Synthesis and Antiproliferative Evaluation of Novel Hybrids of Dehydroabietic Acid Bearing 1,2,3-Triazole Moiety
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
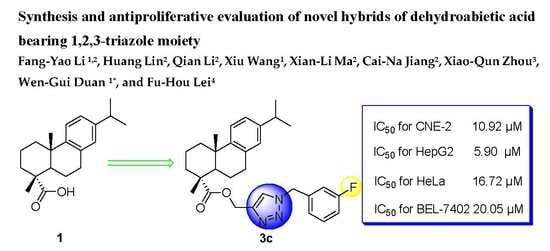
2.2. In Vitro Assay of Antiproliferative Activity
3. Experimental Section
3.1. General Information
3.2. Synthesis of Dehydroabietic Acid Propynyl Ester 2
3.3. General Procedure for the Synthesis of the Target Compounds 3a–p
3.4. In Vitro Antiproliferative Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, H.; Lei, H.M.; Ma, B.; Liu, M.; Guo, D.; Liu, X.; Hu, L.H. Synthesis and cytotoxicity evaluation of 4′-amino-4′-dehydroxyloleandrin derivatives. Fitoterapia 2016, 113, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, X.; Wang, T.F.; Xiao, J.Q. Quinolone hybrids and their anti-cancer activities: An overview. Eur. J. Med. Chem. 2019, 165, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.L.; Guan, X.H.; Ma, H.; Cong, H.; Zhang, W.N.; Miao, Z.Y. Small molecule-drug conjugates: A novel strategy for cancer-targeted treatment. Eur. J. Med. Chem. 2019, 163, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, M.; Ren, X.C.; Zhou, X.B.; Shang, Q.; Lu, W.Q.; Luo, P.; Jiang, Z.H. Synthesis and cardiomyocyte protection activity of crocetin diamide derivatives. Fitoterapia 2017, 121, 106–111. [Google Scholar] [CrossRef] [PubMed]
- González, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar]
- González, M.A. Aromatic abietane diterpenoids: Total syntheses and synthetic studies. Tetrahedron 2015, 71, 1883–1908. [Google Scholar]
- González, M.A. Synthetic derivatives of aromatic abietane diterpenoids and their biological activities. Eur. J. Med. Chem. 2014, 87, 834–842. [Google Scholar]
- Fonseca, T.; Gigante, B.; Marques, M.M. Synthesis and antiviral evaluation of benzimidazoles, quinoxalines and indoles from dehydroabietic acid. Bioorg. Med. Chem. 2004, 12, 103–112. [Google Scholar] [CrossRef]
- Kang, M.S.; Hirai, S.; Goto, T. Dehydroabietic acid, a phytochemical, acts as ligand for PPARs inmacrophages and adipocytes to regulate inflammation. Biochem. Biophys. Res. Commun. 2008, 369, 333–338. [Google Scholar] [CrossRef]
- Helfenstein, A.; Vahermo, M.; Nawrot, D.A.; Demirci, F.; Iscan, G.; Krogerus, S.; Yli-Kauhaluoma, J.; Moreira, V.M.; Tammela, P. Antibacterial profiling of abietane-type diterpenoids. Bioorg. Med. Chem. 2016, 25, 132–137. [Google Scholar] [CrossRef]
- Zhang, W.M.; Yang, T.; Pan, X.Y.; Liu, X.L.; Lin, H.X.; Gao, Z.B.; Yang, C.G.; Cui, Y.M. The synthesis and antistaphylococcal activity of dehydroabietic acid derivatives: Modifications at C12 and C7. Eur. J. Med. Chem. 2017, 127, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Pan, X.Y.; Yang, T.; Zhang, W.M.; Wang, T.Q.; Wang, H.Y.; Lin, H.X.; Yang, C.G.; Cui, Y.M. The synthesis and antistaphylococcal activity of dehydroabietic acid derivatives: Modifications at C-12. Bioorg. Med. Chem. Lett. 2016, 26, 5492–5496. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Roller, A.; Maulide, N. Synthesis and antimicrobial evaluation of novel analogues of dehydroabietic acid prepared by C-H-Activation. Eur. J. Med. Chem. 2017, 126, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.Y.; Duan, W.G.; Lin, G.S.; Liu, L.Z.; Zhang, R.; Li, D.P. Synthesis and antifungal activity of dehydroabietic acid-based 1,3,4-thiadiazole-thiazolidinone compounds. Mol. Divers. 2016, 20, 1–9. [Google Scholar] [CrossRef]
- Pertino, M.W.; Vega, C.; Rolón, M.; Coronel, C.; Arias, A.R.; Hirschmann, G.S. Antiprotozoal activity of triazole derivatives of dehydroabietic acid and oleanolic acid. Molecules 2017, 22, 369. [Google Scholar] [CrossRef]
- González, M.A.; Guaita, D.P.; Royero, J.C.; Zapata, B.; Agudelo, L.; Arango, A.M.; Galvis, L.B. Synthesis and biological evaluation of dehydroabietic acid derivatives. Eur. J. Med. Chem. 2010, 45, 811–816. [Google Scholar]
- Wada, H.; Kodato, S.; Kawamori, M.; Morikawa, T.; Nakai, H.; Takeda, M.; Saito, S.; Onoda, Y.; Tamaki, H. Antiulcer activity of dehydroabietic acid derivatives. Chem. Pharm. Bull. 1985, 33, 1472–1487. [Google Scholar] [CrossRef]
- Roa-Linares, V.C.; Brand, Y.M.; Agudelo-Gomez, L.S.; Tangarife-Castaño, V.; Betancur-Galvis, L.A.; Gallego-Gomez, J.C.; González, M.A. Anti-herpetic and anti-dengue activity of abietane ferruginol analogues synthesized from (+)-dehydroabietylamine. Eur. J. Med. Chem. 2016, 108, 79–88. [Google Scholar] [CrossRef]
- Kim, J.; Kang, Y.G.; Lee, J.Y.; Choi, D.H.; Cho, Y.U.; Shin, J.M.; Park, J.S.; Lee, J.H.; Kim, W.G.; Seo, D.B.; et al. The natural phytochemical dehydroabietic acid is an anti-aging reagent that mediates the direct activation of SIRT1. Mol. Cell. Endocrinol. 2015, 412, 216–225. [Google Scholar] [CrossRef]
- Huang, X.C.; Huang, R.Z.; Liao, Z.X.; Pan, Y.M.; Gou, S.H.; Wang, H.S. Synthesis and pharmacological evaluation of dehydroabietic acid thiourea derivatives containing bisphosphonate moiety as an inducer of apoptosis. Eur. J. Med. Chem. 2016, 108, 381–391. [Google Scholar] [CrossRef]
- Luo, D.J.; Ni, Q.; Ji, A.L.; Gu, W.; Wu, J.H.; Jiang, C.P. Dehydroabietic acid derivative QC4 induces gastric cancer cell death via oncosis and apoptosis. BioMed Res. Int. 2016, 2016, 2581061. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.C.; Wang, M.; Wang, H.S.; Chen, Z.F.; Zhang, Y.; Pan, Y.M. Synthesis and antitumor activities of novel dipeptide derivatives derived from dehydroabietic acid. Bioorg. Med. Chem. Lett. 2014, 24, 1511–1518. [Google Scholar] [CrossRef]
- Reddy, T.S.; Kulhari, H.; Reddy, V.G.; Subba-Rao, A.V.; Bansal, V.; Kamal, A.; Shukla, R. Synthesis and biological evaluation of pyrazolo–triazole hybrids as cytotoxic and apoptosis inducing agents. Org. Biomol. Chem. 2015, 13, 10136–10149. [Google Scholar] [CrossRef] [PubMed]
- Stefely, J.A.; Palchaudhuri, R.; Miller, P.A.; Peterson, R.J.; Moraski, G.C.; Hergenrother, P.J.; Miller, M.J. N-((1-Benzyl-1H-1,2,3-triazol-4-yl)methyl)arylamide as a new scaffold that provides rapid access to antimicrotubule agents: Synthesis and evaluation of antiproliferative activity against select cancer cell Lines. J. Med. Chem. 2010, 53, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Binh, L.H.; Van, N.T.T.; Kien, V.T.; My, N.T.T.; van Chinh, L.; Nga, N.T.; Tien, H.X.; Thao, D.T.; Vu, T.K. Synthesis and in vitro cytotoxic evaluation of new triazole derivatives based on artemisinin via click chemistry. Med. Chem. Res. 2016, 25, 738–750. [Google Scholar] [CrossRef]
- Valdomir, G.; de los Ángeles Fernández, M.; Lagunes, I.; Padron, J.I.; Martin, V.S.; Padron, J.M.; Davyt, D. Oxa/thiazole-tetrahydropyran triazole-linked hybrids with selective antiproliferative activity against human tumour cells. New J. Chem. 2018, 42, 13784–13789. [Google Scholar] [CrossRef]
- Reddy, V.G.; Reddy, T.S.; Nayak, V.L.; Prasad, B.; Reddy, A.P.; Ravikumar, A.; Taj, S.; Kamal, A. Design, synthesis and biological evaluation of N-((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)-1,3-diphenyl-1H-pyrazole-4-carboxamides as CDK1/Cdc2 inhibitors. Eur. J. Med. Chem. 2016, 122, 164–177. [Google Scholar] [CrossRef]
- Yadav, P.; Lal, K.; Kumar, A.; Guru, S.K.; Jaglan, S.; Bhushan, S. Green synthesis and anticancer potential of chalcone linked-1,2,3-triazoles. Eur. J. Med. Chem. 2017, 126, 944–953. [Google Scholar] [CrossRef]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click Chemistry: 1,2,3-triazoles as pharmacophores. Chem.-Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef]
- Wang, X.; Pang, F.H.; Huang, L.; Yang, X.P.; Ma, X.L.; Jiang, C.N.; Li, F.Y.; Lei, F.H. Synthesis and Biological Evaluation of Novel Dehydroabietic Acid-Oxazolidinone Hybrids for Antitumor Properties. Int. J. Mol. Sci. 2018, 19, 3116. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.Y.; Duan, W.G.; Liu, L.Z.; Li, F.Y.; Lu, M.P.; Liu, B.M. Synthesis and antifungal activity of dehydroabietic acid-based thiadiazole-phosphonates. Holzforschung 2015, 69, 1–7. [Google Scholar] [CrossRef]
- Li, F.Y.; Wang, X.; Duan, W.G.; Lin, G.S. Synthesis and in vitro anticancer activity of novel dehydroabietic acid-based acylhydrazones. Molecules 2017, 22, 1087. [Google Scholar] [CrossRef]
- Lin, G.S.; Duan, W.G.; Yang, L.X.; Huang, M.; Lei, F.H. Synthesis and antifungal activity of novel myrtenal-based 4-methyl-1,2,4-triazole-thioethers. Molecules 2017, 22, 193. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Miao, T.T.; Hua, D.W.; Jin, X.Y.; Tao, X.B.; Huang, C.B.; Wang, S.F. Synthesis and in vitro cytotoxic evaluation of new 1H-benzo[d]imidazole derivatives of dehydroabietic acid. Bioorg. Med. Chem. Lett. 2017, 27, 1296–1300. [Google Scholar] [CrossRef]
- Huang, R.Z.; Liang, G.B.; Huang, X.C.; Zhang, B.; Zhou, M.M.; Liao, Z.X.; Wang, H.S. Discovery of dehydroabietic acid sulfonamide based derivatives as selective matrix metalloproteinases inactivators that inhibit cell migration and proliferation. Eur. J. Med. Chem. 2017, 138, 979–992. [Google Scholar] [CrossRef]
- Nagarsenkar, A.; Guntuku, L.; Guggilapu, S.D.; Bai, K.D.; Srinivasulu, G.; Naidu, V.G.M.; Babu, B.N. Synthesis and apoptosis inducing studies of triazole linked 3-benzylidene isatin derivatives. Eur. J. Med. Chem. 2016, 124, 782–793. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2 and 3a–p are available from the authors. |

| Compound | IC50 (μM) | ||||
|---|---|---|---|---|---|
| CNE-2 | HepG2 | BEL-7402 | HeLa | HL-7702 | |
| 3a (R = H) | 33.40 ± 0.33 | >100 | 36.44 ± 070 | 67.88 ± 0.56 | >100 |
| 3b (R = o-F) | 21.44 ± 0.52 | 25.86 ± 0.27 | 18.63 ± 0.82 | 17.76 ± 0.31 | >100 |
| 3c (R = m-F) | 10.92 ± 0.21 | 5.90 ± 0.41 | 16.72 ± 0.06 | 20.05 ± 0.54 | >100 |
| 3d (R = p-F) | 12.20 ± 0.33 | 20.60 ± 0.09 | 14.84 ± 075 | 22.33 ± 0.35 | >100 |
| 3e (R = o-Cl) | 74.88 ± 0.14 | 45.08 ± 0.33 | 25.06 ± 0.32 | 44.28 ± 0.43 | >100 |
| 3f (R = m-Cl) | 59.03 ± 0.28 | 22.27 ± 0.35 | 26.09 ± 0.15 | 53.78 ± 0.32 | >100 |
| 3g (R = p-Cl) | 60.73 ± 066 | >100 | 38.04 ± 0.48 | >100 | >100 |
| 3h (R = o-Br) | 48.30 ± 0.27 | 35.42 ± 0.21 | 27.26 ± 0.36 | 25.90 ± 0.20 | >100 |
| 3i (R = m-Br) | 44.14 ± 0.22 | 40.66 ± 0.62 | 36.88 ± 0.23 | 56.08 ± 0.36 | >100 |
| 3j (R = p-Br) | >100 | 23.40 ± 0.32 | 14.53 ± 0.62 | 43.18 ± 0.22 | >100 |
| 3k (R = o-NO2) | 44.90 ± 0.32 | 6.25 ± 0.37 | 18.62 ± 0.26 | >100 | >100 |
| 3l (R = m-NO2) | 11.45 ± 0.18 | 15.83 ± 0.64 | 15.39 ± 0.51 | 67.37 ± 0.33 | >100 |
| 3m (R = p-NO2) | 19.61 ± 0.38 | >100 | 22.81 ± 0.22 | 22.48 ± 0.35 | >100 |
| 3n (R = m-CH3) | >100 | 60.18 ± 0.39 | 23.61 ± 0.44 | 25.32 ± 0.81 | >100 |
| 3o (R = p-CH3) | >100 | 51.78 ± 0.43 | 41.89 ± 0.72 | 42.51 ± 0.37 | >100 |
| 3p (R = m-OCH3) | 80.98 ± 0.78 | >100 | 25.03 ± 0.22 | 24.66 ± 0.16 | >100 |
| DHAA | 88.64 ± 0.73 | 80.36 ± 0.84 | 46.70 ± 0.55 | 37.40 ± 0.64 | >100 |
| Cisplatin | 8.75 ± 0.24 | 6.42 ± 0.18 | 12.68 ± 0.33 | 1.94 ± 0.20 | 20.76 ± 0.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.-Y.; Huang, L.; Li, Q.; Wang, X.; Ma, X.-L.; Jiang, C.-N.; Zhou, X.-Q.; Duan, W.-G.; Lei, F.-H. Synthesis and Antiproliferative Evaluation of Novel Hybrids of Dehydroabietic Acid Bearing 1,2,3-Triazole Moiety. Molecules 2019, 24, 4191. https://doi.org/10.3390/molecules24224191
Li F-Y, Huang L, Li Q, Wang X, Ma X-L, Jiang C-N, Zhou X-Q, Duan W-G, Lei F-H. Synthesis and Antiproliferative Evaluation of Novel Hybrids of Dehydroabietic Acid Bearing 1,2,3-Triazole Moiety. Molecules. 2019; 24(22):4191. https://doi.org/10.3390/molecules24224191
Chicago/Turabian StyleLi, Fang-Yao, Lin Huang, Qian Li, Xiu Wang, Xian-Li Ma, Cai-Na Jiang, Xiao-Qun Zhou, Wen-Gui Duan, and Fu-Hou Lei. 2019. "Synthesis and Antiproliferative Evaluation of Novel Hybrids of Dehydroabietic Acid Bearing 1,2,3-Triazole Moiety" Molecules 24, no. 22: 4191. https://doi.org/10.3390/molecules24224191
APA StyleLi, F.-Y., Huang, L., Li, Q., Wang, X., Ma, X.-L., Jiang, C.-N., Zhou, X.-Q., Duan, W.-G., & Lei, F.-H. (2019). Synthesis and Antiproliferative Evaluation of Novel Hybrids of Dehydroabietic Acid Bearing 1,2,3-Triazole Moiety. Molecules, 24(22), 4191. https://doi.org/10.3390/molecules24224191