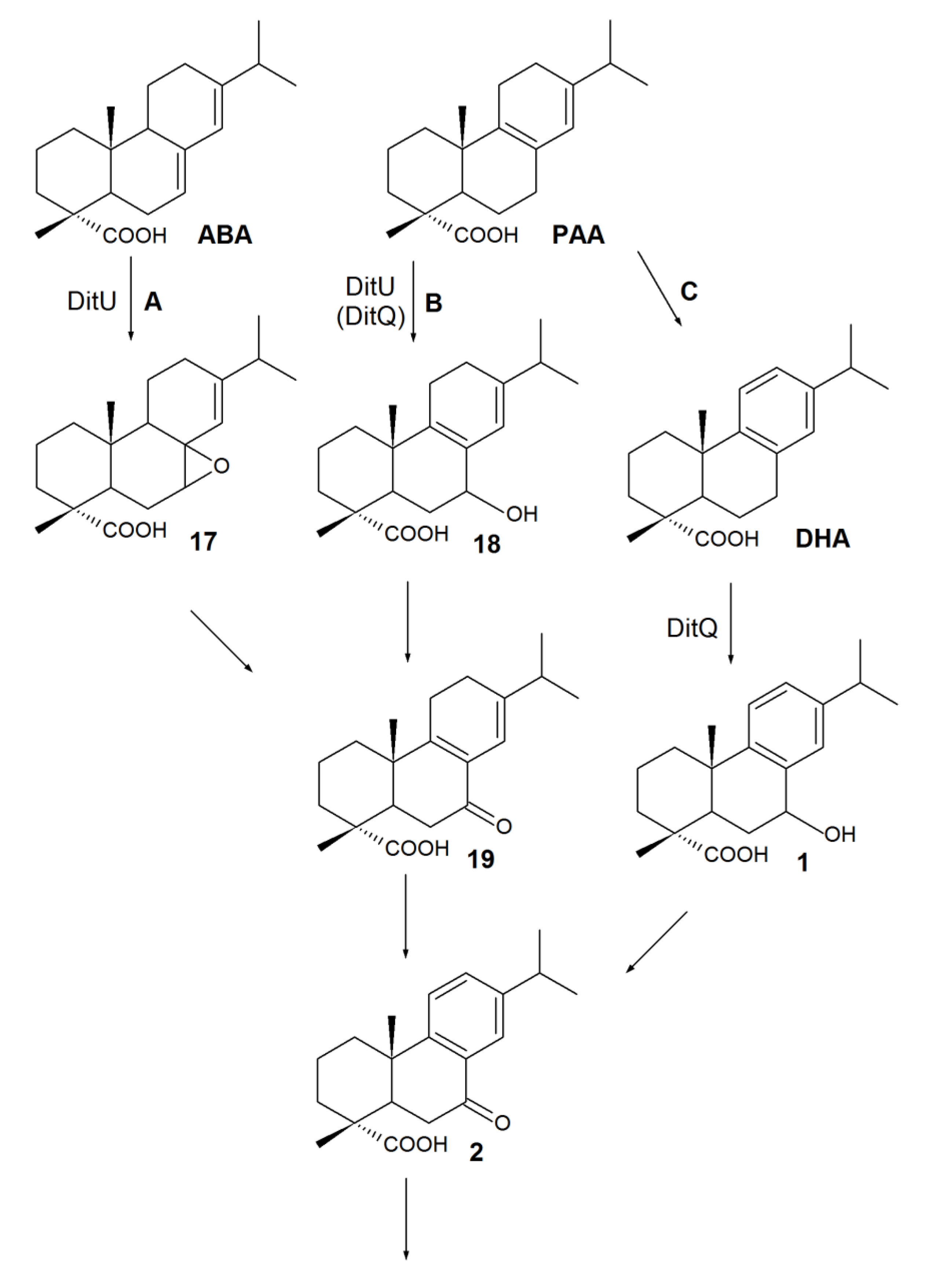
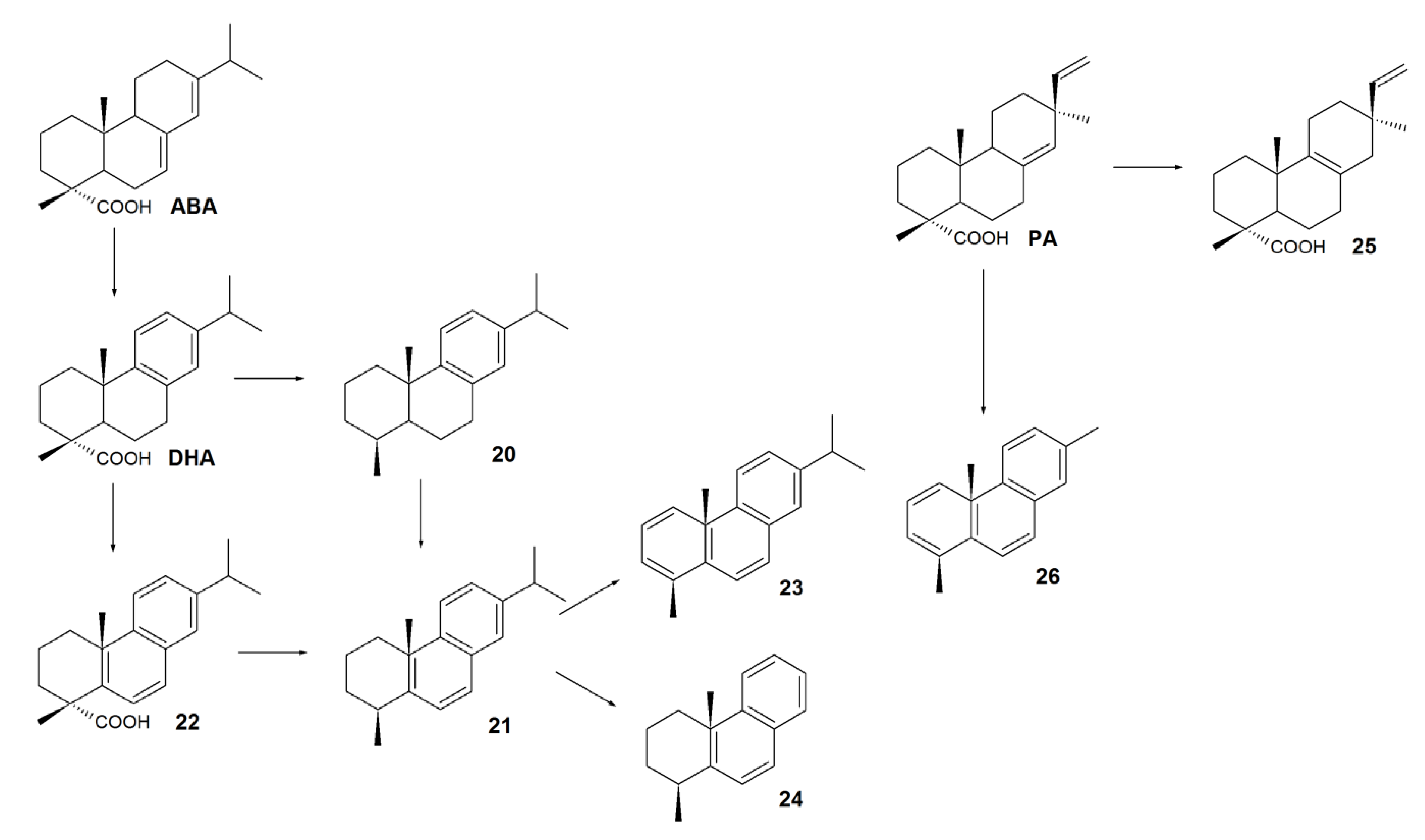
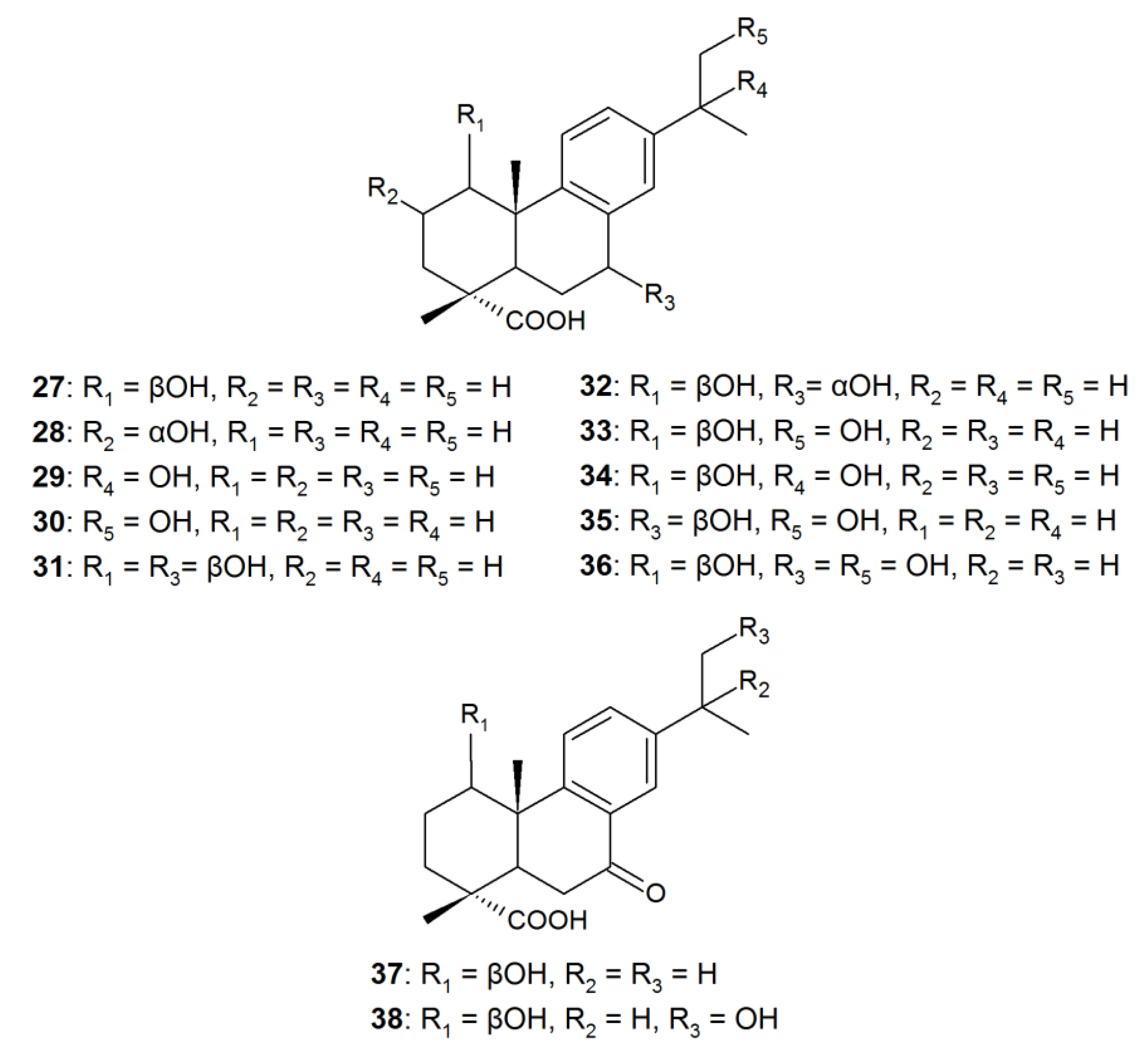
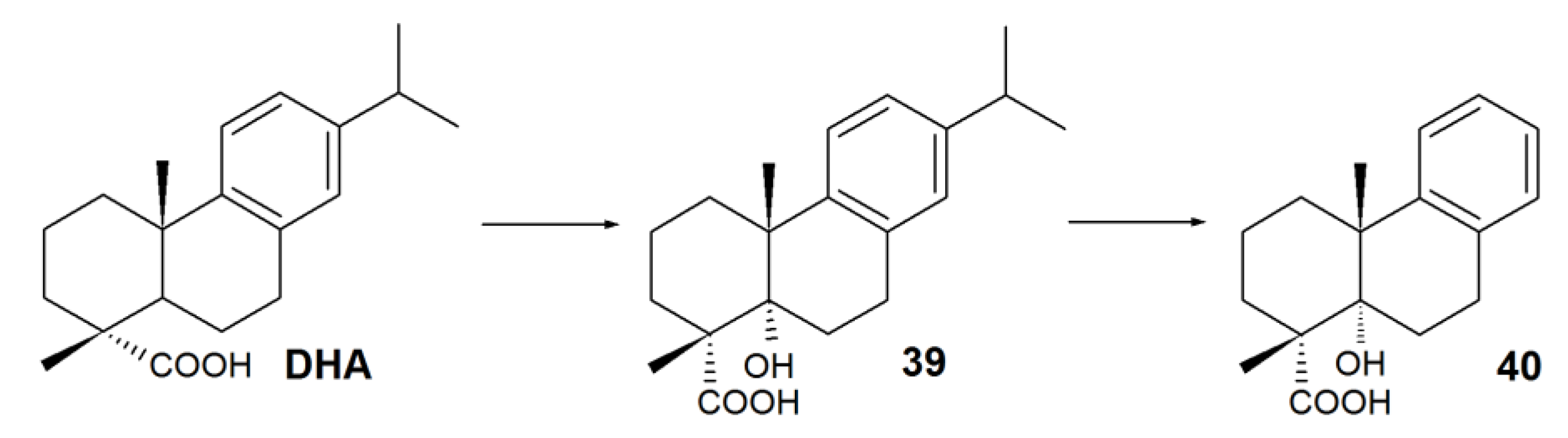
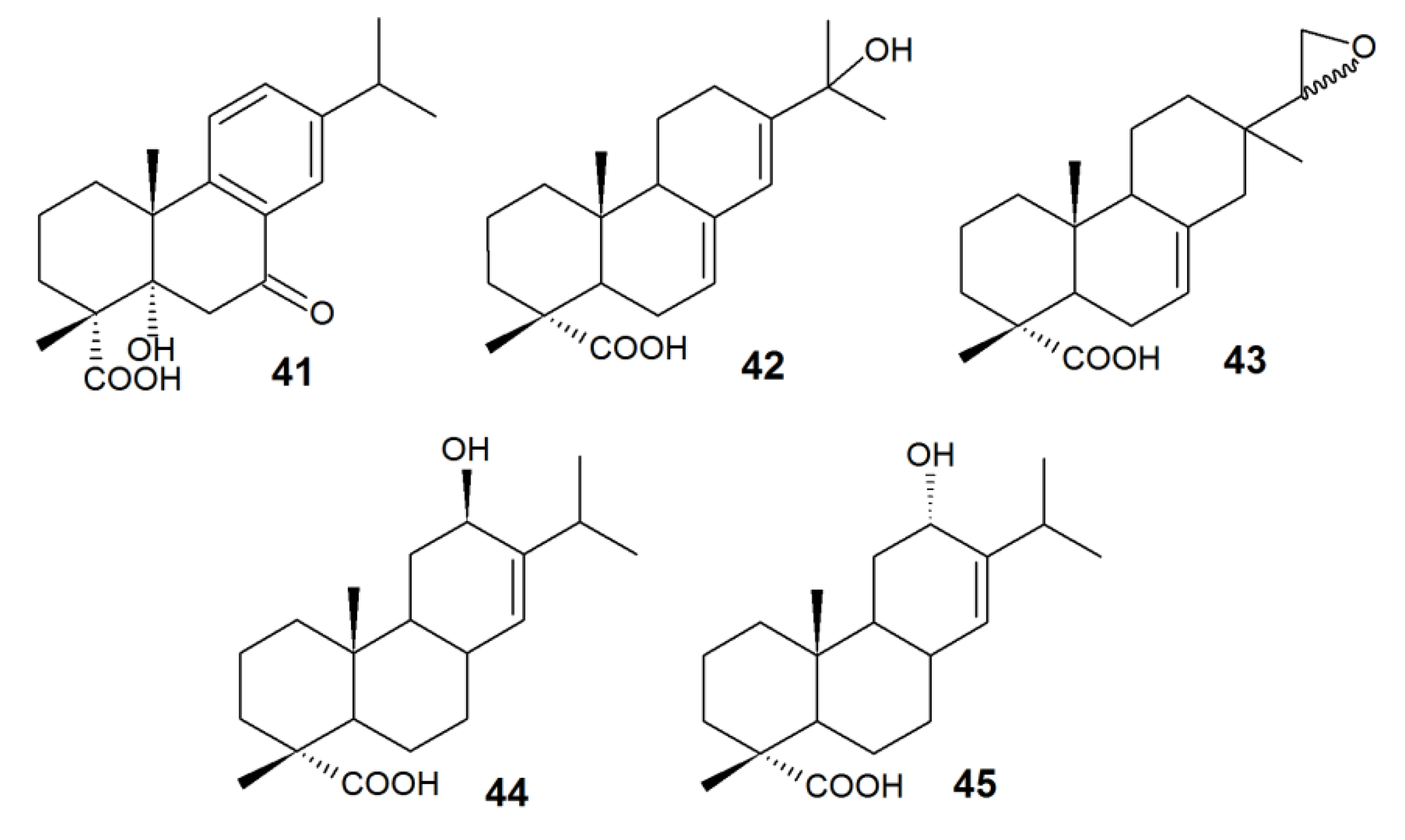
| Acid | Scots Pine P. silvestris | Ordinary Spruce P. excelsa | Maritime Pine P. pinaster |
|---|---|---|---|
| Abietic | 7.86 | 13.95 | 16.10 |
| Dehydroabietic | 64.58 | 50.08 | 23.50 |
| Pimaric | 10.86 | 7.57 | 10.80 |
| Isopimaric | 8.26 | 18.83 | 6.90 |
| Unidentified | 8.43 | 9.55 | – |
| RA | Solubility, mg/L | Acute Toxicity (LD50), mg/L | ||||
|---|---|---|---|---|---|---|
| Daphnia Daphnia magna, 48 h | Rainbow Trout Oncorhynchus mykiss, 96 h | Red Salmon O. nerka, 96 h | Silver Salmon O. kisutch, 96 h | Fathead Minnow Pimephales promelas, 96 h | ||
| DHA | 5.11 | 1.28–6.35 | 0.77–1.32 | 0.50–2.10 | 0.75–1.85 | 2.10–3.20 |
| ABA | 2.75 | 0.68 | 0.72–1.53 | 0.20 | 0.40 | 2.38 |
| LPA | 2.54 | 0.50 | 0.61–1.00 | – | – | – |
| NAA | 2.31 | 0.35 | 0.63–0.71 | – | – | 1.30–1.70 |
| PA | 2.17 | 0.26 | 0.74–1.23 | – | 0.32 | – |
| SPA | 1.82 | 0.13 | – | – | 0.36 | – |
| IPA | 1.70 | 0.07 | 0.40–1.00 | 0.70 | 0.20 | – |
| Study Object | Concentration | Conditions | Reference |
|---|---|---|---|
| Fine flounder Paralychthys adspersus Small-eyes flounder P. microps | |||
| Bile | 30.5–41.9 µg/g, total RA content | Caught near the PPM effluent discharge site | [4] |
| Rainbow trout O. mykiss | |||
| Bile | <200 µg/g DHA | After 57 days of exposure to PPM effluents | [32] |
| Blood plasma | 155–318 µg/g DHA | After 4 days of exposure to DHA (1.2 mg/L) in water | [31] |
| Liver | 98–103 µg/g DHA | After 4 days of exposure to DHA (1.2 mg/L) | |
| 202–351 µg/g, total RA content | After 2 days of exposure to a mixture of RAs (1.4 mg/L) in water | ||
| Kidney | 47–114 µg/g DHA | After 4 days of exposure to DHA (1.2 mg/L) | |
| 72–115 µg/g, total RA content | After 2 days of exposure to a mixture of RAs (1.4 mg/L) in water | ||
| Strain | Substrate | Reference |
|---|---|---|
| Gram-negative | ||
| Alcaligenes sp. D11-13 | DHA | [53] |
| Betaproteobacterium sp. DhA-71, DhA-73 | DHA | [57] |
| Burkholderia cepacia F45L5 | DHA, ABA, IPA | [58] |
| Burkholderia sp. DhA-54 | DHA | [59] |
| Burkholderia sp. IpA-51 | IPA | [59] |
| B. xenovorans LB400 | DHA, ABA, PA | [60,61,62] |
| Pseudomonas abietaniphila BKME-9 | DHA, ABA | [52,63] |
| P. fluorescens NRRL B21432 | Mixture of RAs | [64] |
| P. marginalis E-001624 | Mixture of RAs | [65] |
| P. mohnii IpA-2T, P. moorei RW10T | IPA | [66] |
| “Pseudomonas multiresinivorans” * (P. nitroducent) IpA-1 * | IPA | [67] |
| P. reinekei Mt1 | IPA | [66] |
| Pseudomonas sp. A19-6a | ABA | [53] |
| Pseudomonas sp. DhA-92 | DHA | [55] |
| Pseudomonas sp. IpA-2 | IPA | [67] |
| Pseudomonas sp. IpA-93, IpA-95 | IPA | [55] |
| P. vancouverensis Dha-51 | DHA | [59] |
| Ralstonia sp. BKME-6 | DHA | [52] |
| Serratia marcescens NRRL B21429 | Mixture of RAs | [64] |
| Sphingomonas sp. DhA-33 | DHA | [54,68] |
| Sphingomonas sp. DhA-95 | DHA | [55] |
| Xanthomonas campestris NRRL B21430 | Mixture of RAs | [64] |
| Zoogloea ramigera DhA-35 | DHA | [68] |
| Gram-positive | ||
| Bacillus psychrophilus | DHA | [69] |
| Dietzia maris IEGM 55T | DHA | [56] |
| Gordonia rubripertincta IEGM 104, IEGM 105, IEGM 109 | DHA | [51] |
| G. terrae IEGM 150 | DHA | [51] |
| Mycobacterium sp. DhA-55 | DHA | [54] |
| Mycobacterium sp. IpA-13 | IPA | [67] |
| Rhodococcus erythropolis IEGM 267 | DHA | [51] |
| R. rhodochrous IEGM 107 | DHA | [51] |
| R. ruber IEGM 80 | DHA | [51] |
| Compound | Biological Activity | Biocatalyst | Reference |
|---|---|---|---|
| 1β-hydroxy-DHA (27) | Antimicrobial, inhibitory activity against α-glucosidase | Aspergillus niger, Cephalosporium aphidicola, Cunninghamella elegans, Fusarium moniliforme, F. oxysporum, Gibberella fujikuroi, Neurospora crassa, Phlebiopsis gigantea, Rhizopus stolonifera | [22,90,91,92,93] |
| 2α-hydroxy-DHA (28) | Antimicrobial, selective antitumor | Mucor ramannianus | [91] |
| 7β-hydroxy-DHA (1) | Antimicrobial, fungicidal, antitumor | A. niger, N. crassa | [78,79,90,91,94] |
| 15-hydroxy-DHA (29) | Anti-inflammatory. An intermediate of antiviral and antitumor agent synthesis | C. aphidicola, C. elegans, G. fujkuroi, R. stolonifera | [22,83] |
| 16-hydroxy-DHA (30) | Antimicrobial | C. aphidicola, C. elegans, G. fujkuroi, R. stolonifera | [22] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luchnikova, N.A.; Ivanova, K.M.; Tarasova, E.V.; Grishko, V.V.; Ivshina, I.B. Microbial Conversion of Toxic Resin Acids. Molecules 2019, 24, 4121. https://doi.org/10.3390/molecules24224121
Luchnikova NA, Ivanova KM, Tarasova EV, Grishko VV, Ivshina IB. Microbial Conversion of Toxic Resin Acids. Molecules. 2019; 24(22):4121. https://doi.org/10.3390/molecules24224121
Chicago/Turabian StyleLuchnikova, Natalia A., Kseniya M. Ivanova, Ekaterina V. Tarasova, Victoria V. Grishko, and Irina B. Ivshina. 2019. "Microbial Conversion of Toxic Resin Acids" Molecules 24, no. 22: 4121. https://doi.org/10.3390/molecules24224121
APA StyleLuchnikova, N. A., Ivanova, K. M., Tarasova, E. V., Grishko, V. V., & Ivshina, I. B. (2019). Microbial Conversion of Toxic Resin Acids. Molecules, 24(22), 4121. https://doi.org/10.3390/molecules24224121







