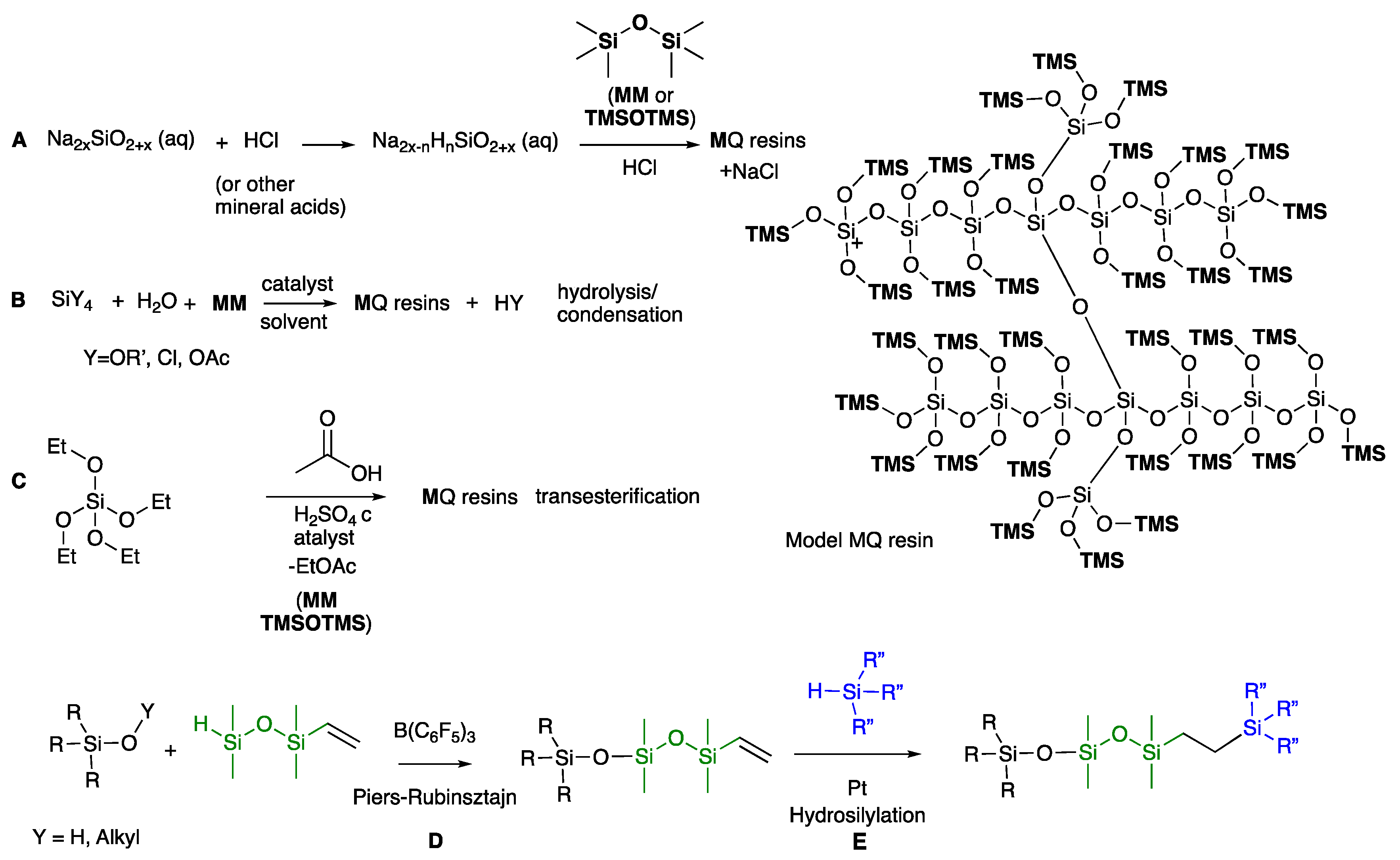
4.2. Characterization Methods
1H NMR and 13C NMR experiments were recorded at room temperature and performed on a Bruker Avance 600 MHz nuclear magnetic resonance spectrometer. Note: in the 1H NMR of compounds formed by hydrosilylation, only the data for the major, terminal hydrosilylation isomer (SiCH2CH2Si) are reported, which comprises >90% of the product. The minor peaks from the other isomer (SiMeCHSi) were only identifiable in low generation molecules.
High-resolution mass Spectrometry was performed with a Hi-Res Waters/Micromass Quattro Global Ultima (Q-TOF mass spectrometer (MS)). MALDI mass spec was collected on a Bruker Autoflex III at McGill University by Dr Nadim Saade with dithranol as the matrix used for most of the samples. ESI-MS Instrument: Exactive Plus Orbitrap (Thermo Scientific, Waltham, Massachusetts). Sample concentration used were~50–100 μM.
GPC data was collected on a Viscotek GPC Max (VE 2001 GPC Solvent/Sample Module) using a Viscotek VE 3580 RI Detector and a Viscotek 270 Dual Detector using a PolyAnalytik SupeRes PAS-101 (8 mm × 30 cm) column with a hard styrene-divinylbenzene gel; single pore, 6nm particle size, a plate count >18,000, and an exclusion limit of 1.5K. The samples were run in toluene. Polystyrene was used as standard for GPC calibration.
Young’s modulus data was collected using a MACH-1 micromechanical testing instrument equipped with a hemispherical indenter (diameter:0.5 mm). The results were analyzed by the add-on software Mach-1 Analysis. The analysis model (Elastic model in indention) for finding the Young’s modulus from an indentation test was provided by the vender (Biomomentum), which was developed based on the mathematical model established by Hayes et al. [
25]. The elastomers in 12-well plate (thickness: 5 mm) were measured in triplicate, with error bars representing the standard deviation of the replicate measurements.
The adhesion strength of elastomeric materials was measured using a MACH-1 micromechanical testing instrument equipped with a punch (diameter:13mm) following the method and optimized conditions described in the literature [
26,
27]. Briefly, the elastomers, formed in a 12-well plate, were mounted onto the stage of the instrument. The steel punch moved down to come in contact with the elastomer to be tested. When the contact stress reached 1.5 N, the punch was programed to hold at that position for 3 min to establish sufficient contact. The probe was then pulled away at a de-bonding velocity of 1 mm s
−1. The displacement of the punch and the force applied on it was collected by the instrument. The results are shown in
Table S3.
To normalize the influence of Young’s modulus on the tack strength, work of adhesion was calculated with Young’s modulus and tack strength using the following equation (
Table 1,
Table S4).
where W (J/m
2) is the work of adhesion; a is the radius of the punch (6.5 × 10
−3 m); P is the tack strength measured (Pa); E is the Young’s modulus of the samples. Viscosity measurements were carried out on a TA Instruments Discovery HR-2 Hybrid Rheometer with a 40 mm steel Peltier plate. The shear rate was set between 0 and 500 s
−1 (
Table S2).
4.3. Synthesis of All Compounds
4.3.1. Synthesis of 1
Allyltrimethoxysilane (5.0328 g, 31.017 mmol) was added to a 500 mL oven-dried round-bottomed flask. Dry hexanes (~10 mL) were added to the reaction and the flask was capped and flushed with nitrogen. BCF catalyst solution (110 μL, 0.0365 g/mL toluene, 7.84 × 10−3 mmol) was added before slowly adding in excess pentamethyldisiloxane (22.7582 g, 153.401 mmol). After 2 h the reaction was shown to be complete by 1H NMR and ~1.5 g of neutral alumina was added to the flask to remove the BCF. The solution was left to stir for ~1 h before filtering the product through Celite and rinsing with hexane. Hexanes were removed under reduced pressure, yielding 14.6443 g of 1 (85% yield). 1H NMR (CDCl3, 600 MHz): δ 6.13 (dd, 1H, J = 14.8, 20.4 Hz), 5.93 (dd, 1H, J = 3.8, 14.8 Hz), 5.74 (dd, 1H, J = 3.8, 20.4 Hz), 1.57 (d, 2H, J = 7.9 Hz), 0.19–0.05 (m, 46H) ppm
4.3.2. Synthesis of 2
Allyltrimethoxysilane (5.0087 g, 30.868 mmol) was added to a 500 mL oven-dried round-bottomed flask. Dry hexanes (~10 mL) were added to the reaction and the flask was capped and flushed with nitrogen. BCF catalyst solution (600 μL, 0.0400 g/mL toluene, 4.68 × 10−2 mmol) was added before slowly adding excess bis(trimethylsiloxy)methylsilane (34.4652 g, 154.900 mmol). The reaction was kept stirring for 3 h, and then neutral alumina (~1 g) was added to the flask. The solution was left to stir for ~1 h before filtering the product through Celite and rinsing with hexane. Hexanes were removed under reduced pressure. Excess bis(trimethylsiloxy)methylsilane was removed by distillation at 120 °C for ~45 min yielding 19.4302 g of 2 (81% yield). 1H NMR (CDCl3, 600 MHz): δ 6.13 (dd, 1H, J = 14.8, 20.4 Hz), 5.93 (dd, 1H, J = 3.8, 14.8 Hz), 5.74 (dd, 1H, J = 3.8, 20.4 Hz), 1.57 (d, 2H, J = 7.9 Hz), 0.20–0.02 (m, 70H) ppm.
4.3.3. Synthesis of 3
In an oven-dried round bottomed flask, 1 (5 g, 8.94 mmol) was added to a solution of tetramethyldisiloxane (6 g, 44.7 mmol) in 15 mL of dry hexane. A drop of platinum (0) 1,3-divinyl-1,1,3,3-tetramethyldisiloxane catalyst (10 μl of a 2% by wt in xylenes solution, 2.24 × 10−2 mmol) was added. The solution was treated with activated charcoal (∼1 g) and the mixture allowed to stir for 1 h. The solution was then filtered and concentrated in vacuo. The residue was then heated to 100 °C under high vacuum (1 mmHg) to ensure complete removal of excess starting materials, yielding colorless liquid 3 (4.95 g, 80% yield). 1H NMR (CDCl3, 600 MHz): δ 4.70 (m, 1H,) 1.45 (m, 2H), 0.61 (m, 4H), 0.21–0.02 (m, 57H) ppm. High resolution mass spectrometry (ES (electrospray) Positive mode): m/z [M + NH4]+ calc. = 710.2914; found = 710.2909.
4.3.4. Synthesis of 4
In an oven-dried round bottom flask, 2 (10 g, 12.7 mmol) was added to a solution of tetramethyldisiloxane (8.59 g, 63.9 mmol) in 20 mL of dry hexane. A drop of platinum (0) 1,3-divinyl-1,1,3,3-tetramethyldisiloxane catalyst (10 μl of a 2 wt% in xylenes solution, 2.24 × 10−2 mmol) was added. The solution was stirred at room temperature under a nitrogen atmosphere for 3 h. The solution was treated with activated charcoal (∼1 g) and the mixture allowed to stir for 1 h. The solution was then filtered and concentrated in vacuo. The residue was then heated to 100 °C under high vacuum (1 mmHg) to ensure complete removal of excess starting materials, yielding colorless liquid 4 (10.35 g, 89% yield). 1H NMR (CDCl3, 600MHz): δ 4.60 (m, 1H,) 1.45 (m, 2H), 0.63 (m, 4H), 0.17–0.06 (m, 75H) ppm. High resolution mass spectrometry High resolution mass spectrometry (ES Positive mode): m/z [M]+ calc. = 915.3251; found = 915.3218.
4.3.5. Synthesis of 5
Allyltrimethoxysilane (0.622 g, 3.83 mmol) was added to a 500 mL oven-dried round-bottomed flask. Dry hexanes (~10 mL) were added to the reaction and the flask was capped and flushed with nitrogen. BCF catalyst solution (140 μL, 0.025 g/mL toluene, 6.83 × 10−3 mmol) was added before slowly adding 3 (8 g, 11.5 mmol). After 2 h the reaction was shown to be complete by 1H NMR and ~1.5 g of neutral alumina was added to the flask to remove the BCF. The solution was left to stir for ~1 h before filtering the product through Celite and rinsing with hexane. Hexanes were removed under reduced pressure. The residue was subjected to kugelrohr distillation at 170 °C to remove impurities, yielding 7.09 g of 5 (84% yield). 1H NMR (CDCl3, 600 MHz): δ 5.78 (m, 1H), 4.94–4.90 (m, 1H), 4.88–4.85 (m, 1H), 1.54–1.52 (m, 2H), 1.41–1.39 (m, 6H), 0.64–0.57 (m, 12H), 0.10–0.04 (m, 171H) ppm. High resolution mass spectrometry (ES Positive mode): m/z [M + Na]+ calc. = 2213.7391; found = 2213.7465.
4.3.6. Synthesis of 6
Allyltrimethoxysilane (0.177 g, 1.09 mmol) was added to a 250 mL oven-dried round-bottomed flask. Dry hexanes (~10 mL) were added to the reaction and the flask was capped and flushed with nitrogen. BCF catalyst solution (13 μL, 0.025 g/mL toluene, 0.63 × 10−3 mmol) was added before slowly adding 4 (3 g, 3.27 mmol). After 2 h the reaction was shown to be complete by 1H NMR and ~1.5 g of neutral alumina was added to the flask to remove the BCF. The solution was left to stir for ~1 h before filtering the product through Celite and rinsing with hexane. Hexanes were removed under reduced pressure. The residue was subjected to kugelrohr distillation at 170 °C to remove impurities, yielding 2.53 g of 6 (82% yield). 1H NMR (CDCl3, 600 MHz): δ 5.83–5.75 (m, 1H), 4.9–4.90 (m, 1H), 4.8–4.85 (m, 1H), 1.5–1.52 (m, 2H), 1.4–1.40 (m, 6H), 0.6–0.60 (m, 12H), 0.20–0.01 (m, 225H) ppm. High resolution mass spectrometry (ES Positive mode): m/z [M + Na]+ calc. = 2880.9161; found = 2880.9275.
4.3.7. General Procedure for Synthesis of 8–13 (Shown for 9)
In an oven-dried round bottom flask, 6 (4.836 g, 1.69 mmol) was added to a solution of tetramethyldisiloxane (0.144 g, 0.84 mmol) in 5 mL of dry hexane. A drop of platinum (0) 1,3-divinyl-1,1,3,3-tetramethyldisiloxane catalyst (10 μl of a 2 wt% in xylenes solution, 2.24 × 10−2 mmol) was added. The solution was stirred at room temperature under a nitrogen atmosphere for 3 h. The solution was treated with activated charcoal (∼1 g) and the mixture allowed to stir for 1 h. The solution was then filtered and concentrated in vacuo. The residue was subjected to Kugelrohr distillation at 170 °C to remove impurities, yielding colorless liquid 9 (4.46 g, 88% yield). 1H NMR (CDCl3, 600 MHz): δ 1.43–1.38 (m, 16H), 0.64–0.56 (m, 32H), 0.14–0.01 (m, 462H) ppm. High resolution mass spectrometry (ES Positive mode): m/z [M + Na]2+ calc. = 2943.9518; found = 2943.9576.
1H NMR and HR-MS for 8 (Yield: 95%):1H NMR (CDCl3, 600 MHz): δ 1.45–1.38 (m, 16H,), 0.65–0.55 (m, 32H), 0.11–0.01 (m, 354H) ppm. HR-MS (ES Positive mode): m/z [M + Na]+ calc. = 4538.5774; found = 4538.5563.
1H NMR and HR-MS for 10 (Yield: 94%):1H NMR (CDCl3, 600 MHz): δ 7.50–7.48 (m, 2H), 7.34–7.31 (m, 1H), 7.29–7.26 (m, 2), 1.43–1.38 (m, 24H,), 0.64–0.56 (m, 48H), 0.14–0.01 (m, 532H) ppm. High resolution mass spectrometry (ES Positive mode): m/z [M + Na]2+ calc. = 3475.1757; found = 3475.1699
1H NMR and HR-MALDI for 11 (Yield: 94%):1H NMR (CDCl3, 600 MHz): δ 7.50–7.48 (m, 2H), 7.34–7.31 (m, 1H), 7.29–7.26 (m, 2), 1.43–1.38 (m, 24H,), 0.64–0.56 (m, 48H), 0.14–0.01 (m, 694H) ppm. MALDI: m/z [M]+ calc.= 8900.853, found = 8900.311
1H NMR and HR-MALDI for 12 (Yield: 93%):1H NMR (CDCl3, 600 MHz): δ 1.42–1.35 (m, 32H), 0.63–0.53 (m, 64H), 0.08–0.01 (m, 708H) ppm. MALDI: m/z [M]+ calc.= 9091.083, found = 9091.182
1H NMR and HR-MALDI for 13 (Yield: 92%):1H NMR (CDCl3, 600 MHz): δ 1.42–1.35 (m, 32H), 0.63–0.53 (m, 64H), 0.09–0.01 (m, 924H) ppm. MALDI: m/z [M]+ calc.= 11,755.759, found = 11,755.567.
4.3.8. General Procedure for Synthesis of 14–16 (Shown for 14)
Mono-vinyl terminated PDMS
7 was prepared according to the literature procedure [
14]. The Mn of this polymer was 3165 g mol
−1 and dispersity (
ĐM) was 1.2. In an oven-dried round bottom flask, mono-vinyl-terminated PDMS (4 g, 1.27 mmol) was added to a solution of tetramethyldisiloxane (0.085g, 0.632 mmol) in 5 mL of dry hexane. The
1H NMR experiment for this reaction mixture was carried out to make sure that the stoichiometry ratio between Si-H and vinyl group was exactly 1:1. Then, a drop of platinum (0) 1,3- divinyl-1,1,3,3-tetramethyldisiloxane catalyst (10 μl of a 2% by wt in xylenes solution, 2.24 × 10
−2 mmol) was added. The solution was stirred at room temperature under a nitrogen atmosphere for 3 h. The solution was treated with activated charcoal (∼1 g) and the mixture allowed to stir for 1 h. The solution was then filtered and concentrated in vacuo. The residue was subjected to Kugelrohr distillation at 170 °C to remove impurities, yielding colorless liquid
14 (3.47 g, 85% yield).
1H NMR (CDCl
3, 600 MHz): δ 1.26–1.21 (m, 8H) 0.80 (t, 6H,
J = 6.8 Hz), 0.48 (t, 4H,
J = 6.7 Hz) 0.37–0.32 (m, 8H)) 0.09–0.04 (m, 510H) ppm. GPC: Mn: 7330, Mw: 7960, and PDI: 1.08.
1H NMR and GPC for 15 (Yield: 89%):1H NMR (CDCl3, 600 MHz): δ 7.50–7.48 (m, 2H), 7.34–7.31 (m, 1H), 7.29–7.26 (m, 2), 1.35–1.29 (m, 12H) 0.93–0.86 (m, 9H), 0.55–0.53 (m, 6H), 0.47–0.39 (m, 12H) 0.37–0.32 (m, 8H),0.09–0.04 (m, 852H). GPC: Mn: 10835, Mw: 11745, and PDI: 1.08.
1H NMR and GPC for 16 (Yield: 90%):1H NMR (CDCl3, 600 MHz): δ 1.26–1.21 (m, 16H) 0.80 (m, 12H), 0.48 (m, 8H) 0.37–0.32 (m, 16H), 0.09–0.04 (m, 1120H) ppm. GPC: Mn: 13,105, Mw: 16,250, and PDI: 1.24.
4.3.9. General Procedure for Synthesis of 17–18 (Shown for 17)
In an oven-dried round bottom flask, 5 (4 g, 1.39 mmol) was added to a solution of DMS-H11 from Gelest (0.823 g, 0.698 mmol) in 5 mL of dry hexane. The 1H NMR experiment for this reaction mixture was carried out to make sure that the stoichiometry ratio between Si-H and vinyl group was exactly 1:1. Then, a drop of platinum (0) 1,3- divinyl-1,1,3,3-tetramethyldisiloxane catalyst (10 μL of a 2 wt% in xylenes solution, 2.24 × 10−2 mmol) was added. The solution was stirred at room temperature under a nitrogen atmosphere for 3 h. The solution was treated with activated charcoal (∼1 g) and the mixture allowed to stir for 1 h. The solution was then filtered and concentrated in vacuo. The residue was subjected to kugelrohr distillation at 170 °C to remove impurities, yielding colorless liquid 17 (4.08 g, 83% yield). 1H NMR (CDCl3, 600 MHz): δ 1.42–1.35 (m, 10H), 0.63–0.53 (m, 20H), 0.09–0.01 (m, 568H) ppm. GPC: Mn:7160, Mw: 9590, PDI:1.33.
1H NMR and HR-MALDI for 18 (Yield: 90%):1H NMR (CDCl3, 600 MHz): δ 1.42–1.35 (m, 10H,), 0.63–0.53 (m, 20H), 0.09–0.01 (m, 1046H) ppm. GPC: Mn:14,000, Mw: 19,895, PDI:1.42.
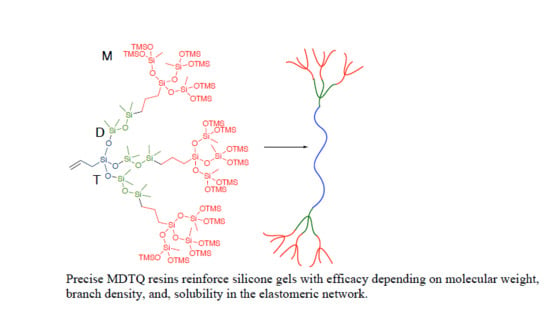
4.3.10. Preparation of MTDQ Resin/Filler Silicone Elastomers
A master batch of hydrosilylation cure base was made by mixing vinyl-terminated silicone DMS V31 (50.0 g, H
2C=CH-SiMe
2(OSiMe
2)
nOSiMe
2CH=CH
2, vinyl 4.6mmol) with crosslinker hydrosilicone crosslinker HMS 301 (Me
3Si(OSiMeH)
x(OSiMe
2)
yOSiMe
3, 0.928 g SiH 4.6mmol). A control elastomer
19 was prepared by adding diluted Karstedt’s catalyst solution (2 µL, concentration: 1 mg/ml) to 2.0 g of the batch mixture. No reinforcing agents were used. The mixture was then degassed by a vacuum desiccator, heated at 80 °C overnight. In a typical synthesis of MTDQ resin reinforced/filled silicone elastomer (10 wt% loading), the premixed base (1.8 g) and synthesized unreactive silicone resin (0.2 g) were added in a 12-well plate. Diluted Karstedt’s catalyst solution (2 µL, 20 ppm, concentration: 1 mg ml
−1) was added to the mixture, followed by rapid mixing and degassing in a vacuum desiccator. The gelation was achieved in ~10 min after mixing at room temperature. The plate was then placed in a 80 °C oven overnight to achieve complete cure. Young’s modulus data and adhesion data to be found in
Tables S3 and S4.